Patient Empowerment in Neuro-Oncology: New Perspectives on an Emerging Concept
Abstract
Patient empowerment focuses on the control the individual patient exerts on his/her own health and illness. The empowerment approach is based on the recognition that patients have the capacity to influence their own health behavior and health focused on long-term surveillance and treatment.
An Integrated review was performed using PubMed and EMBASE databases. The search was limited to studies published between 1990 and 2014. Keywords and/or Medical Subject Headings (MeSH) terms used were (("Patient Participation"[Mesh])) AND "Brain Neoplasms"[Mesh]). Papers were excluded when empowerment concepts were not addressed to primary brain tumors.
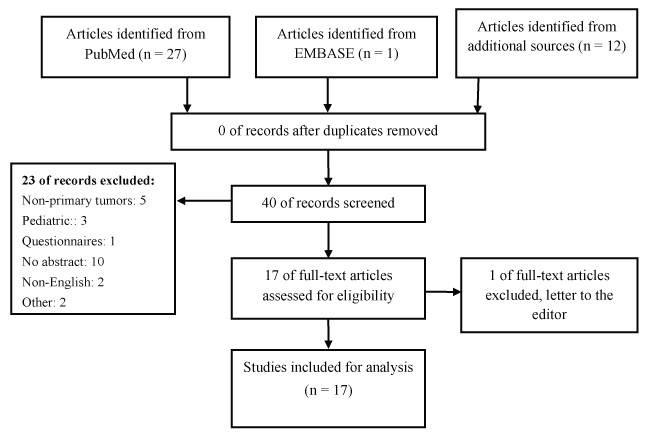
A total of 40 articles were found: 28 citations by database searching and 12 citations by hand searching. No duplicates were reviewed on title. A total of 23 papers were excluded. The remaining 17 papers after title selection and abstract were reviewed.
Using integrative literature review, we examined how empowerment strategies can be taken under consideration. We proposed an orbit model where patients are placed in the center of the orbit and two major orbits surround the patient as the nucleolus.
Patient empowerment and self-management programs are resources of supporting patients with chronic diseases. Significant efforts should be directed towards improving QoL of patients living with brain cancer.
Keywords
Empowerment, Self-management, Chronic care model, Neuro-oncology, Quality of life
Abbreviations
QoL: Quality of Life; WHO: World Health Organization; CCM: Chronic Care Model; SNIP: Standard Nursing Intervention Protocol
Introduction
Patient empowerment focuses on the control the individual patient exerts on his/her own health and illness, including the use of health care organizations, communities and the broader health care system. This concept also includes patient self-help and mutual aid programs. The fundamental premise underlying the patient empowerment approach to care is that patients, along with their health care professionals, have significant input regarding the treatment decisions addressing their health problems [1].
During the last 25 years, the roles of physicians and patients have changed substantially [2-4]. When the majority of morbidity and mortality was caused by acute illnesses, the patient was largely a passive recipient of medical care, but this is the age of chronic disease where the patient and his/her family are far more involved the prevention and treatment of chronic illnesses [5,6]. With chronic diseases, patients are responsible for the daily self-management of their illness over the long-term. Their success in self-management is significantly affected by the environmental context in which they manage their illness [7].
The ecological approach suggested by Fisher, et al. for diabetes patients, involves integrating the skills and choices of individual patients with the services and support they receive from the social environment of family (friends, worksites, organizations and cultures) and the physical and policy environments of neighborhoods, communities and governments; following key resources and support for self-management: individualized assessment, collaborative goal setting, skills enhancement, follow-up and support, access to resources and continuity of quality clinical care [7].
The empowerment approach is based on the recognition that patients have the capacity to positively influence their own health behavior and subsequently their health [8,9]. To be effective, supportive and rehabilitation services must provide appropriate follow-up support to alleviate side effects of treatment and concomitant psychological problems such as fear of disease recurrence, as well as, physical problems such as pain [1,10]. Empowering patients and their family can also have a positive impact on health care costs. Research by Kennedy, et al. demonstrated a small economic saving; however, no long-term prospective data is yet available [11,12]. This is a matter of great priority, since the working disability costs from the World Bank and the World Health Organization (WHO) have been reported to exceed one trillion dollars (US) [13].
As consequence of the technological advances on cancer screening, more patients experience malignancy as a chronic illness in which the new paradigm focuses on long-term surveillance and treatment [10,14]. According to the 2005 Institute of Medicine report, six major phases of cancer care were described: prevention, early detection, diagnosis, treatment, survivorship and end-of-life care [10,15]. The chronic disease model is intended to enrich the patient's Quality of Life (QoL) defined by the WHO as the subjective perception of one's own position in life in the context of the culture and values where you live and in relation to one's own objectives, hopes, habits and matters, frequently used as an index of disease severity or outcome [16,17].
Methods
We conducted an integrated review, defined by Whitte more and Knafl as a specific review method that summarizes past empirical or theoretical literature to provide a more comprehensive understanding of a particular phenomenon of health care problem [18,19]. As this paper examines how empowerment or related concepts in Neuro-Oncology have been described, both empirical and theoretical data were needed.
Search strategy
PubMed and EMBASE database were used. The search was limited to studies publishes between 1990 and 2014. Published material with patients < 18 years old were excluded. Keywords and/or Medical Subject Headings (MeSH) terms used were (("Patient Participation"[Mesh]) AND "Brain Neoplasms"[Mesh]).
Inclusion and exclusion criteria
Manuscripts that gathered empowerment or empowerment-related concepts in relation to brain tumor patients were included. Papers were excluded when empowerment concepts were not addressed to primary brain neoplasms (i.e metastasis), when empowerment or related concepts were only treated by professional caregivers, when questionnaires applications were used, when the study population consisted of patients with psychiatric or cognitive disorders/impairment or depression, not available abstract and when the paper was not written in English.
Data extraction
One of the authors (S.M.) initially identified and reviewed citation on title. Two authors (J.R. and R.A.) added empowerment related manuscripts that were not included in the MeSH search.
Results
Included studies
Using the PRISMA protocol, we initially identified a total of 40 articles: 28 citations by database searching and 12 citations by hand searching. No duplicates were reviewed on title. A total of 23 papers were excluded. The remaining 17 papers after title selection and abstract were reviewed for full text reading. Only two clinical trials were found and two other empirical manuscripts were included (Figure 1) [20].
Empowerment in relation to neuro-oncologic patients
We found no prior description of this concept focusing in Neuro-Oncologic patients. Only two manuscripts described the relationship between empowerment and cancer. Other two papers defined empowerment in cancer described as a chronic illness.
Change of paradigm: from paternalism to patient empowerment
In the treatment of acute illnesses, health care providers have historically told patients what to do and patients were expected to unquestioningly follow their physician's recommendations. Nowadays, effective health care requires a long-term commitment to extending care well past the acute phase. The acute care approach to patient care is not effective for treatment of chronic illnesses. Because the patients' behavior has such a significant impact on their health and QoL, chronic disease care requires a collaborative relationship between physicians and their patients [5,10].
Barlow elegantly defines patient self-management as the patient's ability to manage the symptoms and the consequences of living with chronic conditions. Successful self-management of chronic conditions includes treatment as well as physical, social and lifestyle changes [8,10,21]. This model enables the patient to manage most of the medical aspects of the illness and to cope with the psychological consequences of living with a chronic illness.
Patients must acquire new skills to manage a chronic illness, such as problem-solving, decision-making, resource utilization, forming partnerships with health care providers and taking action when necessary. This is a lifelong dynamic process of self-monitoring and self-evaluation [6,22].
Linked with the advances in interdisciplinary management of brain tumors that include radio- and chemotherapy and techniques to achieve greater tumor resection with less damage of non-involved areas of the brain (molecular biology, intraoperative imaging, fluorescence, ultrasonic surgical aspiration, and neuronavigation), other interventions are directed to different patients needs, including home caregivers, rehabilitators, social resources, care for wounds, nutrition, and adequate use of medications such as steroids and diverse treatments for pain, seizure control, delirium, and agitation [23-34]. These processes also involve identification and management of neurological deterioration, clinical complications, rehabilitation, and psychosocial issues with an interdisciplinary approach [35]. Socioeconomic evaluations have shown that these interventions have positive cost-effective and have an encouraging effect on QoL.
The chronic care model
The Chronic Care Model (CCM) was designed by Wagner and colleagues to support the delivery of high-quality health care [16]. It has six components: self-management support, delivery system design, decision support, clinical information systems, health care organization, and community resources. When applied as designed, these components converge to stimulate and support activated patients by proactive practice teams [36]. Practice teams integrate evidence-based reminders of clinical decision-making and determine what services are needed to develop collaborative care plans [10].
Self-Management Phases
Self-management during treatment
Adjuvant and neo-adjuvant nonsurgical treatments for cancer such as chemotherapy and radiation have proven to be safe enough to no longer require hospital stay. Most of these treatments are currently monitored in the outpatient setting. Treatment in the outpatient setting is designed to encourage patients to take active roles in preventing or managing treatment- (i.e., chemotherapy) related symptoms [10]. Physical activity has also been shown to be beneficial during and after cancer treatment [37,38].
Oncology nurse researchers designed the PRO-SELF Program as a self-care intervention for common symptoms associated with chemotherapy (oral microsites, nausea, vomiting and infection). No significant differences in outcomes were found between groups, but patients in the PRO-SELF arm reported benefits. The same PRO-SELF Program was re-tested for pain control to encourage patients to titrate analgesics to keep their pain from worsening. Participants in the program reported significant less pain intensity than control subjects (p < 0.001) [10,39]. Pain is associated with anxiety, depression, and sleep disturbances and strongly influences the patient's QoL. Studies indicate that 31 to 65% of patients suffer from inadequate pain control [18].
Patients managing the psychological, emotional and existential consequences of cancer treatment benefit from the support provided when they participate in self-help groups. As demonstrated by Braden, et al. self-help interventions increased level of self-care, self-help, psychological adjustment and confidence in women with breast cancer undergoing adjuvant treatment (p < 0.003) [10,40]. These authors modified the interventions to include early recognition and self-management of disabling symptoms. Significant improvements were found in the reduction of fatigue, pain and nausea (p = 0.04) [10,40].
The "Standard Nursing Intervention Protocol" (SNIP) provided as a home care intervention was designed to assess and monitor potential complications. It was also used to teach complex skills to patients and their families to manage their own care and coordinate resources after the debilitating effect of cancer surgeries. The use of SNIP increased survival for late-stage patients in the intervention group (p = 0.0001) [41].
Self-Management during the post-treatment phase
Post-treatment self-management is designed to reduce oncology visits, help patients understand the signs and symptoms of disease recurrence and manage the late-term effects of cancer and cancer treatment. During this phase, it is important to restore patients' social roles and normal routines. It is also important to help patients treat residual psychological distress to reduce its negative impact on QoL [10].
An intervention provided 2,838 home visits by a neuro-oncological team reach out 197 patients with brain tumors, that included clinical evaluation though scales and home care changes at the different stages of disease including low (weekly home access or contact by phone) to high intensity, defined as changes according to the appropriate needs at the different stages of disease. A magnetic resonance image was performance every 3 months before terminal progression of the disease, with visits according with the intensity of the progression. They report a positive impact on caregivers in 97% of cases, for nursing in 95%, communication in 90%, rehabilitation at home in 92%, social work help in 85%, and 72% had improvement in their quality of life scores due to rehabilitation [34].
Using the SF-36 Health Survey to measure the energy and fatigue of a cohort of breast cancer patients in a self-help group who received the education component, significantly less cancer-related distress in a 6-month follow-up period was reported (p = 0.037) [42]. Web-based interventions aimed to increase either patient empowerment or physical activity has shown promising results in other studies [37,38,43].
Self-Management at the end of life
Good palliative care avoids excessive invasive intervention as well as unneeded expenses and surgeries. The natural history of cancer is associated with physical changes that result in loss of the capacity to manage aspects of medical regimens and the normal activities of daily living. A home care intervention for patients with cancer by advance practice oncology nurses trained to prescribe care and address symptoms, physical function, emotional needs and psychological needs showed less distress (p = 0.03) and greater independence (p = 0.02) than participants in the usual care groups [10,44]. The home palliative care and end of life topics have provided evidence proceeding from observational and cohort studies.
Health-oriented lifestyle modifications during the post-treatment period have primarily focused on cancers with good prognoses and extended survival [10]. Mishra, et al. reviewed 40 trials with 3694 patients randomized to an exercise (n = 1927) or comparison (n = 1764) group. Cancer diagnoses in the study included breast, colorectal, head and neck, lymphoma and others. Mode of exercise consisted of strength training, resistance training, walking, cycling, yoga, Qigong, or Tai Chi. The authors concluded that exercise appears to have beneficial effects on QoL [38].
Religion and spirituality
The letting-go process includes emotional and spiritual concerns. Religion and spirituality help patients cope and help their families comfort them during their suffering. Although no data are currently available to support the efficacy of spirituality and prayer, they are universally accepted as playing an important role in helping patients cope with cancer [45].
Caution must be taken when invoking religion because some religions may lead people to interpret their illness as a sanction, rejection, or punishment from a divine source (i.e., God), which can lead people to experience feelings of abandonment, guilt and emotional distress and eventually reduce their capacity to face life events [45,46].
A recent Cochrane review included 1130 participants in a total of five randomized clinical trials and found inconclusive evidence that the intervention with spiritual or religious components in adults in terminal phase of a disease may or may not enhance well being [47]. Prayer is one of the most ancient interventions used with the intention of alleviating illness and promoting good health. Whether this may contribute towards proving or disproving the existence of God is unclear [47]. Roberts, et al. reviewed ten studies (7646 patients) comparing intercessory prayer plus standard care vs. standard care alone and found there was no clear effect of intercessory prayer on death (RR 0.73 CI 0.38-1.38) [48].
Discussion
Using integrative literature review, we examined how empowerment concepts in brain neoplasm (high-grade glioma) strategies can be taken under consideration. Inevitably, the end-of-life phase will come when tumor directed treatment is no longer possible and the patient's condition declines [49]. The debilitating effect of cancer surgeries on function and other parameters of QoL have prompted studies to address support for patients and families after surgery. The role of nurses in delivering interventions with positive outcomes has been confirmed in other studies. Most medical centers that provide neuro-oncologic treatment programs do not have a designated team to address a supportive patient empowerment program. The absence of standardization has been a major limitation in establishing such programs in cancer care. Patients and families need to be assessed to determine their willingness and ability to manage their cancer care themselves, including managing treatment, schedules, side effects, emotional commotion and family dynamics [10].
To date, there has been no analysis of these types of interventions in developing countries. Considering the links between poverty, health and disability in low-income countries, interventions that support and empower patients and their families may be of particular relevance to persons with disabilities [50,51]. Unfair distribution of and access to power, wealth and social resources in developing countries are often tolerated by social norms, policies and practices [50].
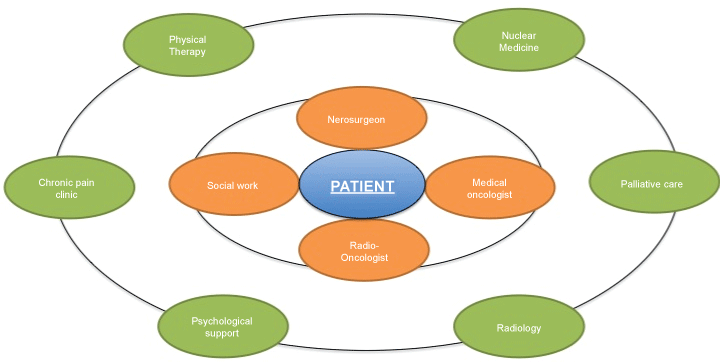
We propose an orbit model where patients are placed in the center of the orbit and two major orbits surround the patient as the nucleolus. The orbit model requires eliminating physician paternalism in patient care. The orbit approach puts the patient in the center of his/her universe surrounded by the first orbit. Te Boveldt, et al. also examined an empowerment concept limited to pain management, describing a two-cycle model with a central role for the patient as well as the clinician [18].
The orbit model
The first orbit consists of the major disciplines involved in the diagnosis and treatment of brain tumors (high-grade tumors). It should include brain surgeons, oncologists, radio-oncologists and social services. The brain surgeon is essential to provide symptom relief from brain edema and obtaining tissue samples for the neuro-pathologist to establish the final diagnosis. After the diagnosis is made, the medical oncologist will concomitantly start treatment with radiotherapy, i.e., high-grade gliomas [26]. There is a transition zone from the immediate period after surgery and hospital discharge, when major functional and psychological impairment is observed to transit to the second orbit (Figure 2).
The second orbit will function as the first line of contact with the patient after discharge and will follow the patient and family, mainly until the end. It includes all the interdisciplinary efforts to provide the best QoL with physical rehabilitation, chronic pain clinic, palliative care, psychology and follow-up imaging studies by the radiology department (MR and PET-CT). Cancer survivors are 1.4 times likely to be unemployed than healthy persons. Patients with cancer experience return-to-work benefits from multidisciplinary interventions [52]. Follow-up is essential for a persistent state of alarm secondary to the fear of tumor progression or negative life perspectives [53].
For brain cancer patients, The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire 30 (EORTC QLQ-C30) and the 20-item EORT QLQ-Brain Neoplasm (QLQ-BN20) are used to evaluate and analyzed the change of QoL. The QLQ-C30 questionnaire was comprised of 5 functional scales (physical, role, emotional, cognitive and social), 3 symptoms scales (fatigue, nausea/vomit and pain), 6 single-item scales (dyspnea, insomnia, appetite loss, constipation and financial effect on treatment) and a global QoL. The QLQ-BN20 questionnaire consisted of 11 items, grouped into 4 domains (future uncertainly, visual disorder, communication deficit and motor dysfunction) and 7 single-item (headache, seizure, drowsiness hair loss, itching, weakness of both legs and difficulty controlling bladder function) [54].
Exercise has a beneficial effect on physical function, role function, independence, social function and fatigue [53,55]. Patients with a primary brain tumor often experience depression. No eligible studies proved the benefit or harms of any pharmacological treatment of depression and, in most cases, are administered with caution of potential side effects [56].
Brain tumors (benign and non-benign) do not respect or predict functional areas of invasion. Before or after surgery, the patient may experience motor function loss, sensory deficiency, and cognitive decline and/or language deficit. Giovagnoli, et al. demonstrated that cognitive pattern was marked by impaired mental flexibility and memory, indicating the coexistence of multiple brain dysfunctions caused by mass effect, drugs, radiotherapy and chemotherapy [17]. In the same study, this author reported glioblastoma patients to have the worst disease perception, effective well-being, role/sociability and overall QoL compared with other subgroups of brain tumors [17].
This requires transition to the second orbit. The aim of the second orbit is to avoid complications, carry out disease management and follow-up treatment and prepare the patient and the family towards the inevitable events. Pain control is essential to provide the patient with an adequate QoL. Radiology departments play a key role in obtaining the best medical imaging to detect early complications and tumor recurrences [57]. End-of-life care discussion should not be delay until the patient becomes incompetent to participate. Sizoo, et al. described that 4 months after diagnosis 15-23% of patient were unable to decide [49,58]. Family plays an essential and dynamic role; it bounces indistinctly from the nucleolus to any rim.
Until this moment there has not been published any prospective protocol using this orbit model. The aim of this paper is to settle a multidisciplinary effort plan to bring to the brain tumor patient the summary of all resources to achieve the best QoL. No prospective data exists regarding economical savings; this can be an ideal platform to investigate that topic.
Conclusion
Patient empowerment and self-management programs have emerged as a viable resource to address the challenges of supporting patients with chronic diseases. As the number of cancer survivors increases due to better medical and surgical treatments, patients and families can benefit significantly fromen rolling in non-paternalist supportive follow-up programs. There has not been a level I evidence study that correlates patient empowerment with economic savings. Additional studies need to be done to test this hypothesis. Scientific research has not demonstrated that religion and spirituality provide a benefit but are generally accepted to have a positive impact on patients and families. End-of-life care is aimed to maintain quality of life with medical decisions for the prevention and relief of suffering. The most challenging part of the orbit model will be bringing all medical and surgical specialties together and placing the patient as the center of interest. With the growth in cancer survival rates, significant efforts should be directed towards improving QoL of patients living with cancer.
Ethics
This manuscript was not submitted to an ethics committee because is a proposed theory model and no human (nor animal) subjects were used to elaborate this paper.
Author's Contributions
1. Study concept and design: Manrique
2. Acquisition of data: Manrique
3. Analysis and interpretation of data: Manrique and Rodriguez-Flores
4. Drafting of the manuscript: Manrique and Rodriguez-Flores
5. Critical revision of the manuscript for important intellectual content: Rodriguez-Saldaña and Anderson
6. Statistical analysis: None
7. Administrative, technical, and material support: Manrique
8. Study supervision: Anderson
Financial Disclosure
All authors have no direct financial payment, ownership of share or act as personal consultant for any companies and/or organization(s) with financial interest for promotion.
Funding/Support
None.
References
- McAllister M, Dunn G, Payne K, et al. (2012) Patient empowerment: the need to consider it as a measurable patient-reported outcome for chronic conditions. BMC Health Serv Res 12: 157.
- Kaplan SH, Greenfield S, Ware JE (1989) Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care 27: S110-S127.
- Funnell MM (2000) Helping patients take charge of their chronic illnesses. Fam Pract Manag 7: 47-51.
- Alexander JA, Hearld LR, Mittler JN, et al. (2012) Patient-physician role relationships and patient activation among individuals with chronic illness. Health Serv Res 47: 1201-1223.
- Alwan A, Maclean DR (2009) A review of non-communicable disease in low- and middle-income countries. Int Health 1: 3-9.
- Yach D, Hawkes C, Gould CL, et al. (2004) The global burden of chronic diseases: overcoming impediments to prevention and control. JAMA 291: 2616-2622.
- Fisher EB, Brownson CA, O'Toole ML, et al. (2005) Ecological approaches to self-management: the case of diabetes. Am J Public Health 95: 1523-1535.
- Funnell MM, Anderson RM, Arnold MS, et al. (1991) Empowerment: an idea whose time has come in diabetes education. Diabetes Educ 17: 37-41.
- Anderson RM (1995) Patient empowerment and the traditional medical model. A case of irreconcilable differences? Diabetes Care 18: 412-415.
- McCorkle R, Ercolano E, Lazenby M, et al. (2011) Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin 61: 50-62.
- Kennedy A, Reeves D, Bower P, et al. (2007) The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 61: 254-261.
- Foster G, Taylor SJ, Eldridge SE, et al. (2007) Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev.
- Saunders SL, Nedelec B (2014) What work means to people with work disability: a scoping review. J Occup Rehabil 24: 100-110.
- Spitzer WO, Dobson AJ, Hall J, et al. (1981) Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. J Chronic Dis 34: 585-597.
- Grant M, Economou D, Ferrell B, et al. (2012) Educating health care professionals to provide institutional changes in cancer survivorship care. J Cancer Educ 27: 226-232.
- Wagner EH, Austin BT, Von Korff M (1996) Organizing care for patients with chronic illness. Milbank Q 74: 511-544.
- Giovagnoli AR, Meneses RF, Silvani A, et al. (2014) Quality of life and brain tumors: what beyond the clinical burden? J Neurol 261: 894-904.
- Te Boveldt N, Vernooij-Dassen M, Leppink I, et al. (2014) Patient empowerment in cancer pain management: an integrative literature review. Psychooncology 23: 1203-1211.
- Whittemore R, Knafl K (2005) The integrative review: updated methodology. J Adv Nurs 52: 546-553.
- Liberati A, Altman DG, Tetzlaff J, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 151: 65-94.
- Barlow J, Wright C, Sheasby J, et al. (2002) Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 48: 177-187.
- Ryan P, Sawin KJ (2009) The Individual and Family Self-Management Theory: background and perspectives on context, process, and outcomes. Nurs Outlook 57: 217-225.
- Hegi ME, Murat A, Lambiv WL (2006) Brain tumors: molecular biology and targeted therapies. Ann Oncol 17: 191-197.
- Lacroix M, Abi-Said D, Fourney DR, et al. (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95: 190-198.
- Stummer W, van den Bent MJ, Westphal M (2011) Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien) 153: 1211-1218.
- Stupp R, Tonn JC, Brada M, et al. (2010) High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21: 190-193.
- Sanai N, Mirzadeh Z, Berger MS (2008) Functional outcome after language mapping for glioma resection. The New England Journal of Medicine 358: 18-27.
- Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62: 753-764.
- Stupp R, Mason WP, van den Bent MJ, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
- Watanabe R, Nakasu Y, Tashiro H, et al. (2011) O6-methylguanine DNA methyltransferase expression in tumor cells predicts outcome of radiotherapy plus concomitant and adjuvant temozolomide therapy in patients with primary glioblastoma. Brain Tumor Pathol 28: 127-135.
- Kotsarini C, Griffiths PD, Wilkinson ID, et al. (2010) A systematic review of the literature on the effects of dexamethasone on the brain from in vivo human-based studies: implications for physiological brain imaging of patients with intracranial tumors. Neurosurgery 67: 1799-1815.
- Tremont-Lukats IW, Ratilal BO, Armstrong T, et al. (2008) Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev.
- Kerrigan S, Grant R (2011) Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev.
- Pompili A, Telera S, Villani V, et al. (2014) Home palliative care and end of life issues in glioblastoma multiforme: results and comments from a homogeneous cohort of patients. Neurosurg Focus 37: E5.
- Catt S, Chalmers A, Fallowfield L (2008) Psychosocial and supportive-care needs in high-grade glioma. Lancet Oncol 9: 884-891.
- Coleman K, Austin BT, Brach C, et al. (2009) Evidence on the Chronic Care Model in the new millennium. Health Aff (Millwood) 28: 75-85.
- Kuijpers W, Groen WG, Aaronson NK, et al. (2013) A systematic review of web-based interventions for patient empowerment and physical activity in chronic diseases: relevance for cancer survivors. J Med Internet Res 15: e37.
- Mishra SI, Scherer RW, Geigle PM, et al. (2012) Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev.
- Miaskowski C, Dodd M, West C, et al. (2004) Randomized clinical trial of the effectiveness of a self-care intervention to improve cancer pain management. J Clin Oncol 22: 1713-1720.
- Braden CJ, Mishel MH, Longman AJ (1998) Self-Help Intervention Project. Women receiving breast cancer treatment. Cancer Pract 6: 87-98.
- McCorkle R, Strumpf NE, Nuamah IF, et al. (2000) A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc 48: 1707-1713.
- Ganz PA, Kwan L, Stanton AL, et al. (2004) Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst 96: 376-387.
- Samoocha D, Bruinvels DJ, Elbers NA, et al. (2010) Effectiveness of web-based interventions on patient empowerment: a systematic review and meta-analysis. J Med Internet Res 12: e23.
- McCorkle R, Benoliel JQ, Donaldson G, et al. (1989) A randomized clinical trial of home nursing care for lung cancer patients. Cancer 64: 1375-1382.
- Vonarx N, Hyppolite SR (2013) Religion, spirituality, and cancer: the question of individual empowerment. Integr Cancer Ther 12: 69-80.
- Pargament KI, Koenig HG, Perez LM (2000) The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol 56: 519-543.
- Candy B, Jones L, Varagunam M, et al. (2012) Spiritual and religious interventions for well-being of adults in the terminal phase of disease. Cochrane Database Syst Rev.
- Roberts L, Ahmed I, Hall S, et al. (2009) Intercessory prayer for the alleviation of ill health. Cochrane Database Syst Rev.
- Sizoo EM, Pasman HR, Buttolo J, et al. (2012) Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer 48: 226-232.
- Borg J, Bergman AK, Ostergren PO (2013) Is 'legal empowerment of the poor' relevant to people with disabilities in developing countries? An empirical and normative review. Glob Health Action 6: 22854.
- Marmot M (2005) Social determinants of health inequalities. Lancet 365: 1099-1104.
- de Boer AG, Taskila T, Tamminga SJ, et al. (2011) Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev.
- Ooi AL, Mazlina M (2013) Functional status and health-related quality of life in patients with primary intracranial tumour. Med J Malaysia 68: 448-452.
- Kim CW, Joo JD, Kim YH, et al. (2016) Health-Related Quality of Life in Brain Tumor Patients Treated with Surgery: Preliminary Result of a Single Institution. Brain Tumor Res Treat 4: 87-93.
- Mishra SI, Scherer RW, Snyder C, et al. (2012) Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev.
- Rooney A, Grant R (2010) Pharmacological treatment of depression in patients with a primary brain tumour. Cochrane Database Syst Rev.
- Wen PY, Macdonald DR, Reardon DA, et al. (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28: 1963-1972.
- Triebel KL, Martin RC, Nabors LB, et al. (2009) Medical decision-making capacity in patients with malignant glioma. Neurology 73: 2086-2092.
Corresponding Author
Salvador Manrique-Guzman, Department of Neurosurgery, ABC Neurological Center, Sur 136 No 116, Col Las Americas, Delegación Alvaro Obregon, Mexico, Mexico ZC: 01120.
Copyright
© 2017 Manrique-Guzman S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.