Serum Homocysteine Levels in Patients with Retinal Vein Occlusion in a Spanish Population
Abstract
Purpose
Our aim was a) to compare serum Hcy levels in patients with RVO and population-based controls, and b) to evaluate whether hyperhomocysteinemia is a risk factor for RVO.
Patients and methods
Prospective case-control study of all patients diagnosed with RVO at a tertiary-care hospital, and age-and sex-matched controls taking part in a population-based prospective cohort in the same geographic area. Differences in serum Hcy between both groups were analyzed by a general linear model adjusted for age, body mass index (BMI), glomerular filtration rate (GFR), hypertension, dyslipidemia, diabetes, tobacco use and alcohol intake. Besides, we tested the relationship between hyperhomocysteinemia (> 15 µmol/L) and RVO, by a stepwise logistic regression analysis.
Results
RVO patients (n = 85) had a higher prevalence of hypertension (p = 0.002), diabetes (p = 0.008), and dyslipidemia (p = 0.04) than controls (n = 82). Adjusted median Hcy levels were higher in RVO patients (p < 0.0001). Adjusted OR for hyperhomocysteinemia were 4.4 (95% CI, 2.0-9.3; p < 0.0001) in the overall sample, and 2.6 (95% CI, 1.04-6.6; p = 0.04) and 6.1 (95% CI, 1.7-21.6; p = 0.005) for men and women, respectively.
Conclusion
Patients with RVO have higher serum Hcy levels than age- and sex-matched population-based controls. Hyperhomocysteinemia seems to be a risk factor for RVO, independent of age, BMI, GFR and classical vascular risk factors.
Keywords
Retinal vein occlusion, Homocysteine, Hyperhomocysteinemia, Risk factor
Introduction
Retinal vein occlusion (RVO) is one of the most common causes of vision loss in the elderly and has been associated with an increase in cardiovascular mortality [1]. Classical vascular risk factors (VRF) represent the main etiopathogenic factors for RVO. In fact, it is usually thought that the disorder may be considered as a manifestation of systemic atherosclerosis [2].
On the other hand, several studies have suggested that a hypercoagulable state could be involved in some patients with RVO, although this has not been conclusively established [3,4]. In line with this, we have recently shown that acquired thrombophilia (antiphospholipid syndrome and hyperhomocysteinemia) should be considered in the clinical assessment of patients with RVO, whereas genetic thrombophilia must be only ruled out in patients aged < 50 years [5-7].
Homocysteine (Hcy) is a sulfhydryl-containing amino acid formed as an intermediate product of methionine and cysteine metabolism [8]. The first report suggesting a proatherogenic effect of Hcy was published by McCully in 1969 [9]. Since then, increased Hcy levels (above 15 µmol/L) have been related to increased risk of stroke, myocardial infarction and venous thrombosis [10-12].
Several mechanisms have been proposed to explain the possible contribution of serum Hcy to development of RVO, and include the activation of factor V, the increased oxidation of low-density lipoprotein (LDL), the inhibition of plasminogen activator binding, and the activation of protein C [13].
There are some studies analyzing the relationship between serum Hcy levels and RVO [14-19]. Nevertheless, most of them are retrospective in nature, have substantial heterogeneity, adjustment for potential confounder is not well defined, and well-characterized population-based controls are scarce. For that reasons, meta-analysis on the association between serum Hcy and RVO, have reported a high level of heterogeneity among studies, included case-series studies, and only indirectly compared cases and controls. Therefore, the results have been questioned [15,20,21]. In the most recent systematic review and meta-analysis of case-controls studies, Li, et al. [15] founded some evidence that plasma Hcy was related to a moderate increase in RVO. However, the same limitations reported for previous meta-analysis have been found: substantial heterogeneity among included studies, publication and selection bias, lack of adjustment for important confounding factors, unclear definition or selection of controls, and absence of population-based data.
To get deeper insight and shed some light in the association between Hcy and RVO, we have carried out a prospective case-control study with a well-defined cohort of patients diagnosed with RVO and age- and sex-matched population-based controls.
Patients and Methods
We conducted a prospective case-control study, from July 2012 to January 2015, at a tertiary-care center that serves as a reference hospital for a population of 350.000 inhabitants in Northern Spain. Based on clinical, fundoscopic and angiographic criteria, all patients diagnosed with RVO at the Division of Ophthalmology, were referred and assessed at our Internal Medicine outpatient clinic department.
A randomly selected age-and sex-matched control group of 82 subjects taking part in a population-based prospective cohort study in the same geographic area [22] were also screened for plasma Hcy levels between April 2013 and January 2015. Those with renal failure (defined as a glomerular filtration rate < 60 ml/min/1.72 m2 or a previous diagnosis of chronic kidney disease) or previous arterial or venous RVO were excluded from the present study. Serum vitamin B12 or folate deficiency was ruled out at baseline, and none of the cases and controls received vitamin B12 or folic acid supplementation. All the subjects gave written informed consent. The study protocol was approved by the ethical committee and conducted in accordance with the Declaration of Helsinki.
Clinical variables
Data were collected using a prespecified standardized questionnaire, in a computerizing database. We assessed the following clinical variables: age, sex, weight, height, body mass index (BMI), current tobacco use, alcohol intake (> 20 g per day), high blood pressure (equal or greater than 140/90 mm Hg or being on antihypertensive agents), dyslipidemia (serum total cholesterol or triglyceride levels greater than 230 mg/dl and 150 mg/dl respectively or being on lipid-lowering drugs), diabetes mellitus (according to the ADA criteria) [23], past or present history of thromboembolic disease in another location different from retinal, history of ischemic heart disease, stroke or peripheral arterial disease, type of RVO (central or branch), and prescribed treatments.
Laboratory parameters and Hcy measurement
Routine biochemical parameters were measured by standard automated methods in an ADVIA 2400 Chemistry System autoanalyzer (Siemens). Blood samples were obtained from an antecubital vein in the morning, after a requested 12-hour overnight fast.
We estimated glomerular filtration rate (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations [24].
We measured total serum Hcy by chemiluminescent immunoassay (Immulite 2000 Xpi; Siemens®). Serum sample releases Hcy from its binding proteins. It is converted to S-adenosyl-homocysteine (SAH) in the presence of S-adenosyl-L-Hcy hydrolase and dithiothreitol (DTT). After 30 minute incubation, the treated sample is transferred to a second reaction tube containing a SAH-coated polystyrene bead and an alkaline phosphatase-labeled antibody specific for SAH. During 30 minute incubation, the converted SAH from the sample pretreatment competes with immobilized SAH for binding alkaline phosphatase-labeled anti-SAH antibody conjugate. Unbound enzyme conjugate is removed by a centrifugal wash. The bound label is then quantified using the dioxetane substrate to produce light. Light is emitted when the chemiluminescent substrate reacts with the alkaline phosphatase label bound to the bead. The amount of light emitted is inversely proportional to the amount of analyte originally present in the sample. This light emission is detected by the photomultiplier tube (PMT) and results are calculated for each sample. This method have been highly correlated with HPLC (r = 0.97). The range of measurement is 2-50 µmol/L with a sensitivity of 1,2 µmol/L. Samples greater than 50 µmol/L are diluted 1/3 with the specific Hcy diluent; the linearity is 2-150 µmol/L. The precision method was repeatedly assayed in quadruplicate over the course of several days, for a total of 20 runs and 80 replicates. The within-assay coefficients of variation were 12,8% (4,2 µmol/L); 8,1% (11 µmol/L); 5,9% (16,5 µmol/L) and 5,6% (24,5 µmol/L). Serum Hcy levels greater than 15 µmol/L were considered as high levels. Hcy was measured in the first visit at our Internal Medicine Department, 4-5 days after the RVO diagnosis, and always during the first 2-weeks.
Statistical analysis
Baseline characteristics of the population were calculated for the total sample and for men and women separately. Serum Hcy was not normally distributed and was log-transformed before analysis. Results were expressed as mean ± SD, median (interquartile range-IQR-) or percentages, as appropriate. Student's t test or Mann-Whitney U-test were used to determine the differences between groups for continuous variables, and χ2-test for categorical variables. We constructed a general linear model to test the differences in serum Hcy levels between cases and controls. Multivariable models were adjusted for age, BMI, GFR, hypertension, dyslipidemia, diabetes mellitus, and tobacco use and alcohol intake. Besides, further adjustment for past history of ischemic heart disease, stroke, and peripheral arterial disease and thromboembolic disorders was performed.
Finally, we have developed a stepwise logistic regression analysis to test the relationship between hyperhomocysteinemia, (defined as a serum Hcy level above 15 µmol/L) and RVO. Odds ratios (ORs) (95% CI) were calculated and regression models were adjusted for the same covariates above mentioned. All analyses were conducted using SPSS 20.0 (Chicago, IL, USA). A p value < 0.05 was considered statistically significant in all the calculations.
Results
Eighty-five patients (45 men and 40 women; mean age, 69 ± 12 years) and 82 controls (43 men and 39 women; mean age, 67 ± 9 years), were included in the study. Fifty-five patients (64.7%) had central RVO and 30 (35.3%) had branch RVO.
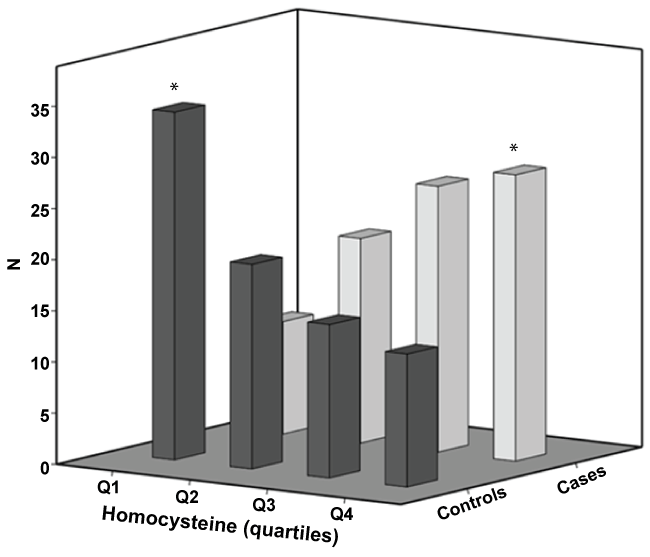
Baseline characteristics of the population are summarized in (Table 1). Overall, classical vascular risk factors (hypertension, type 2 diabetes mellitus and dyslipidemia), were significantly more prevalent in patients with RVO than in population-based controls. There was no differences between patients with central versus branch RVO, regarding Hcy levels (p = 0.8). (Figure 1) shows the distribution of cases and controls across serum Hcy levels quartiles. The number of patients with RVO was the highest in the last quartile whereas it was the lowest in the group of controls. These figures are diametrically opposed in the first serum Hcy quartile.
Median serum Hcy levels as well as hyperhomocysteinemia defined as a cutoff value of 15 µmol/L were higher in cases than in controls (p < 0.0001, respectively). After adjusting for age, BMI, glomerular filtration rate, hypertension, dyslipidemia, diabetes mellitus, tobacco use and alcohol intake, differences in serum Hcy levels remained significant (15.6 vs. 14.2 µmol/L; p = 0.008). (Table 2) shows linear general model adjusted for these covariates and stratified by sex. Associations between serum Hcy and RVO was significant in women (p = 0.02) and borderline significant in the group of men (p = 0.06). Further adjustment for past history of ischemic heart disease, stroke, peripheral arterial disease and thromboembolic disorders, did not virtually change these results (data not shown).
Unadjusted OR for hyperhomocysteinemia was 3.4 (95% CI, 1.7-6.6; p < 0.0001) in the overall sample, and 2.6 (95% CI, 1.1-6.3; p = 0.03) and 4.9 (95% CI, 1.7-14.5; p = 0.003) for men and women respectively. The correspondent figures after adjustment for confounders were 4.4 (95% CI, 2.0-9.3; p < 0.0001); 2.6 (95% CI, 1.04-6.6; p = 0.04), and 6.1 (95% CI, 1.7-21.6; p = 0.005).
Discussion
In the last years, Hcy has been recognized as an atherosclerotic and thrombotic risk factor [25,26]. However, its role in the pathological changes associated with atherosclerosis as well as the intrinsic mechanisms triggered by Hcy accumulation is unclear to date.
Hyperhomocysteinemia would act as an atherosclerotic risk factor in several ways. Firstly, Hcy metabolites can combine with LDL-cholesterol to produce aggregates, which may be taken by macrophages in the arterial intima, and contribute to the development of the atherosclerotic plaque [27]. Nevertheless, the effects of hyperhomocysteinemia on plaque rupture or instability are poorly understood although obstruction of vasa vasorum by aggregates of microorganisms with homocysteinylated low-density lipoproteins has been proposed to cause arterial wall ischemia and a microabscess of the intima, leading to vulnerable atherosclerotic plaque [28]. Secondly, Hcy may activate inflammatory responses that lead to the recruitment of monocytes to the arterial wall [27]. Thridly, high Hcy levels may dysregulate lipid metabolism in vascular cells through activation of the sterol regulatory element-binding protein family of transcription factors [29]. Finally, hyperhomocysteinemia also increases smooth muscle cell proliferation, and its oxidation would form free radicals, inducing endothelial damage [30].
Although RVO pathogenesis remains still unclear, the disease is considered a manifestation of the atherosclerosis process [31]. In this sense, classical vascular risk factors, such as hypertension, diabetes, dyslipidemia and smoking have been reported as the main predisposing factors for RVO [32]. In this sense, it has been suggested that hyperhomocysteinemia may lead to an enhancement of the adverse effects of these classical risk factors [33].
The role of hyperhomocysteinemia as a risk factor for RVO is an ongoing matter of debate. Several publications have found positive associations, but negative results have also been reported [16,18,34]. Besides, three meta-analysis have been published to date, and all of them have suggested that there is some evidence that serum Hcy is associated with RVO. Nevertheless, authors indicate that these analyses should be interpreted cautiously because of marked heterogeneity between the study estimates and possible effect of publication bias. In fact, Li, et al. [15] have included in their systematic review and meta-analysis only 9 studies that reported an association between serum Hcy levels and RVO. They showed that 1 µmol/L increase in Hcy plasma levels was associated with a significant OR of 1.14 (95%CI, 1.07-1.21) in the random-effects model. However, the heterogeneity was higher, so no definite conclusions can be drawn.
In this scenario, the studies analyzing the association between Hcy and RVO have many methodological weaknesses, which may lead to misunderstanding this relationship. Thus, the difficulty to assess Hcy levels regarding to sample management issues (fasting status, extraction and conservation of the sample, etc.; substantial heterogeneity among studies (design, sample size, confounding variables); limitations in the selection of controls or no adequate matchinchg, and absence of population based-studies have been the main limitations found in these studies.
In a recent study from Spain, Martinez, et al. [35], analyzed the overall risk factors associated with RVO, and showed that hyperhomocysteinemia was higher in patients with RVO than in some cohorts from general population and similar to the results obtained in a cohort of venous thromboembolic disease. However, composition of control cohorts was rather heterogeneous, with some missing data for important variables, such as dyslipidemia, and above all, adjustment for confounding variables was lacking. Therefore, this study cannot be compared with ours.
We have designed a case-control study including a well-defined prospective cohort of patients with RVO and a subset of age- and sex-matched controls from the general population of our region. Besides, we were able to control for the main variables reported as confounders for the association between serum Hcy and RVO. We have found that patients with RVO have significant higher serum Hcy concentrations than controls, independently of sex, age, BMI, glomerular filtration rate, hypertension, dyslipidemia, diabetes mellitus, tobacco use and alcohol intake, and even of past history or cardiovascular or thromboembolic diseases. When stratified by sex, this finding was particularly relevant in the group of women. Furthermore, we also found that hyperhomocysteinemia (serum levels above 15 µmol/L) was associated with a 4.5-fold increase in the overall risk for developing RVO (2.6 in the case of men and 6.1 in the group of women).
We found some weaknesses in our study. Firstly, given its cross-sectional design it is not possible to determine the temporal nature of the observed relationships, for which prospective data are certainly needed. Secondly, those derived from the time interval after RVO and serum Hcy extraction. In fact, it has been pointed out that the vascular occlusive event itself could increase plasma Hcy concentration [20]. However, we have obtained the samples at least 4-5 days after the episode of RVO, and during the first 2-week, and this possible effect has therefore been minimized. Thirdly, methylenetetrahydrofolate reductase (MTHFR) C677T genotype has not been determined. Nevertheless, there was no evidence to suggest an association between homozygosity for the MTHFR C677T genotype and RVO [15].
This study was supported in part by grants from the "Fondo de Investigación Sanitaria", Ministerio de Sanidad y Consumo, Spain (FIS: PI05 0125 and FIS: PI08 0183). The authors report no conflicts of interest or financial disclosures regarding this paper.
Conclusion
In summary, RVO may be considered as a manifestation of systemic atherosclerosis, and classical risk factors should be controlled, according to the current guidelines, to avoid relapses. We suggest that serum Hcy should be measured in RVO patients, in order to consider to treat hyperhomocysteinemia, given the few adverse effects of vitamin B12 and folate supplementation. Nevertheless, further prospective and controlled trials, with strong cardiovascular end-points, are needed to ascertain the usefulness of this therapeutic approach in these patients.
References
- Rogers S, McIntosh RL, Cheung N, et al. (2010) The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology 117: 313-319.
- Kolar P (2014) Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol 2014: 724-780.
- Claudia Kuhli-Hattenbach, Inge Scharrer, Marc Lüchtenberg, et al. (2010) Coagulation disorders and the risk of retinal vein occlusion. Thromb Haemost 103: 299-305.
- Squizzato A, Manfredi E, Bozzato S, et al. (2010) Antithrombotic and fibrinolytic drugs for retinal vein occlusion: a systematic review and a call for action. Thromb Haemost 103: 271-276.
- Napal Lecumberri JJ, Sedano Balbas C, Canal Villanueva J, et al. (2013) Thrombophilia and vascular risk factors in retinal vein occlusion. Rev Clin Esp (Barc) 213: 229-234.
- Janssen MC, den Heijer M, Cruysberg JR, et al. (2005) Retinal vein occlusion: A form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost 93: 1021-1026.
- Napal JJ, Neila S, Pérez-Montes R, et al. (2016) The role of coagulation disorders in patients with retinal vein occlusion. QJM 109: 97-102.
- Varga EA, Sturm AC, Misita CP, et al. (2005) Cardiology patient pages. Homocysteine and MTHFR mutations: relation to thrombosis and coronary artery disease. Circulation 111: 289-293.
- McCully KS (1969) Vascular pathology of homocysteinemia: implications for the pathogenesis of artheriosclerosis. Am J Pathol 56: 111-128.
- Kang SS, Wong PW, Malinow MR (1992) Hyperhomocysteinemia as a risk factor for occlusive vascular disease. Annu Rev Nutr 12: 279-298.
- David S Wald, Malcolm Law, Joan K Morris (2002) Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ 325: 1202-1206.
- Homocysteine studies collaboration (2002) Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 288: 2015-2022.
- Hajjar KA (1993) Homocysteine-induced modulation of tissue plasminogen activator binding to its endothelial cell membrane receptor. J Clin Invest 91: 2873-2879.
- Sottilotta G, Siboni SM, Latella C, et al. (2010) Hyperhomocysteinemia and C677T MTHFR genotype in patients with retinal vein thrombosis. Clin Appl Thromb Hemost 16: 549-553.
- Li D, Zhou M, Peng X, et al. (2014) Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism, and risk of retinal vein occlusion: an updated meta-analysis. BMC Ophtalmol 14: 147.
- Lahiri KD, Dutta J, Datta H, et al. (2013) Hyperhomocysteinemia, as an independent risk factor for retinal venous occlusion in an Indian population. Indian J Clin Biochem 28: 61-64.
- Al Wadani F, Khandekar R, Salim G, et al. (2014) Hyperhomocysteinia is a risk factor for retinal venous occlusion: a case control study. Indian. J Ophthalmol 62: 291-294.
- Dong N, Wang B, Chu L, et al. (2013) Plasma homocysteine concentrations in the acute phase after central retinal vein occlusion in a chinese population. Curr Eye Res 38: 1153-1158.
- Minniti G, Calevo MG, Giannattasio A, et al. (2014) Plasma homocysteine in patients with retinal vein occlusion. Eur J Ophthalmol 24: 735-743.
- McGimpsey SJ, Woodside JV, Cardwell C, et al. (2009) Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism, and risk of retinal vein occlusion: a meta-analysis. Ophthalmology 116: 1778-1787.
- Cahill MT, Stinnett SS, Fekrat S (2003) Meta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol 136: 1136-1150.
- Hernandez JL, Olmos JM, Ramos C, et al. (2009) Serum lipids and bone metabolism in Spanish men: the Camargo cohort study. Endocr J 57: 51-60.
- American Diabetes Association (2011) Standards of medical care in diabetes-2011. Diabetes Care 34: S11-S61.
- Levey AS, Stevens LA, Schmid CH, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604-612.
- Salahuddin MI, Ahmed SI (2005) Homocysteine level in patients with established transmural myocardial infarction. J Coll Physicians Surg Pak 15: 520-523.
- Weiss N (2005) Mechanisms of increased vascular oxidant stress in hyperhomocys-teinemia and its impact on endothelial function. Curr Drug Metab 6: 27-36.
- McCully KS (1996) Homocysteine and vascular disease. Nat Med 2: 386-389.
- Kilmer S McCully (2016) Homocysteine metabolism, atherosclerosis and diseases of aging. Compr Physiol 6: 471-505.
- Welch GN, Loscalzo GN (1998) Homocysteine and atherothrombosis. N Engl J Med 338: 1042-1050.
- Mansoor MA, Bergmark C, Svardal AM, et al. (1995) Redox status and protein binding of plasma homocysteine and other aminothiols in patients with early-onset peripheral vascular disease. Homocysteine and peripheral vascular disease. Arterioscler Thromb Vasc Biol 15: 232-240.
- Kaul S, Zadeeh AA, Shah PK (2006) Homocysteine hypothesis for atherothrombotic cardiovascular disease: not validated. J Am Coll Cardiol 48: 914-923.
- Mohamed Q, McIntosh RL, Saw SM, et al. (2007) Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 114: 507-519.
- Ganguly P, Alam SF (2015) Role of homocysteine in the development of cardiovascular disease. Nutr J 14: 6.
- Pinna A, Carru C, Zinellu A, et al. (2006) Plasma homocysteine and cysteine levels in retinal vein occlusion. Invest Ophthalmol Vis Sci 47: 4067-4071.
- Martinez F, Furio E, Fabia MJ, et al. (2014) Risk factors associated with retinal vein occlusion. Int J Clin Pract 68: 871-881.
Corresponding Author
José L Hernández MD, PhD, Department of Internal Medicine, Hospital Marqués de Valdecilla, Avda. Valdecilla s/n, 39008 Santander, Spain, Tel: 34-942-202513.
Copyright
© 2017 Hernández JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.