12-Month Follow Up of Combined Cataract Surgery with Schlemm's Canal Scaffolding for the Treatment of Primary Angle Closure Glaucoma
Abstract
Purpose: The purpose of this study is to assess the potential of a surgical implant to bypass trabecular meshwork and scaffold Schlemm's canalfor the reduction of IOP in eyes with primary angle closure or primary angle closure glaucoma and cataract.
Patients and Methods: Eyes with significant senile cataract and primary angle closure glaucoma (PACG) or primary angle closure (PAC) and at least 90 degrees of appositional or synechialirido-trabecular contact with IOP ≥ 21 mmHg on ≥ 1 medication or ≥ 24 mmHg on zero medications were included. Study eyes received the Hydrus® Microstent (Ivantis, Inc.) after successful phacoemulsification. In cases where the nasal quadrant remained closed after phaco, the angle was opened for 2-3 clock hours using goniosynechialysis to expose the trabecular meshwork for microstent implantation. Patients were followed for 12 months.
Results: Twenty eyes were treated in the study. Mean preoperative IOP was 25.0 ± 4.2 mmHg and mean medications count was 1.4 ± 1.1. Thirteen eyes required goniosynechialysis to expose the nasal quadrant for device implantation. At 12-months, mean IOP was 14.5 ± 2.5 mmHg and 94.7% of eyes were medication free. There was no difference in IOP between eyes that required goniosynechialysis prior to microstent implantation and those that did not. Further, there was no correlation between the degree of baseline angle closure and 12 month IOP, nor was there a correlation between the degree of 12-month angle closure and 12 month IOP.
Conclusions: Implantation of a Schlemm's canal microstent after phacoemulsification in PACG is feasible and results in significant near term IOP and medication reduction.
Keywords
Hydrus® Microstent, Schlemm's canal, Goniosynechialysis PACG, Phacoemulsification
Introduction
Glaucoma is a collection of disorders characterized by progressive loss of visual field due to optic nerve damage, and is the leading cause of irreversible blindness in the world, according to the World Health Organization (WHO) [1]. Population studies have estimated that Asians account for approximately half of the world's glaucoma cases [2] and over 80% of Primary Angle Closure Glaucoma (PACG) cases are in Asia [2,3]. It is currently estimated that 20 million individuals are affected by PACG [2], and the prevalence is expected to increase to approximately 34 million cases, 5.3 millions of whom will be blind, by the year 2040 [4,5].
The pathophysiology of angle closure glaucoma is characterized by the peripheral iris coming into contact with the trabecular meshwork, in either an appositional (intermittent) or synechial (permanent) manner. Irido-trabecular contact may significantly decrease aqueous outflow through the conventional outflow pathway (i.e. through the trabecular meshwork and Schlemm's canal), thereby elevating intraocular pressure (IOP). Severe or chronically elevated IOP can lead to glaucomatous optic neuropathy and associated visual field and visual acuity loss.
Historically, angle closure glaucoma has utilized laser peripheral iridotomy and topical medication as mainstays of disease management and have been the historic standard of care in the management of angle closure. However, several studies havealso shown the potential for cataract extraction as a first-line treatment in eyes with angle closure glaucoma and cataract [6-8]. More recently, the results from a large multi-center randomized controlled trial (EAGLE Study) [9] confirmed the utility of cataract extraction as a first line treatment for PACG. The study was comprised of 419 subjects from 30 centers in 5 countries, with subjects randomized to either crystalline lens extraction or laser iridotomy/medications (standard care) with follow-up for 36 months. The 3-year study results demonstrated significantly higher quality of life scores and intraocular pressure lowering for subjects in the cataract extraction group, compared with subjects in the standard care group [10].
In the EAGLE study, subjects in the lens extraction group achieved a statistically significant reduction in IOP compared to iridotomy. However, the difference was small and may not be clinically meaningful (-1.18 mmHg between-group difference, p = 0.004). The Hydrus Microstent in conjunction with cataract surgery has been shown to significantly reduce IOP compared to cataract surgery alone in 2 separate prospective randomized studies [11,12]. The Hydrus Microstentwas developed for the purpose of decreasing IOP by enhancing outflow through the canalicular outflow system. It provides a direct path through the trabecular meshwork and scaffolds Schlemm's canal over approximately 25% of its circumference. In this manner, the Hydrus provides both bypasses the most significant barrier to outflow while also providing access to multiple aqueous collector channels. The Hydrus mechanism of action is therefore complementary to cataract surgery and may provide additional IOP lowering in PACG. Previous studies have shown that the risk of glaucoma progression is reduced 10% for every 1 mm Hg reduction in IOP [13]. The aim of this study is to assess the IOP lowering potential of the Hydrus Microstent in addition to lens extraction in angle closure glaucoma population.
Methods
Study design
This was a prospective, single arm feasibility trial of the Hydrus Microstent for the treatment of Primary Angle Closure (PAC) and Primary Angle Closure Glaucoma (PACG) with adjunctive cataract. Approval of the institutional research board was secured prior to the start of the study and was conducted to the tenets of the Declaration of Helsinki. Study subjects were recruited from the cataract surgery population of the Asian Eye Institute and written informed consent was obtained prior to subject participation in study related examinations. Potential subjects were screened for eligibility at 2 preoperative examinations and scheduled for surgery if all criteria were met. All eyes had phacoemulsification and intraocular lens (IOL) implantation. Eyes with synechial closure in the target quadrant received goniosynechialysis to open approximately 2 clock hours to expose the trabecular meshwork.
Inclusion criteria
Consenting patients who had age related cataract and a diagnosis of mild to moderate primary angle closure (PAC) or primary angle closure glaucoma (PACG)with appositional or synechialirido-trabecular contact of 90 were eligible. A preoperative IOP ≥ 21 mmHg on ≥ 1 medication or ≥ 24 mmHg on 0 medications was required for the participation in the study.
Exclusion criteria
Exclusion criteria included all forms of glaucoma other than PAC or PACG, advanced glaucoma defined as a visual field mean deviation worse than -15 dB, or visual field depression within the central 5 of fixation, or a cup-to-disc ratio 0.9. Prior corneal, refractive, or glaucoma surgery or other significant ocular pathology (diabetic retinopathy, corneal dystrophy, age-related macular degeneration) were excluded. Prior laser iridotomy was allowed.
Patient follow-up
Follow-up visits were conducted at 1 day, 7 days, and 1, 3, 6, and 12 months postoperative. Study subjects could return for unscheduled postoperative visits at any time without limitation. Slit-lamp findings, BCVA, and IOP were assessed at all postoperative visits, and gonioscopy was performed on all but the Day 1 visit.
Study device
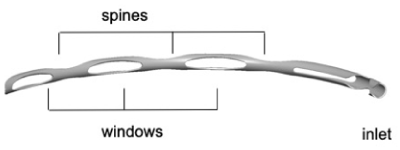
The Hydrus Microstent is an aqueous drainage device made from nitinola metal alloy composed of nearly equal parts of nickel (Ni) and titanium (Ti). Nitinol has been used extensively in a variety of implantable vascular and orthopedic devices for its proven properties of flexibility, strength and biocompatibility. As a shape memory alloy, nitinol has super elastic properties making it suitable as a support structure in Schlemm's canal. The Hydrus Microstent was thermally set to a curvature consistent with Schlemm's canal (SC) as shown in (Figure 1). The device is designed for ab interno placement through the trabecular meshwork (TM) into SC. The inlet segment of the device resides in the anterior chamber (AC) and provides a direct aqueous pathway into SC. The stent dilates a quadrant of Schlemm's canal, and three windows face the TM, providing additional pathways for aqueous to enter SC. The device is semicircular in cross section to avoid the possibility of collector channel obstruction. The device is delivered with a hand-held injector controlled directly by the surgeon. The tip of the injector cannula is designed to perforate the trabecular meshwork and create an entry into Schlemm's canal. The implant is manually advanced into SC through the cannula.
Surgical procedure
Patients were administered preoperative antibiotics, dilating agents and anti-inflammatory medications according to the local standard practice for cataract surgery. Clear corneal phacoemulsification was performed followed by implantation of an IOL. Any complicated cataract surgeries (capsule tears, IOL not placed in the bag, etc.) were excluded from the study. Prior to implanting the microstent, the patient's head was tilted nasally to provide a clear view of the nasal angle structures through a surgical gonioprism. A cohesive ophthalmic viscoelastic device (OVD) was introduced to fill the AC and expand the angle after phacoemulsification. If the trabecular meshwork in the nasal quadrant was not exposed due to the cataract surgery, focal goniosynechialysis was performed to create an access point for the Hydrus. The microstent injector was then introduced into the AC through the clear corneal incision. Using the injector cannula, the trabecular meshwork was incised and the microstent was advanced into Schlemm's canal. Upon visual confirmation of proper device positioning in the canal, the delivery system was withdrawn, the OVD was removed and replaced with balanced salt solution.
Postoperative medications
Postoperatively, a topical antibiotic was administered for 7 days and a tapering dose of a topical corticosteroid for up to 4 weeks. If required, topical hypotensive medications were reintroduced post operatively. If more than one medication was required, medications were initiated one at a time. Medications were removed if warranted by IOP findings at later visits.
Endpoints
The primary effectiveness measure was the proportion of eyes with unmedicated mean IOP reduction ≥ 20% compared to baseline. The secondary effectiveness endpoint was the mean change in IOP from preoperative to follow-up. Other outcome measures include mean IOP, changes in mean medication count, and overall proportion of eyes medication free. Safety evaluation rates of intra operative complications, changes in BCVA, ocular health, and the observed rate of ocular adverse events.
Statistical analysis
Only eyes with successful implants are included in the analysis. Mean and standard deviation for measurements are presented as continuous variables. Differences between preoperative and postoperative measurements were tested with the use of two sample t-tests. For categorical variables, counts and percentages are presented according to treatment group and comparisons made with the use of the Fisher's exact test for binary variables.
Results
Baseline characteristics of the study patients
Patient demographics are summarized in (Table 1). A total 20eyes were included in the study. Study subjects were 75% female, mean age was 66.8 ± 6.5 years, and 100% were Asian. Preoperative ocular characteristics are presented in (Table 2). Study subjects had either primary angle closure (45%) or primary angle closure glaucoma (55%) with mild to moderate severity. Five patients were newly diagnosed at the screening visit and were not taking glaucoma medications. The average preoperative IOP (based on 2 visits) was 25.0 ± 4.2 mmHg (range 21-38 mmHg). The mean amount of angle closure was 298 ± 79°; most eyes had >180° of circumferential angle closure and 11/20 (55%) of eyes had complete 360° closure. All treated eyes completed each scheduled follow-up visit through 6 months. One patient died after the 6-month visit, and so 19/20 treated subjects were available for evaluation at 12 months.
Device implantation
The device was placed within Schlemm's canal in the nasal quadrant in 20eyes after phacoemulsification. While the phacoemulsification procedure opened the angle to a large degree, 13/20 eyes (65%)still required 2-3 clock hours of goniosynechialysis (GSL) in order to expose the trabecular meshwork adequately to allow for device placement. In 1 case the angle view was obscured by blood after GSL, resulting in multiple attempts before the device was placed in position. This case resulted in postoperative layered hyphema of 1-2 mm, which resolved by the 1week visit. Corneal edema was noted in 3 cases on the first day postoperative, which is not uncommon following cataract surgery. All edema was resolved by the 1 week visit without treatment or sequelae.
Endpoints
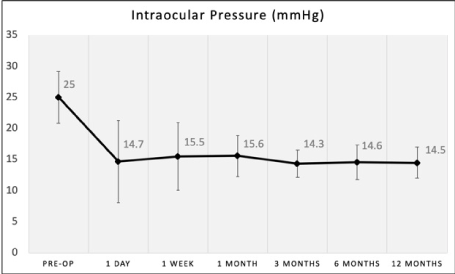
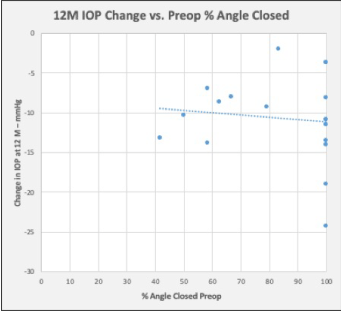
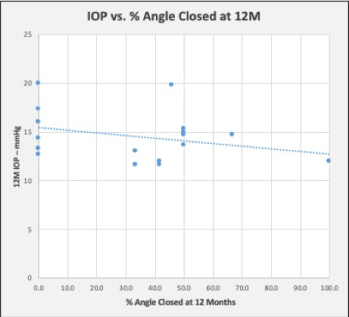
IOP and medication findings for each visit are presented in (Table 3). Mean IOP was stable throughout the follow up period (range 14.3 – 15.6 mmHg), as shown in (Figure 2). The mean IOP at 12months was 14.6 ± 2.5 mmHg (range 11.7 – 20.0 mmHg), a reduction of42% compared to preoperative. This is equivalent to a mean decrease of 10.4 ± 5.3 mmHg (95% CI xx to yy, p = 0.00xx).There was no difference in 12-month mean IOP between eyes that required GSL to access the trabecular meshwork and those that did not (14.1 ± 2.4 vs. 15.7 ± 2.5 mmHg respectively, p = 0.15), although the sample size was small. Medication use was nearly eliminated; a single subject was taking a single medication from 3 to 12 months, and the 12-month IOP in this subject was 12 mmHg. Twelve months' effectiveness outcomes are summarized in (Table 4). Procedure success, defined as no medications and ≥ 20% reduction in IOP, was achieved in 15/19 (78%) subjects. All 19 eyes had a 12-month IOP of < 21 mmHg, 17/19 (89%) had IOP ≤ 18 mmHg, and 11/19 (59%) had IOP of ≤ 15 mmHg. There were no cases of secondary glaucoma surgery or loss in BCVA.As shown in (Table 5), the amount of residual angle closure postoperatively was stable from 1 to 6 months. Among eyes with < 180° of residual angle closure postoperatively, there was some tendency for the angle to re-close slightly from 6 to 12 months. , the proportion of eyes with >180° of closure remained stable throughout the first year of follow up, and complete reclosure was seen in only 1 eye. This eye had full appositional angle closure at baseline, and phaco did not open the angle. Goniosynechialysis was required to create approximately 30° of trabecular access at the site of the Hydrus insertion. This segment of the angle remained open through 6 months, but re-closed completely by 12 months. Interestingly, the IOP in this eye remained stable between 12-14 mmHg from 1 month through 12 months without medication, and the Hydrus inlet remained visible despite the angle closure. While the highest preoperative IOP values observed in the study were among eyes with 100% angle closure, there was no correlation between either preoperative or postoperative angle closure and 12 month IOP. As shown in (Figure 3), the degree of preoperative angle closure and did not affect the amount of IOP lowering observed at 12 months. Further, there was no correlation between the degree of angle closure and the observed IOP as shown in (Figure 4).
Safety
Vision and ocular health were assessed at each visit. By the first postoperative week, 100% of eyes had best corrected visual acuity of 20/40 or better, and there were no instances of BCVA loss throughout follow up. Mild cell and flare were present in 6/20 (30%) of eyes at the 1 day visit which persisted through 1 week but resolved thereafter in 2 cases. There were neither significant changes in VF nor optic nerve appearance through the 12-month follow-up period. There were very few adverse event findings, as summarized in (Table 6). There were no reports of hypotony, flat or shallow chamber, choroidal detachment, or device-corneal contact at any time point. There was one case of IOP elevation that required paracentesis on day 1. There was no device-corneal touch, and there were no instances of device malposition or migration noted during follow up. As might be expected with PACG, we observed 3/20 (15%) cases of non-obstructive iris tissue or PAS formation near the inlet and one case of PAS obstructing the device inlet. The tissue adhesions did not have any apparent effect on IOP. None of these four cases required adjunctive medical therapy and IOP remained under control (16, 12, 13, and 12 mmHg) at 12 months. There were no secondary surgical interventions of any kind during the follow-up period.
Discussion
While the effectiveness of the Hydrus Microstent in POAG has been established in 2 randomized clinical trials,[11,12] this is the first prospective case series of the Hydrus Microstent in PACG. We found consistent and significant IOP lowering through 12 months. Mean IOP was reduced by 10.6 mmHg (41%) from preoperative levels, and 95% of eyes were medication free by the 3-month visit and remained so through 12 months. We observed uniformity of effect in PAC and PACG.
A critical question for this feasibility study was the ability of the device to provide durable aqueous outflow through Schlemm's canal in narrow or closed angles. The design and placement of the device inlet is especially important in this setting. In 12/20 cases (60%), 2-3 clock hours of goniosynechialysis (GSL) was needed to expose the trabecular meshwork adequately prior to implantation of the microstent. There was no significant difference in the apparent IOP lowering capability between eyes that required GSL and those that did not, suggesting that the device was functioning in both situations.
We did not observe a correlation between the amount of preoperative angle closure and IOP reduction. While there was some evidence of progressive closure during the course of follow up, we did not observe a correlation between 12-month IOP and degree of angle closure at 12 months. In 4 cases where PAS was identified near the inlet, the IOP was not affected. Indeed, in a case where the angle reclosed completely, the IOP remained stable and the patient did not require medication. This finding supports the concept that the inlet design is accommodates a certain amount of re-closure and tissue obstruction.
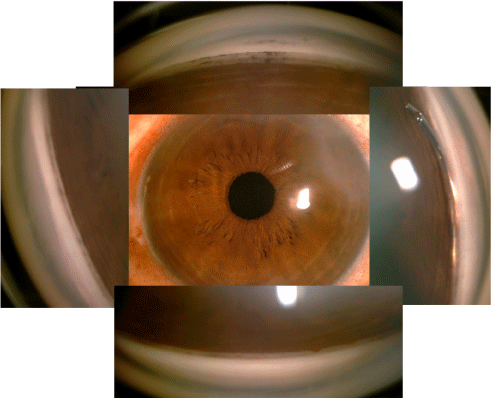
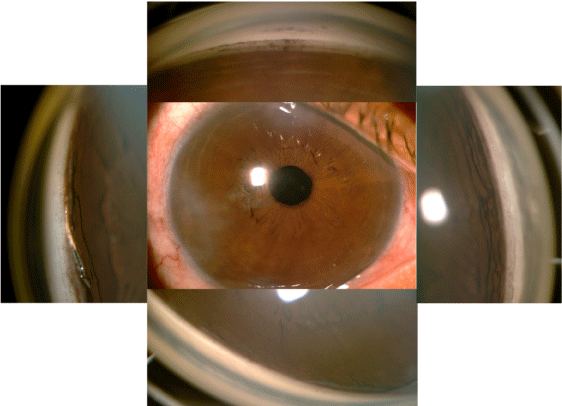
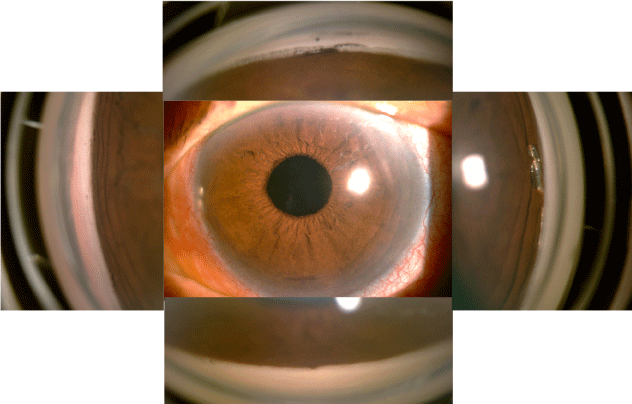
Varying amounts of post-procedure angle closure were observed in the study, from none to 360 of closure. Examples of the varying degrees of post-operative angle closure are shown in (Figures 5-Figure 7). Complete and partially closed angles are shown in (Figures 6, Figure 7), respectively. The device position resembles what would be observed in open angles. The most important finding in this study is that the aqueous inlet remains visible and functional even in when the angle reclosed around the device postoperatively, as shown in (Figure 7).In this eye, the angle was closed 360 with an IOP of 26 mmHg preoperatively. Approximately 90 was opened during the procedure to place the device, but by 1 month was closed over 330. At 12 months, IOP was 14 mmHg with no medications, suggesting the preserved functionality despite re-closure of the angle.
An important question left to be investigated is the contribution of phacoemulsification alone to the results. The EAGLE study showed superior IOP lowering compared to iridotomy as a primary treatment in phakic eyes. However, even in the phaco group, mean pressure was 15.7 mmHg at 6 months and about 40% of eyes required topical medications. It is possible that addition of the Hydrus device reduces medication use and IOP more than phaco alone in PACG in the same manner as previously shown in POAG.
Study limitations
This study was designed to address the feasibility of the procedure, and further investigation is required to definitively establish the role of stenting with phacoemulsification in PACG.
Acknowledgement
None.
Funding
This study was supported by a grant from Ivantis, Inc., Irvine, California, USA.
Disclosures
Robert Edward T. Ang, MD has received research grant from Ivantis, Inc, Irvine, California, USA.
Compliance with Ethic Guidelines
This study received approval from the St. Frances Cabrini Medical Center-Asian Eye Institute Ethics Review Committee and was performed in accordance with the Helsinki Declaration of 1964, and its later amendments. Dr. Ang confirmed that all subjects provided informed consent to participate in this study and consent for publication if any identifying information are included in the manuscript.
Data Availability
The datasets generated during and/or analyzed during the study are available from the corresponding author on reasonable request.
Copyright Permission
This manuscript including the figures and tables are intellectual property of the author. Permission is granted for this material to be shared for non-commercial, educational purposes, provided that this copyright statement appears on the reproduced materials and notice is given that the copying is by permission of the author. To disseminate otherwise or to republish requires written permission from the author.
Prior Presentation
The material in this manuscript has not been published previously. Parts of the material have been presented at medical conferences. Six-month results were presented at the Asia Pacific Association of Cataract and Refractive Surgeons (APACRS ) in July 2018 (Chang Mai, Thailand), World Glaucoma Conference in March 2019, (Melbourne, Australia), American Society of Cataract and Refractive Surgeons (ASCRS) in May 2019 (San Diego, California, USA), Asia Pacific Association of Cataract and Refractive Surgeons (APACRS) in October 2019 (Kyoto, Japan), and European Society of Cataract and Refractive Surgeons (ESCRS) in September 2019 (Paris, France).
Retrospectively Registered
ClinicalTrials.gov Identifier: NCT04622605 (November 10, 2020)
References
- (2012) Global Data on Visual Impairments 2010 (WHO/NMH/PBD/12.01). Geneva: World Health Organization.
- Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90: 262-267.
- Wong TY, Loon SC, Saw SM (2006) The epidemiology of age related eye diseases in Asia. Br J Ophthalmol 90: 506-511.
- Zhou Q, Friedman DS, Lu H, et al. (2007) The epidemiology of age-related eye diseases in Mainland China. Ophthalmic Epidemiol 14: 399-407.
- Tham YC, Li X, Wong TY, et al. (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121: 2081-2090.
- Gunning FP, Greve EL (1998) Lens extraction for uncontrolled angle-closure glaucoma: Long-term follow-up. J Cataract Refract Surg 24: 1347-1356.
- Tham CC, Kwong YY, Leung DY, et al. (2008) Phacoemulsification versus combined phacotrabeculectomy in medically controlled chronic angle closure glaucoma with cataract. Ophthalmology 115: 2167-2173.
- Tham CC, Kwong YY, Leung DY, et al. (2009) Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataract. Ophthalmology 116:725-731.
- Azuara-Blanco A, Burr JM, Cochran C, et al. (2011) The effectiveness of early lens extraction with intraocular lens implanation for the treatment of primary angle-closure glaucoma (EAGLE): Study protocol for a randomized controlled trial. Trials 12:133.
- Azuara-Blanco A, Burr J, Ramsay C, et al. (2016) Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): A randomised controlled trial. Lancet 388: 1389-1397.
- Pfeiffer N, Feijoo JG, Martinez JG, et al. (2015) A randomized trial of a Schlemm's canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 122: 1283-1293.
- Samuelson TW, Chang DC, Marques R, et al. (2019) A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract: The HORIZON Study. Ophthalmology 126: 29-37.
- Heijl A, Leske MC, Bengtsson B, et al. (2002) Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 120: 1268-1279.
Corresponding Author
Robert Edward T Ang, MD, Asian Eye Institute, 8th Floor Phinma Plaza Bldg, Rockwell Center, Makati City 1200, Philippines, E-mail: rtang@asianeyeinstitute.com
Copyright
© 2021 Robert E T Ang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.