Post-Operative Complications after Non-Valved Glaucoma Device Implantation Due to Rapid Polysorb™ Suture Absorption Time
Abstract
Purpose
The characteristics of the suture used in glaucoma implant surgery can play a critical role in the success of the surgery and rate of complications. The properties of Vicryl™ make it an excellent candidate for external tube ligation in glaucoma tube-shunt surgery. Polysorb™ is an absorbable suture manufactured by Covidien with reported similar properties of Vicryl. The purpose of this study is the retrospectively review cases of non-valved glaucoma implants using Polysorb suture and report surgical outcomes.
Methods, intervention, testing
All patients included underwent surgical implantation of a non-valved glaucoma implant with external tube ligation using Polysorb suture.
Main outcome measures
Basic demographic and pre-operative history was collected including age, sex, ocular history, and previous treatment. Pre and post-operative data was obtained including visual acuity, intraocular pressure, estimated time of tube opening, complications, and subsequent treatment needed.
Results
The patients in this study all had uncontrolled and progressive glaucoma on maximal medical therapy. All patients had non-valved glaucoma implant devices placed; Six had Baerveldt Glaucoma Implant 250 mm2 (BGI) and two had Molteno3 (M3). The average intraocular pressure after tube opening was 7.9 (95% CI 5.3-10.4). Five patients (62.5%) had tube opening prior to the 1 month post-op visit. All patients had tube opening by post-operative day 41. Four patients had post-operative complications from early tube opening and hypotony. Choroidal effusions were present in all four patients (50% of total cases), AC shallowing was present in three patients (37.5% of total patients), aqueous misdirection occurred in one patient (12.5% of total patients). Two patients required additional surgery. One patient required irido-zonulo-hyaloidectomy for aqueous misdirection. One patient required eventual Penetrating Keratoplasty (PKP) for corneal decompensation.
Conclusions
This series analyzes a change in suture from Vicryl to Polysorb and a noticeable difference in tube opening time and post-operative complications. We hypothesize these complications were secondary to early suture release prior to adequate fibrous capsule formation over implant plate.
Keywords
Glaucoma, Glaucoma Implant, Baerveldt, Molteno, Polysorb
Introduction
Glaucoma is the most common optic neuropathy of adulthood. When conservative options including drops and/or lasers are not sufficient, surgery is needed. One surgical option to lower intraocular pressure (IOP) is the use of glaucoma drainage devices to allow secondary outflow through an artificial drainage system [1]. These implants shunt aqueous humor from the anterior chamber and can be valved on non-valved [2]. Non-valved devices must be regulated to prevent free flow of aqueous fluid during the early postoperative period while a fibrous capsule is forming around the implant plate. This capsule takes approximately 4-6 weeks to mature, at which point it provides resistance to aqueous outflow and prevents hypotony [3,4]. Methods to regulate outflow during this time include two-staged implantation, internal tubal ligation "rip-cord", and external tubal ligation [5]. External tubal ligation is accomplished by placing an occlusive suture around the tube. Non-absorbable suture can be used to accomplish this, but requires a releasable knot or later suture lysis. Alternatively, absorbable suture can be used without secondary procedure.
The characteristics of the suture used in external tube ligation can play a critical role in the success of the surgery and rate of complications. If the suture absorbs too quickly, it can result in early opening of the tube and hypotony. Hypotony can result in choroidal effusions, choroidal hemorrhages, shallow or flat anterior chamber, and maculopathy [6]. The properties of Vicryl™ make it an excellent candidate for external tube ligation in glaucoma tube-shunt surgery and are the generally accepted suture of choice. Vicryl is an absorbable suture composed of polyglycolic and polylactic acid and is coated with caprolactone. Its reported absorption time is 56-70 days [7,8]. Polysorb™ is an absorbable suture manufactured by Covidien. Polysorb is also composed of polyglycolic and polylactic acid but is coated with caprolactone glycolide copolymer and calcium stearoyl lactylate. It has same reported absorption time of 56-70 days [9].
The purpose of this study is the retrospectively review 8 sequential cases of non-valved glaucoma implants using Polysorb suture and report surgical outcomes.
Methods
The project was submitted to the Institutional Review Board and designated as "exempt", IRB#: PRO17040028. Using PubMed and University of Pittsburgh Health Science Library, a complete literature review was performed. Clinical records of eight patients at UPMC Shadyside Hospital between 9/1/2016-11/30/2016 were reviewed. These were the only cases at this institution who underwent surgical implantation of a non-valved glaucoma implant with external tube ligation using Polysorb suture. Pre and post-operative data was reviewed.
Results
The patients in this study all had uncontrolled and progressive glaucoma on maximal medical therapy. They ranged from 47 to 82-years-old and had various forms of glaucoma, the most common being primary open angle glaucoma (POAG). Two patients had previous Baerveldt implants (BGI). Three patients had cycloablative laser including Endocyclophotocoagulation (ECP) and Micropulse® Cyclophotocoagulation (MP3). Demographics and preoperative characteristics are shown in (Table 1).
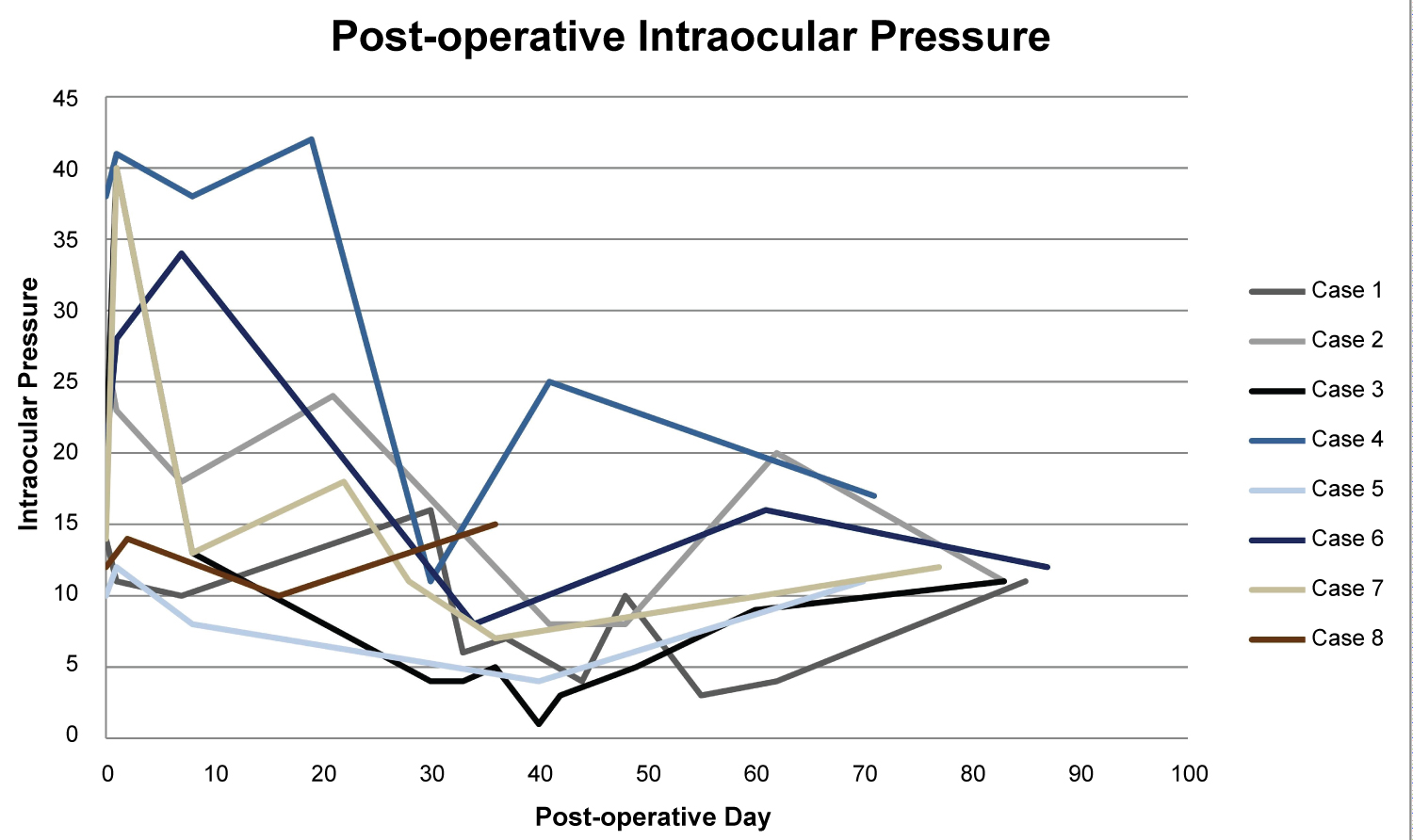
All patients had non-valved glaucoma implant devices placed; Six had Baerveldt Glaucoma Implant 250 mm2 (BGI) and two had Molteno3 (M3). Two different glaucoma trained ophthalmologists performed the surgeries at the same surgical center. All tubes were confirmed to have complete occlusion of tube with ligature at the time of surgery. No vent slits were made in the tubes. Three cases had combined surgeries with phacoemulsification and intraocular lens implantation. There was no evidence of post-operative wound leak. The average pre-operative intraocular pressure was 19.4 (95% CI 12.9-25.8). The average intraocular pressure after tube opening was 7.9 (95% CI 5.3-10.4). Five patients (62.5%) had tube opening prior to the 1 month post-op visit. All patients had tube opening by post-operative day 41. Pre and post-surgical data is shown in (Table 2). Graphic illustration of pre and post-operative IOP is shown in (Figure 1).
Four patients had post-operative complications from early tube opening and hypotony. Choroidal effusions were present in all four patients (50% of total cases), AC shallowing was present in three patients (37.5% of total patients), aqueous misdirection occurred in one patient (12.5% of total patients). Two patients required additional surgery. One patient required irido-zonulo-hyaloidectomy for aqueous misdirection. One patient required eventual Penetrating Keratoplasty (PKP) for corneal decompensation. Surgical data is shown in (Table 3).
Discussion
We report 8 sequential cases of non-valved glaucoma implant surgeries with external tubal ligation using Polysorb suture. This series analyzes a change in suture from Vicryl to Polysorb and a noticeable difference in tube opening time and post-operative complications. We hypothesize these complications were secondary to early suture release prior to adequate fibrous capsule formation over implant plate. These were the only cases using Polysorb suture at the institution. Due to observed increased rates of complication, it was decided to switch back to the previously used Vicryl suture.
Vicryl is the generally accepted suture of choice for external tube ligation in tube-shunt surgery. Vicryl suture absorption occurs by hydrolysis. It begins with loss of tensile strength and eventual absorption of mass [9,10]. Suture is analyzed for this tensile strength and absorption time, both of which play a role in surgical decision making. For ligature in tube shunt surgery, the tube will open after a critical loss of tensile strength, but before the reported absorption time. Ethicon reports the tensile strength retention in vivo to be approximately 75% at 2 weeks, 50% at 3 weeks, and 25% remains at 4 weeks [9,10]. Final absorption time is reported at 56-70 days. The manufacturer data for Polysorb shows it is stronger out of the package and during the first 2 weeks compared to Vicryl. However, after these first 2 weeks, Polysorb loses strength more quickly than Vicryl [7,8]. Polysorb's tensile strength is listed at 80% at 2 weeks, 30% at 3 weeks, and less than 15% by week 4 [7,8]. It is this loss of strength after 2 weeks that we feel is playing a critical role in early tube opening after tube-shunt surgery.
In a study evaluating different tube ligation methods, Kawamorita reports a mean tube ligation release of 48 day. Although they report many patients with tubes opening in the 5th postoperative week, this is still much longer than our series which showed an average of 35 days. This number is likely much lower considering five patients had tube opening prior to the approximate 1 month post-operative visit.
This early tube opening also led to increased post-operative hypotony. Kawamorita reported ocular hypotension (< 6 mmHg) 23.3% (vs. 37.5% in this series) [11]. Early post-operative complications after Baerveldt glaucoma implant are commonly related to hypotony. In the Tube vs. Trab study, the most common complications were choroidal effusion (16%), persistent corneal edema (16%), and shallow or flat anterior chamber (11%) [12]. The rates of complications in this study were much higher with choroidal effusions present in 50% and AC shallowing present in three patients at 37.5%. One patient required PKP for non-clearing corneal edema secondary to AC shallowing. The TVT study did not report aqueous misdirection as a complication, but it has been reported in 2% of Baerveldt glaucoma implants [13]. One patient (12.5%) in this study had post-operative aqueous misdirection refractory to medical management and required irido-zonulo-hyaloidectomy.
Two patients in this study had Molteno3 implants. Although there was early tube opening in these cases, there was no post-operative hypotony or ocular complications. Considered a non-valved device, the Molteno3 implants have primary drainage area to act as a "biologic valve". The literature does not show clear evidence to support this [14,15].
This is a retrospective case review of eight patients. This study is limited by the study design and small number of patients. The goal of this paper is to inform other ocular surgeons to prevent future ophthalmic complications. It is unknown how this has impacted other hospitals/practices and we feel further evaluation is necessary to identify a possible preventable risk factors in the future. A randomized and controlled comparison between Vicryl and Polysorb would be needed to further evaluate this problem.
Financial Support
None.
Conflict of Interest
The authors have no conflicts of interest, acknowledgements, or funding to disclose.
References
- Lloyd MA, Baerveldt G, Heuer DK, et al. (1994) Initial experience with the Baerveldt implant in complicated glaucomas. Ophthalmology 101: 640-650.
- Schwartz KS, Lee RK, Gedde SJ (2006) Glaucoma drainage implants: A critical comparison of types. Curr Opin Ophthalmol 17: 181-189.
- Molteno AC (1971) A new implant for glaucoma. Effect of removing implants. Br J Ophthalmol 55: 28-37.
- Molteno AC (1969) New implant for drainage in glaucoma. Clinical trial. Br J Ophthalmol 53: 606-615.
- Trible JR, Brown DB (1998) Occlusive ligature and standardized fenestration of a Baerveldt tube with and without antimetabolites for early postoperative intraocular pressure control. Ophthalmology 105: 2243-2250.
- Sharkawi E, Oleszczuk JD, Barton K (2016) Systematic occlusion of shunts: Control of early postoperative IOP and hypotony related complications following glaucoma shunt surgery. J Glaucoma 25: 54-61.
- http://www.covidien.com/imageServer.aspx?contentID=14357&contenttype=application/pdf
- https://www.hospeq.com/v/vspfiles/photos/pdfs/covidien-polysorb-sutures.pdf
- http://www.ethiconinstitute.com/sites/default/files/ETHICON%c2%ae%20Family%20of%20Sutures%20Instructions%20for%20Use_5.pdf
- http://media.xn--benersttning-lcb.se/2012/04/Ethicon-wound-closure-manual.pdf
- Kawamorita S, Hamanaka T, Sakurai T (2014) The early postoperative complications of two different tube ligation methods in baerveldt implant surgery. J Curr Glaucoma Pract 8: 96-100.
- Gedde SJ, Schiffman JC, Feuer WJ, et al. (2007) Treatment outcomes in the tube versus trabeculectomy study after one year of follow-up. Am J Ophthalmol 143: 9-22.
- Patel S, Pasquale LR (2010) Glaucoma drainage devices: A review of the past, present, and future. Semin Ophthalmol 25: 265-270.
- Valimaki JO, Ylilehto AP (2014) Molteno3 implantation as primary glaucoma surgery. J Ophthalmol 2014: 167564.
- Valimaki J (2012) Surgical management of glaucoma with Molteno3 implant. J Glaucoma 21: 7-11.
Corresponding Author
Julia K Polat, MD, UPMC Eye Center, Eye and Ear Institute, University of Pittsburgh Medical Center, 203 Lothrop Street, Pittsburgh, PA 15213, USA, Tel: 412-647-2200; 412-647-5119
Copyright
© 2020 Sorrentino DM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.