Immunohistochemical and Biomolecular Characteristics of Craniopharyngiomas: A Single Institution Experience from Southern Italy
Abstract
In the present paper, The Authors discuss the histological and immunohistochemical aspects observed in a casuistry of craniopharyngiomas (CP), identifying not only subtypes (papillary or adamantinomatous), but also their proliferative activity and molecular pathways. For this purpose, a series of thirty-seven was retrieved during the last decade (2007-2017) from files of our pathology department; there were 21 female and 16 male patients (age range 5-75 years, mean age 43.48), but 7 cases were recorded in childhood. A prevalent adamantinomatous pattern (ACP) (34 cases) was encountered, while only 3 exhibited papillary variant (PCP).
After a pretreatment, the immunostaining protocol was performed by Ventana BenchMark ultraimmunostainer to investigate β-catenin, Ki67 and BRAF V600E mutant epitope. Successively, in order to evaluate the mutational status concerning BRAF, tissue sections were microdissected by scalpel, subjected to DNA extraction and subsequent analysis utilizing the BRAF codon 600 mutation analysis kit to identify five somatic mutations in codons 600.
Twenty-five ACP cases exhibited β-catenin immunostaining; moreover, nine additional cases of ACP were unreactive for β-catenin, but showed a Ki67 labelling index > 5%. Although none of PCP cases showed β-catenin immunoreaction, a cytoplasmic immunopositivity for BRAF V600E mutant epitope was recorded only in all PCP cases, which also harboured BRAF V600E mutations; in these latter cases, a less favorable outcome was documented since one patient died for the disease and two presented loco-regional recurrences. Therefore, we suggest that some immunohistochemical markers and biomolecular signatures in CP histologic variants may be utilized as hallmarks to identify improved treatment modalities.
Keywords
Craniopharyngioma, Histotype, Immunohistochemistry, BRAF status, Prognosis
Introduction
Craniopharyngiomas (CP) are benign epithelial tumors, histogenetically arising from Rathke's residues and frequently developed in the suprasellar region [1-5]. Nevertheless, intrasellar or sphenoidal osseous localizations have been reported [1-5]. It is well known that CP may develop in all ages, although young adults are the most frequent affected patients [6]. Grossly, the tumor exhibits a common cystic appearance with a lobular surface, but rarer totally solid presentation [7]. The neoplastic dimensions are ranging from few to many centimeters, although a reactive gliosis is always documented as well as a definite expansion and/or compression to adjacent nervous tissues, such as optic nerve, chiasm and the third ventricle [2-6]. This latter aspect greatly influences neurosurgical procedures and it may predict the final outcome [1,3]. Moreover, together with the local neoplastic infiltration, the identification of histological subtypes may also suggest a severe long term morbidity, stressing the attempt to identify immunohistochemical and biomolecular characteristics useful for the management of these unusual tumors as a peculiar aim of the present study.
Materials and Methods
From archival files of our Department located in advanced teaching hospital (University of Messina & Azienda Ospedaliera Universitaria "Polyclinic G. Martino") 37 CPs were retrieved with reference the period 2007-2017. Total or sub-total surgically resected specimens were obtained from adult and pediatric neurosurgical sections; in detail, patients were 21 female and 16 male (age range 5-75 years, mean age 43.48 yrs). 30 cases exhibited a cystic appearance and 7 were represented by childhood cases. Accordingly to WHO (2016), 34 CP were histologically classified as adamantinomatous variant (ACP), while only 3 presented a papillary picture (PCP). For all cases follow-up data were available.
From same neoplastic tissue blocks utilized for the routine H&E staining, 4 mµ thick seriate silane-coated sections were obtained and then immunostained using a Ventana BenchMark ultraimmunostainer (Ventana, Tucson, AZ, USA) with the following antibodies: monoclonal mouse anti- β-catenin (Cell Marque, clone 14, working dilution 1:100, Darmstadt, Germany), Ki 67 (Dako, clone MIB-1, w.d. 1:150, Glostrup, Denmark) and monoclonal mouse recognizing the BRAF V600E mutant epitope (BRAF V600E-specific clone VE1, Ventana, w.d. 1:200, Tucson, AZ, USA). The staining protocol included pretreatment with cell conditioner 1 (pH 8.4) for 64 minutes, incubations with antibodies at 36 ℃ for 16 minutes, followed by primary antibodies detection using the UltraView Universal DAB detection kit (Ventana, Tucson, AZ, USA). Finally, the nuclear counterstain by haematoxylin for 4 minutes. The stained sections were then dehydrated in the ascending alcohols, cleared in xylene and mounted with Permount (Sigma-Aldrich, Darmstadt, Germany).
In order to evaluate BRAF mutational status, four 10 mµ thick haematoxylin & eosin stained sections were microdissected by a scalpel using an inverted microscope to collect only regions with the highest neoplastic representation. DNA extraction was performed by QIAamp DNA FFPE Tissue kit (Qiagen, GmbH, Hilden, Germany) according to the manifacturer's recommendations and DNA quantified by fluorometry with the Qubit platform (Life Technologies, Rockville, Maryland, USA). DNA samples were then subjected to BRAF mutational analysis utilizing the BRAF Codon 600 Mutation Analysis Kit II (EntroGen Inc, Los Angeles, CA, USA) that allows to identify five BRAF somatic mutations in codons 600 (V600D, V600E, V600K, V600M, V600R). The amplifications were carried out in a StepOnePlus Real-Time PCR system (Life Technologies, Rockville, Maryland, USA), following the manifacturer's procedures as well as according to the recommendations of both the Italian Association of Medical Oncology (AIOM) and the Italian Society of Pathology and Cytopathology (SIAPEC) [8].
Results
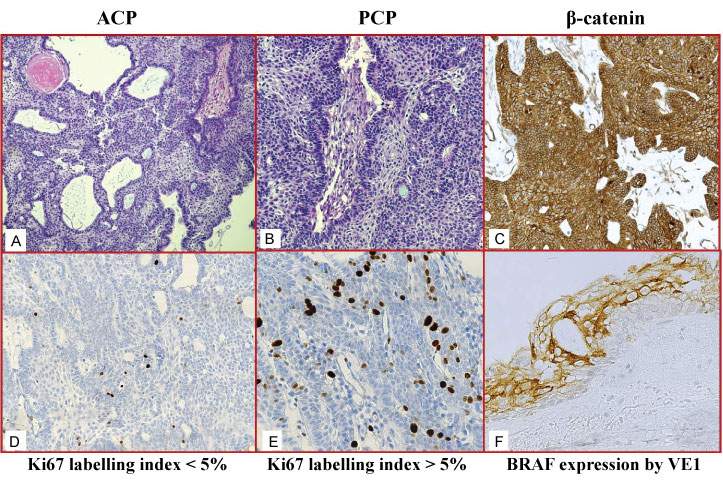
Among 37 CP patients, sub-total excision was performed in 19 cases (51.35%), while a gross total excision was done in the remaining 18 cases (48.65%). The follow-up observational period ranged from 5-122 months (mean 49 months). During this interval, four patients experienced recurrences (2 ACP and 2 PCP), two patients died (1 ACP for neurosurgery related events, 1 PCP for the disease), while the remaining were alive, some of them with significant morbidities. Histologically ACP, representing the great majority of our casuistry, exhibited an adamantinomatous pattern characterized by stratified epithelium with a palisading arrangement of the basal cells, with presence of keratin and microcystic changes (Figure 1a). Three cases of PCP presented papillary areas composed by stratified squamous epithelium resting upon a connective stromal tissue (Figure 1b). A reactive, sometimes exuberant, gliosis with Rosenthal fibers was encountered in the adjacent cerebral tissue.
By immunohistochemistry, twenty-five (73.52%) ACP cases showed an evident β-catenin immunostaining with its peculiar cytoplasmic/membranous distribution and without any shift to nuclear accumulation (Figure 1c); in these cases, Ki67 immunoexpression rate was constantly < 5% (Figure 1d). interestingly, The additional 9 cases of ACP were unreactive for β-catenin, but showed a Ki67 labelling index > 5%, (Figure 1e). The two ACP cases that exhibited recurrences were present either in positive β-catenin group either in negative one, independently from the Ki growth fraction. None of the PCP cases showed β-catenin immunostaining.
Moreover, with the VE1 antibody cases were considered positive if there was unequivocal diffuse cytoplasmic staining in > 85% of tumour cells (Figure 1f). The intensity of staining of BRAF expression in tumour cells was recorded on a 0-3 scale. Strong cytoplasmic staining was scored as 3, medium cytoplasmic staining as 2, weak cytoplasmic staining as 1 and the absence of staining was scored as 0. Scores of 1-3 represented positive staining, while scores less than 1 were considered negative staining. A cytoplasmic immunopositivity for anti-VE1 antibody was encountered only in all 3 PCPs; these latter cases also harboured BRAF V600E mutations and presented a less favorable outcome, since 1 patient died for the disease and two manifested recurrences.
Discussion
In intracranial tumours, the management of CPs remains one of the most hot point due to their pattern of growth as well as high recurrence rate and co-morbidities [9-11]. Although a complete resection may be considered largely curative in order to preserve patient's long-term quality of life, the risk of involvement for other brain structures often represents an adverse event [12,13]. Moreover, recurrent CPs may result refractory to additional surgery as well as to adjuvant radio-chemotherapy; therefore, emerging molecular targeted therapy should be addressed to fight CP in reducing morbidity [14-18]. In particular, the discovery of new molecular pathways involved in CPs, such as mutations in the gene encoding β-catenin or in the BRAF gene, has raised a great interest to provide potential targets for new treatments [19-22].
It has been reported that nuclear translocation of β-catenin was associated to more aggressive CPs, as documented by an intense β-catenin expression in cases with generalized recurrences [23-26]. In our series, a β-catenin cytoplasmic/membranous immunostaining, without shift towards nuclear accumulation, was encountered in 73.52% ACP cases, while Ki67 immunoexpression rate was constantly < 5%. The additional 9 cases of ACP (26.48%), showing a Ki67 labelling index > 5%, were unreactive for β-catenin. Consequently, the occurrence of CTNNB1 mutation is not always present as rule in CPs. However, our immunohistochemical observations offered an advantage of Sanger sequencing in the detection of β-catenin gene mutations, even though a relationship between this marker and growth fraction performed by Ki67 was not revealed. In fact, the two ACP patients with recurrences belonged to positive β-catenin ACP group as well as to negative one. On the other hand, our data were not so surprising since other analyses failed to demonstrate such a predictive value [27].
It is well known the BRAF gene encoded a serine/threonine-protein kinase B-Raf (BRAF), which belongs to the family of growth signal transduction non-receptor protein kinases [28]. BRAF mutations have been identified in different types of cancer, such as colon carcinoma, melanoma, papillary thyroid carcinoma and some lymphomas [28]. Recently, a BRAF V600E mutant-specific antibody VE1 became available for immunohistochemical screening of such BRAF mutant tumors [29,30]. Testing by this specific antibody our CP cohort, we observed a cytoplasmic immunopositivity was recorded only in all 3 PCPs; moreover, these cases also harboured BRAF V600E mutations and they presented a less favorable outcome, since 1 patient died for the disease and two manifested recurrences. According to this molecular event, the presence of BRAF mutation in PCPs might suggest new therapeutic approaches; recently in fact, among different treatment options, some studies have shown relevant response of PCPs using BRAF inhibitors or combination therapy with BRAF (vemurafenib and dabrafenib) and MEK (trametinib) inhibitors [22,31]. These data may suggest the opportunity to surgically access either before or after targeted therapy in order to significantly lower chances of recurrence while maintaining superior quality of life. Specifically, trials with BRAF inhibitors such as vemurafenib and dabrafenib preceding or following surgical procedures for CPs (mainly PCPs) may lead to improved clinical outcomes.
Finally, further pluri-institutional prospective studies will help to elucidate the role of targeted therapy in CPs, improving the management in cases with aggressive or recurrent behavior, such as BRAF positive PCPs, according to the present although limited observations.
Conflict of Interest
The authors declare no conflict of interests.
References
- Coury JR, Davis BN, Koumas CP, et al. (2018) Histopathological and molecular predictors of growth patterns and recurrence in craniopharyngiomas: A systematic review. Neurosurg Rev 41: 1-8.
- Qiao N (2018) Endocrine outcomes of endoscopic versus transcranial resection of craniopharyngiomas: A system review and meta-analysis. Clin Neurol Neurosurg 169: 107-115.
- Mrowczynski OD, Langan ST, Rizk EB (2018) Craniopharyngiomas: a systematic review and evaluation of the current intratumoral treatment landscape. Clin Neurol Neurosurg 166: 124-130.
- Sahm F, Reuss DE, Giannini C (2018) WHO 2016 classification: changes and advancements in the diagnosis of miscellaneous primary CNS tumours. Neuropathol Appl Neurobiol 44: 163-171.
- Solari D, Morace R, Cavallo LM (2016) The endoscopic endonasal approach for the management of craniopharyngiomas. J Neurosurg Sci 60: 454-462.
- Cohen LE (2016) Update on childhood craniopharyngiomas. Curr Opin Endocrinol Diabetes Obes 23: 339-344.
- Pakneshan S, Salajegheh A, Smith RA, et al. (2013) Clinicopathological relevance of BRAF mutations in human cancer. Pathology 45: 346-356.
- Ieni A, Barresi V, Cardia R, et al. (2016) The micropapillary/hobnail variant of papillary thyroid carcinoma: a review of series described in the literature compared to a series from one southern Italy pathology institution. Rev Endocr Metab Disord 17: 521-527.
- Koutourousiou M, Fernandez-Miranda JC, Wang EW, et al. (2018) The limits of transsellar/transtuberculum surgery for craniopharyngioma. J Neurosurg Sci 62: 301-309.
- Lauretti L, Legninda Sop FY, Pallini R, et al. (2018) Neuroendoscopic treatment of cystic craniopharyngiomas: a Case series with systematic review of the literature. World Neurosurg 110: e367-e373.
- Alli S, Isik S, Rutka JT (2016) Microsurgical removal of craniopharyngioma: endoscopic and transcranial techniques for complication avoidance. J Neurooncol 130: 299-307.
- Conti A, Pontoriero A, Ghetti I, et al. (2019) Benefits of image-guided stereotactic hypofractionated radiation therapy as adjuvant treatment of craniopharyngiomas. A review. Childs Nerv Syst 35: 53-61.
- Hage M, Lombès M, Chanson P (2014) Craniopharyngiomas: progress in pathogenesis and therapeutics. Ann Endocrinol (Paris) 75: S46-S54.
- Müller HL, Merchant TE, Puget S, et al. (2017) New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat Rev Endocrinol 13: 299-312.
- Müller HL (2010) Childhood craniopharyngioma--current concepts in diagnosis, therapy and follow-up. Nat Rev Endocrinol 6: 609-618.
- Clark AJ, Cage TA, Aranda D, et al. (2013) A systematic review of the results of surgery and radiotherapy on tumor control for pediatric craniopharyngioma. Childs Nerv Syst 29: 231-238.
- Clark AJ, Cage TA, Aranda D, et al. (2012) Treatment-related morbidity and the management of pediatric craniopharyngioma: A systematic review. J Neurosurg Pediatr 10: 293-301.
- Yang I, Sughrue ME, Rutkowski MJ, et al. (2010) Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus 28: E5.
- Kurppa KJ, Catón J, Morgan PR, et al. (2014) High frequency of BRAF V600E mutations in ameloblastoma. J Pathol 232: 492-498.
- Brown NA, Rolland D, McHugh JB, et al. (2014) Activating FGFR2- RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 20: 5517-5526.
- Brastianos PK, Taylor-Weiner A, Manley PE, et al. (2014) Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 46: 161-165.
- Brastianos PK, Shankar GM, Gill CM, et al. (2015) Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J Natl Cancer Inst 108.
- Hara T, Akutsu H, Takano S, et al. (2018) Clinical and biological significance of adamantinomatous craniopharyngioma with CTNNB1 mutation. J Neurosurg 1: 1-10.
- Prieto R, Pascual JM (2018) Can tissue biomarkers reliably predict the biological behavior of craniopharyngiomas? A comprehensive overview. Pituitary 21: 431-442.
- Martinez-Barbera JP (2015) Molecular and cellular pathogenesis of adamantinomatous craniopharyngioma. Neuropathol Appl Neurobiol 41: 721-732.
- Apps JR, Martinez-Barbera JP (2016) Molecular pathology of adamantinomatous craniopharyngioma: review and opportunities for practice. Neurosurg Focus 41: E4.
- Duò D, Gasverde S, Benech F, et al. (2003) MIB-1 immunoreactivity in craniopharyngiomas: a clinico-pathological analysis. Clin Neuropathol 22: 229-234.
- Dankner M, Rose AAN, Rajkumar S, et al. (2018) Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 37: 3183-3199.
- Bartels S, Adisa A, Aladelusi T, et al. (2018) Molecular defects in BRAF wild-type ameloblastomas and craniopharyngiomas-differences in mutation profiles in epithelial-derived oropharyngeal neoplasms. Virchows Arch 472: 1055-1059.
- Rostami E, Witt Nyström P, Libard S, et al. (2017) Recurrent papillary craniopharyngioma with BRAFV600E mutation treated with neoadjuvant-targeted therapy. Acta Neurochir (Wien) 159: 2217-2221.
- Roque A and Odia Y (2017) BRAF-V600E mutant papillary craniopharyngioma dramatically responds to combination BRAF and MEK inhibitors. CNS Oncol 6: 95-99.
Corresponding Author
Dr. Antonio Ieni, Section of Pathological Anatomy and Cytopathology, Department of Human Pathology of Adult and Evolutive Age "Gaetano Barresi", University of Messina & Azienda Ospedaliera Universitaria "Polyclinic G. Martino", Via Consolare Valeria, 1-Messina, Italy, Tel: 39-90-2212536.
Copyright
© 2019 Ieni A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.