T-DM1 and AVM - Coincidence or Causation: Case Report
Abstract
Background : Trastuzumab emtansine (T-DM1) is a novel agent used for the patients with human epidermal growth factor receptor (HER-2) positive breast cancer. Prolonged use of T-DM1 has been shown to cause rare adverse effects, such as hereditary haemorrhagic telangiectasia (HHT)-like symptoms and vascular malformation. The authors present a case of HHT-like symptoms and new brain arteriovenous malformation (AVM) in the setting of T-DM1 therapy for breast cancer.
Observation : A 77-year-old lady with a background of metastatic breast cancer who developed HHT-like mucocutaneous clinical manifestation during treatment with T-DM1, which regressed at nine months following cessation of T-DM1. She was found to have new brain AVMs four years after commencement of T-DM1 therapy in the absence of other risk factors for AVM.
Conclusion: Given the increasing use of T-DM1 for metastatic breast cancer, it is imperative for clinicians to be aware of the potential development of brain AVM whilst on T-DM1 via HHT linkage.
Keywords
T-DM1, AVM, Arteriovenous malformation
Introduction
Trastuzumab emtansine (T-DM1) is a novel agent that has now emerged as a standard of care for the patients with human epidermal growth factor receptor (HER-2) positive breast cancer in the post-neoadjuvant and metastatic setting [1]. Here, we present a case of brain arteriovenous malformations (AVM) that is potentially attributed to T-DM1. The patient developed hereditary haemorrhagic telangiectasia (HHT)-like symptoms during treatment with T-DM1. HHT patients have been shown to have higher rates of brain AVMs [2]. And this has led us to suspect a correlation between T-DM1 and brain AVM pathogenesis.
Case
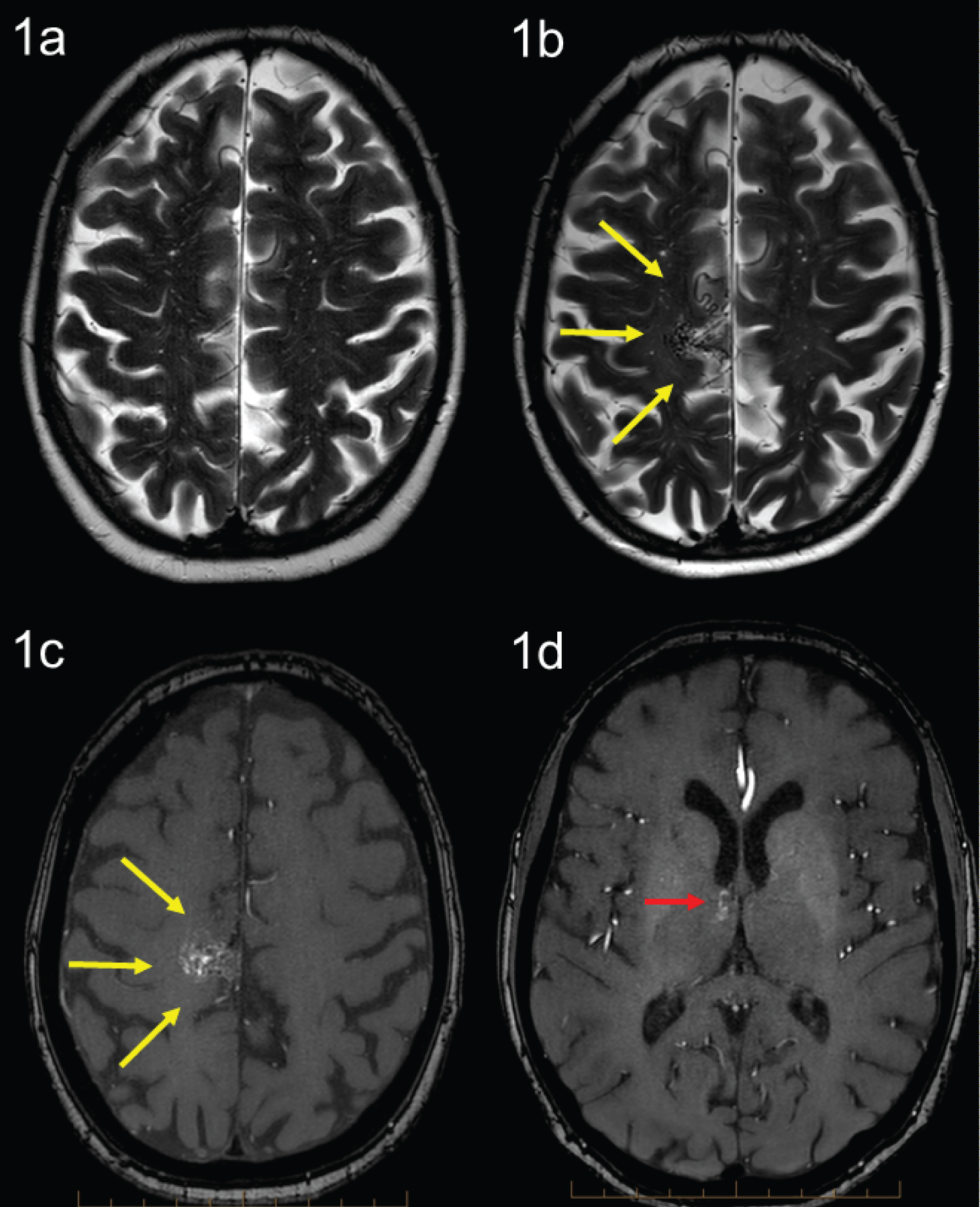
A 77-year-old lady with a background of breast cancer presented with a few days’ history of worsening dizziness and unsteadiness whilst on T-DM1. MRI brain was performed in Oct 2021 which demonstrated a new right posteromesial frontal surface vascular malformation, without evidence of intracranial metastases. A prior MRI brain in 2014 was normal. Subsequent MRI angiography (MRA) confirmed an 18 mm right posteromesial frontal arteriovenous malformation (AVM) supplied from a paracentral branch of the enlarged right callosomarginal artery with superficial venous drainage towards the superior sagittal sinus (Spetzler Martin Grade 2) (Figure 1). There was also a second small 5 mm anteromedial thalamic AVM with deep venous drainage (Spetzler-Martin grade 3) (Figure 1). She had no history of hypertension or any other significant comorbidities. There was no significant family history of HHT.
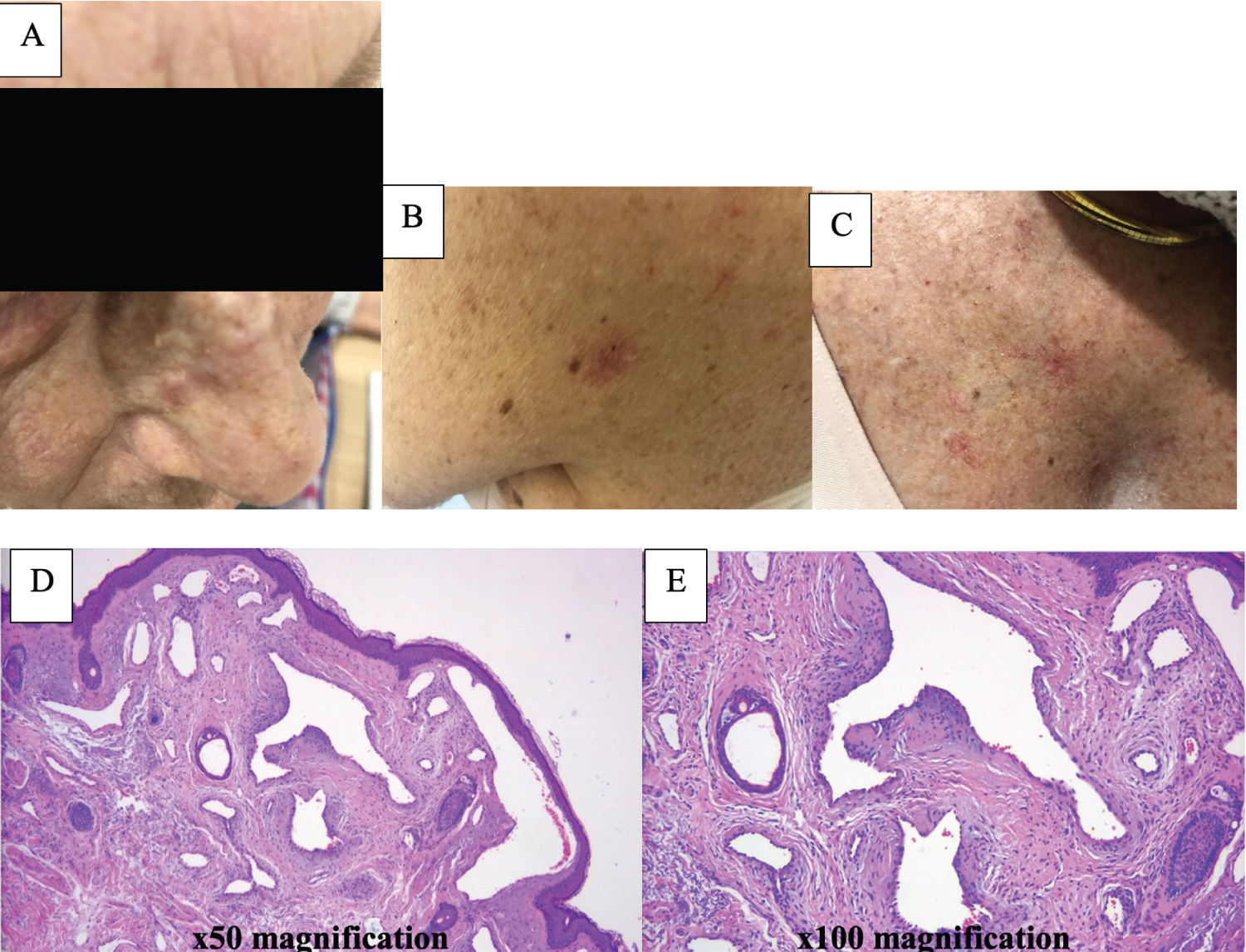
In 1992, she was diagnosed with a right breast invasive ductal carcinoma, ER+/PR-/HER2 unknown which was managed by mastectomy and axillary dissection. In September 2008, she developed a right chest wall recurrence and sternal metastasis. She underwent a resection of the local recurrence (which demonstrated the tumour to be HER2-amplified) followed by radiotherapy to the right chest wall, 50.4Gy in 28 fractions, completed in September 2008. She received systemic treatment with letrozole only. She re-presented in 2014 with progressive metastatic disease with pulmonary, liver, and bony metastases proven on staging CT. Her baseline MRI brain was unremarkable. She received paclitaxel and trastuzumab with good response until July 2017 until she developed further progressive disease. She was commenced on trastuzumab emtansine (T-DM1) which she started in August 2017. After 1 year on T-DM1, she developed frank haematuria, bleeding skin lesions, frequent epistaxis from her left nostril, increased bruising, and a spider angiomata lesions over her upper trunk and neck as well as on her left cheek (Figure 2). Her cheek lesion was biopsied and confirmed to be vascular malformation (Figure 2) and she underwent a cystoscopy which was unremarkable. She has had no further haematuria but continued to experience waxing and waning symptoms until December 2021.
Her metastatic disease burden has responded well to T-DM1, but in December 2021, she developed evidence of liver dysfunction with elevation of alanine aminotransferase. This is a known adverse effect associated with T-DM1 and though liver dysfunction remained grade 1 by CTCAE, due to patient concerns, T-DM1 was ceased and switched to trastuzumab and exemestane.
Her brain AVM diagnosis came to light 4 years after she was commenced on T-DM1, after she presented with an episode of dizziness and unsteadiness. Following discussion at the AVM multidisciplinary tumour board, we have elected to observe the patient with surveillance MRI. She had an MRI brain 9 months post-discontinuation of T-DM1 which demonstrated stable appearance of the right posteromesial frontal and right anteromesial thalamic vascular malformations. However, she has reported no further epistaxis and has noticed regression of her vascular cutaneous lesions. The patient will continue to be followed up closely with surveillance MRI/MRA brain.
Discussion
There is increasing evidence that AVMs can be de novo lesions that are dynamic in nature [3,4]. A recent review by Dalton, et al. identified multiple associated factors with AVM development including ischaemic stroke, moyamoya, intracerebral haemorrhage, brain tumours, seizures, head trauma, previous AVM treatment, and haemorrhagic telangiectasia [3,4]. Patients with hereditary haemorrhagic telangiectasia (HHT) are at a notably increased risk of AVM, with up to 23% of patients with HHT developing cerebral vascular malformations [5]. These lesions tend to be smaller with an increased likelihood of having single draining veins, be located superficially and can be multiple [6].
The chronological relationship between the exposure of T-DM1 and clinical manifestation of hereditary haemorrhagic telangiectasia (HHT)-like symptoms and AVM in our patient suggest that it is potentially attributed to T-DM1.
T-DM1 is an antibody-drug conjugate that incorporates the HER2-targeted antitumor properties of trastuzumab with the cytotoxic activity of the microtubule-inhibitory agent DM1 (derivative of maytansine) via a stable linker molecule [1]. T-DM1 has been shown to significantly prolong progression-free and overall survival with less toxicity compared to lapatinib and capecitabine in patients with HER2-positive advanced breast cancer previously treated with trastuzumab and a taxane [1]. The results of this study, EMILIA, led to T-DM1 becoming the standard of care agent in the second line of metastatic HER2 positive breast cancer. The adverse effects of T-DM1 include fatigue, nausea, diarrhoea, elevated transaminases, anaemia and thrombocytopenia with secondary haemorrhage [7]. There have been some case reports about previously unknown toxicities from prolonged use of T-DM1 including portal hypertension [8,9] and pulmonary hypertension [10].
Extensive mucocutaneous telangiectasia as described in our patient with T-DM1 has been reported in the literature [10,11]. Telangiectasia can occur in the gastrointestinal tract, nasal or oral mucosa and haemorrhages, induced by vascular malformations [8,9] similar to what is the literature [10,11]. Telangiectasia can occur in the gastrointestinal tract, nasal or oral mucosa and haemorrhages, induced by vascular malformations [8,9] similar to what is observed in HHT [12,13]. The link between telangiectasia and T-DM1 can be supported by theories including the role for disruption of endothelial, impaired angiogenesis and cytoskeletal microtubules by emtansine have been reported [12,14]. Although spider angiomata could be related to the degree of liver damage, associated with the use of T-DM1, our patient interestingly developed her cutaneous stigmata one year into her T-DMI regimen without any notable derangement in liver function [12,15]. Therefore, this suggests that her spider angiomata lesions are related to T-DMI use via HHT linkage.
Pertaining to AVM, the exact signalling pathway and downstream targets that contribute to the development of AVM and HHT pathogenesis are not fully understood [16]. Several studies suggest that HHT is linked to aberrant reactivation of angiogenesis, and the overactivation of pro-angiogenic pathways such as vascular endothelial growth factor (VEGF) signalling, which contributes to the development of vascular pathology models of HHT [17]. Thus, we postulate that overactivation of VEGF signalling may have been involved in the pathogenesis of telangiectasia and AVM with T-DM1 therapy.
The optimal management of unruptured AVM remains controversial because of insufficient high-quality evidence on the lifetime risk of intracranial haemorrhage and the predictors and risks associated with treatment. The only randomised controlled trial (ARUBA), enrolled 266 patients with unruptured AVM to medical management alone or interventional therapy (e.g. resection, embolization or SRS or in combination) [18]. This trial was terminated early after a mean follow up of 33 months, as the risk of stroke or death was > 3 times higher with surgical intervention than in the medical management group. For our patient, we have elected for expectant monitoring with the understanding that there would be a risk of haemorrhage [19]. There have been reports of the adverse effects including bleeding events resolving after discontinuation of T-DM1 [10]. The AVM in our patient was stable on her subsequent MRI brain 9 months following discontinuation of her T-DMI with resolution of her vascular cutaneous lesions and no further report of epistaxis. The brain AVMs will be monitored with annual MRA.
Conclusion
In this case report, we report a case of HHT-like manifestation and AVM with T-DM1 treatment. Given the increasing use of T-DM1 for metastatic breast cancer. A limitation of this case report is that it is a single case study.
Compliance with Ethical Standards
Nil conflict of interest.
Informed consent has been obtained from the patient. This case report has been approved by Sir Charles Gairdner Hospital ethics committee.
References
- Verma S, Miles D, Gianni L, et al. (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367: 1783-1791.
- Brinjikji W, Iyer VN, Yamaki V, et al. (2016) Neurovascular manifestations of hereditary hemorrhagic telangiectasia: A consecutive series of 376 patients during 15 years. AJNR Am J Neuroradiol 37: 1479-1486.
- Florian IA, Beni L, Moisoiu V, et al. (2021) 'De Novo' brain AVMs-hypotheses for development and a systematic review of reported cases. Medicina (Kaunas) 57: 201.
- Dalton A, Dobson G, Prasad M, et al. (2018) De novo intracerebral arteriovenous malformations and a review of the theories of their formation. Br J Neurosurg 32: 305-311.
- Vella M, Alexander MD, Mabray MC, et al. (2020) Comparison of MRI, MRA, and DSA for Detection of Cerebral Arteriovenous Malformations in Hereditary Hemorrhagic Telangiectasia. AJNR Am J Neuroradiol 41: 969-975.
- Kim H, Su H, Weinsheimer S, et al. (2011) Brain arteriovenous malformation pathogenesis: A response-to-injury paradigm. Acta Neurochir Suppl 111: 83-92.
- Kowalczyk L, Bartsch R, Singer CF, et al. (2017) Adverse events of trastuzumab emtansine (T-DM1) in the treatment of HER2-positive breast cancer patients. Breast Care (Basel) 12: 401-408.
- Fujii Y, Doi M, Tsukiyama N, et al. (2020) Sinusoidal obstruction syndrome post-treatment with trastuzumab emtansine (T-DM1) in advanced breast cancer. Int Cancer Conf J 9: 18-23.
- Monteiro GVB, Mussi MCL, Nardelli MJ, et al. (2021) Portal hypertension after prolonged use of trastuzumab emtansine: Nodular regenerative hyperplasia with autoimmune features. Research, Society and Development. 10.
- Kwon Y, Gomberg-Maitland M, Pritzker M, et al. (2016) Telangiectasia and pulmonary arterial hypertension following treatment with trastuzumab emtansine: A case report. Chest 149: 103-105.
- Sibaud V, Vigarios E, Combemale P, et al. (2015) T-DM1-related telangiectasias: A potential role in secondary bleeding events. Ann Oncol 26: 436-437.
- Sibaud V, Niec RE, Schindler K, et al. (2014) Ado-trastuzumab emtansine-associated telangiectasias in metastatic breast cancer: A case series. Breast Cancer Res Treat 146: 451-456.
- James WD (2020) Cutaneous vascular diseases. Andrews’ diseases of the skin.
- Yan H, Endo Y, Shen Y, et al. (2016) Ado-Trastuzumab emtansine targets hepatocytes via human epidermal growth factor receptor 2 to induce hepatotoxicity. Mol Cancer Ther 15: 480-490.
- Milam P, Berger M, Ramaswamy B, et al. (2019) Spider telangiectases and palmar erythema as harbingers of structural liver changes in three breast cancer patients on ado-trastuzumab emtansine. J Clin Aesthet Dermatol 12: 23-26.
- Schimmel K, Ali MK, Tan SY, et al. (2021) Arteriovenous malformations-current understanding of the pathogenesis with implications for treatment. Int J Mol Sci 22: 9037.
- Ardelean DS, Letarte M (2015) Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front Genet 6: 35.
- Mohr JP, Parides MK, Stapf C, et al. (2014) Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): A multicentre, non-blinded, randomised trial. Lancet 383: 614-621.
- Ogilvy CS, Stieg PE, Awad I, et al. (2001) AHA scientific statement: Recommendations for the management of intracranial arteriovenous malformations: A statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke. 32: 1458-1471.
Corresponding Author
Dr Anika Mittal, Peter MacCallum Cancer Centre, Victoria, Australia, Tel: +61 3 8559 5000.
Copyright
© 2023 Mittal A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.