Cynara cornigera Fruit Extracts Mediated Green Synthesized Silver Nanoparticles (AgNPs): Comparison Investigation on Characterizations and Synthesis Techniques
Abstract
The role of green chemistry in nanotechnology and nanoscience fields is very significant in synthesizing various nanomaterials. The effect of the synthesis technique on the size of Ag-NPs have been reported. Ag nanoparticles has been produced by using two techniques: Green synthesis and uv-irradiation green synthesis methods, respectively. The current research included to use a stabilizer and reducing agents from plant extract to get the non-toxic and environmentally friendly product, via using Cynara cornigera . The characterization of AgNPs was conducted with ultraviolet-visible (UV-vis) spectroscopy, powder X-ray diffraction (XRD), and scanning electron microscopy (SEM). The results showed that AgNPs were successfully synthesized by Cynara cornigera extract through the two methods. However, the results improved that, the AgNPs synthesis process was effectively enhanced by UV irradiation. The UV-vis spectrum of the AgNPs colloids showed an absorption peak at around 400 nm. The XRD patterns confirm the presence of Ag nanoparticles with a cubic structure and SEM images showed AgNPs with an average diameter of 16 ± 5 nm and 10 ± 2 nm. It was concluded that Cynara cornigera extract could be used as a bio-reducing agent, an alternative to chemical agents. In addition, the UV irradiation improved the concentration of AgNPs and the reaction rate. The synthesized AgNPs could be tested and applied in applications such as antimicrobial agents for further research and investigation.
Keywords
Silver nanoparticles, Bio-reducing agent, Extract, Green synthesis, UV-irradiation
Introduction
Nanoscience and nanotechnology represent a great research area that involves structures, systems, and devices with novel properties and functions due to the arrangement of their atoms on the 1-100 nm scale [1]. In recent years, nanoparticles have been expanded into a broad and promising range of clinical applications, such as anticancer and antimicrobial agents, because of their unique properties [1-7]. Silver nanoparticles (AgNPs) are notable nanometals that provide a greater capacity and a higher surface area than the bulk form. At the nanoscale, this material exhibits unique electrical, optical, and catalytic properties [3]. AgNPs are used in many fields, such as medicine, bioremediation studies, catalysis applications, food, cosmetics, industry, agricultural activities, and electronics [4]. Several conventional synthesis methods, including green and chemical, have been successfully employed in nanoparticle production [5]. The nanoparticles can be prepared through chemical and physical methods. For chemical methods, chemical reduction methods are used to synthesis the nanoparticles [5-7]. Most of the chemical methods use toxic chemical materials, which can cause serious damage to the environment.
Recently, replacing these methods with green synthesis, environmentally friendly, and low-cost methods has attracted the researchers's concerns. Physical methods for the preparation metal nanoparticles as a green method can be done using irradiation as a reducing agent, including gamma irradiation [8], ultraviolet (UV) irradiation [9], microwave irradiation [10], and ultrasonic waves [11]. Physical irradiation could enhance the formation rate of AgNPs [12,13]. The photoreduction method of the metallic ions to metallic nanoparticles has been successfully applied to produce nanometal colloids, including silver and gold. The proposed methods needed light at different wavelengths to promote the reduction of the metals in solution, as well as the presence of both reductants and surface capping agents as stabilizers of the obtained colloid nanoparticles [14].
For instance, plant extracts, bacteria, fungi, and algae are widely used for the green synthesis of nanoparticles [15,16]. In response to the challenge of green synthesis, which uses plant extracts instead of industrial chemical agents to reduce metal ions, the method has been developed to be more beneficial than traditional chemical synthesis because it costs less, decreases pollution, and increases environmental and human health safety [17]. Recent investigations reveal that plant extracts possess organic compounds such as terpenoids, flavonoids, phenols and dihydric phenols, which can be employed for metal reduction [18]. However, Cynara cornigera (the artichoke) is a dwarf plant that grows in Mediterranean countries. In Libya, it is one of the wild plants distributed in the eastern part of the country and used as food and in folk medicine. The plant is rich in natural antioxidants, mainly polyunsaturated fatty acids, vitamins C, A, and E. Moreover, it is rich in polyphenols and flavonoid compounds [19-21].
This research presents the synthesis of AgNPs by using local Cynara cornigera as a stabilizer with UV irradiation as a reducing agent to enhance the formation of AgNPs and form small-size and highly distributed AgNPs. AgNPs have been characterized via ultraviolet-visible (UV-vis) spectroscopy, powder X-ray diffraction (XRD), and scanning electron microscopy (SEM).
Experimental
Materials and equipments
The Cynara cornigera plant (Figure 1) was collected from Tokra town, Libya. The dried, ground fruit was obtained from the plant and used for the bio-reducing agent preparation. All chemicals used in this study were analytical grade and used without further purification. Silver nitrate, NaOH and deionized water were used in all experiments. UV irradiation lamp (a low-pressure mercury lamp: 93110, E27 of spectral lamp company, λ = 185 nm and P = 6W) for 20 min, these exprementals have been done in the chemistry department laboratory in Benghazi University.
Preparation of bio-reducing agent from Cynara cornigera
The preparation of the bio-reducing agent (the Cynara cornigera extract) was carried out according to the following steps. Firstly, 50 g of the dried and ground Cynara cornigera fruit was mixed with 500 ml of deionized water. Secondly, the mixture was heated at 100 °C for 20 minutes, and then, it was cooled at room temperature (25 °C). Finally, the mixture was filtered using Whatman No.1 filter paper to separate the Cynara cornigera extract, which was used as a bio-reducing agent.
Synthesis of silver nanoparticles (AgNPs)
1 mM aqueous solution of AgNO 3 was prepared and used to synthesise the AgNPs.
For the first method 10 ml of Cynara cornigera extract was added to 90 ml of the AgNO 3 aqueous solution (labeled as Cynara cornigera -AgNO 3 ) at pH = 10 was stirred at room temperature (25 °C) for 120 min at 200 rpm [22].
And for the second method, In this work, Cynara cornigera -AgNO 3 was irradiated using a UV irradiation lamp (a low-pressure mercury lamp: 93110, E27 of spectral lamp company, λ = 185 nm and P = 6W) for 20 min [23].

Characterization
The AgNPs were characterized using ultraviolet-visible (UV-vis) spectroscopy, X-ray diffraction (XRD), and scanning electron microscopy (SEM). The UV-vis spectra were recorded in the range of 300-800 nm with the H.UV.1650 PC, SHIMADZU UV-vis spectrophotometer. The XRD patterns were carried out on a Philips (X'pert, Cu Kα) at a scan speed of 2°/min, and the images were taken with the JEOL JSM-7600F scanning electron microscope (SEM). The ImageJ program has been used to calculate the size of nanoparticles.
Results and Discussion
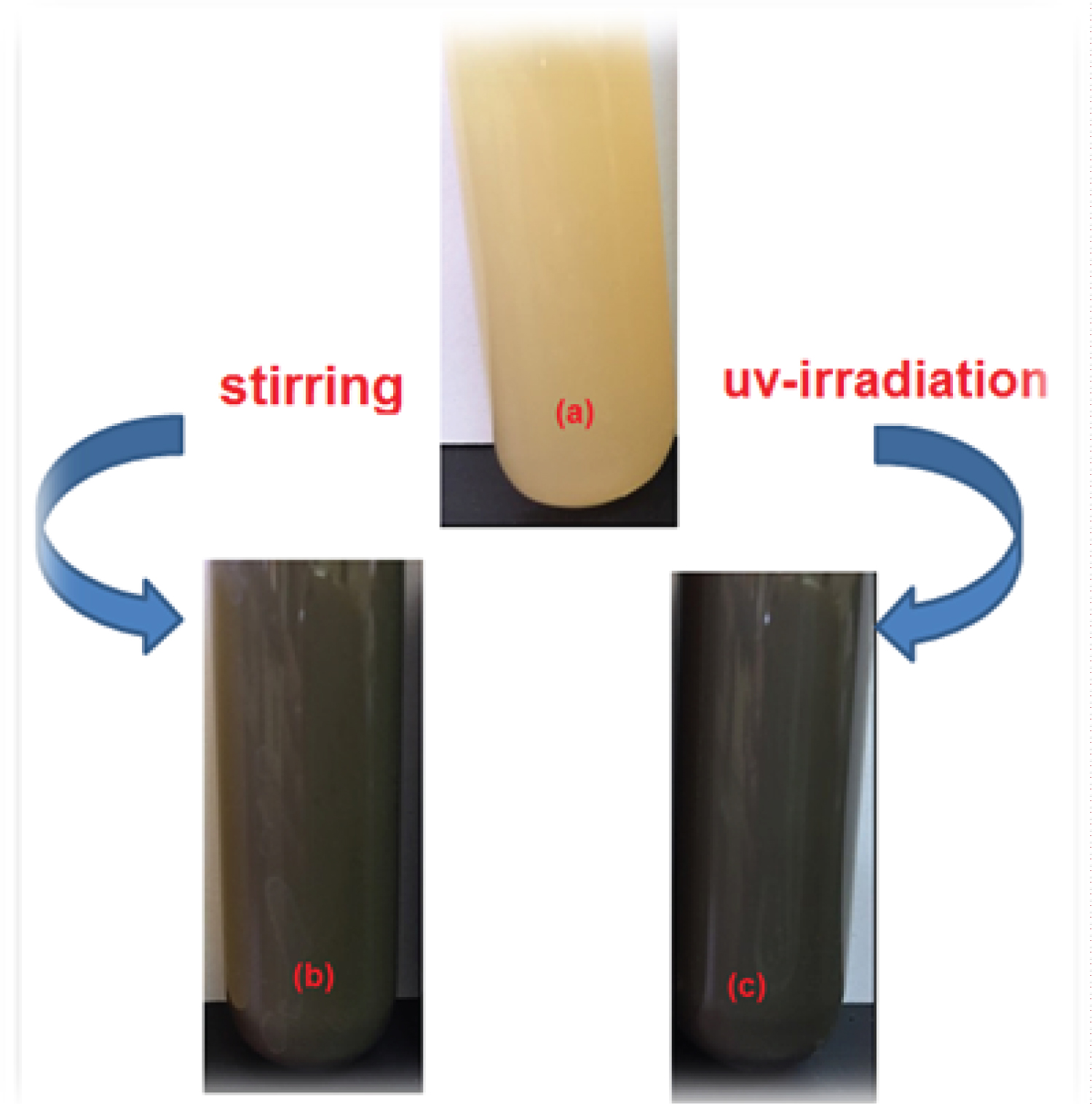
The colour of Cynara cornigera- AgNO 3 , changes from yellow in Figure 2a to dark brown after 120 min stirring at room temperature as illustrated in Figure 2b. In addition, after irradiation of the mixture of Cynara cornigera- AgNO 3 using a UV-lamp for 20 min, it was noticed that the colour of Cynara cornigera- AgNO 3 also changed to dark grey (Figure 2c). The change in the colour was considered as an indication of AgNPs formation by the reduction of the Ag + ion [24]. This result demonstrates that more AgNPs were obtained under a shorter time with UV irradiation which the nanoparticle clusters were disintegrated due to the irradiation. Photoinduced fragmentation of AgNPs has been reported [25] as follows:
(1)
(2)
(3)
where ( Ag ) n is the silver nanocluster containing n silver atoms and is the aqueous electron. After UV irradiation to aqueous solutions of Cynara cornigera /Ag + , a large amount of aqueous electrons was produced, and the silver cations were reduced into AgNPs.
UV-visible spectroscopy analysis
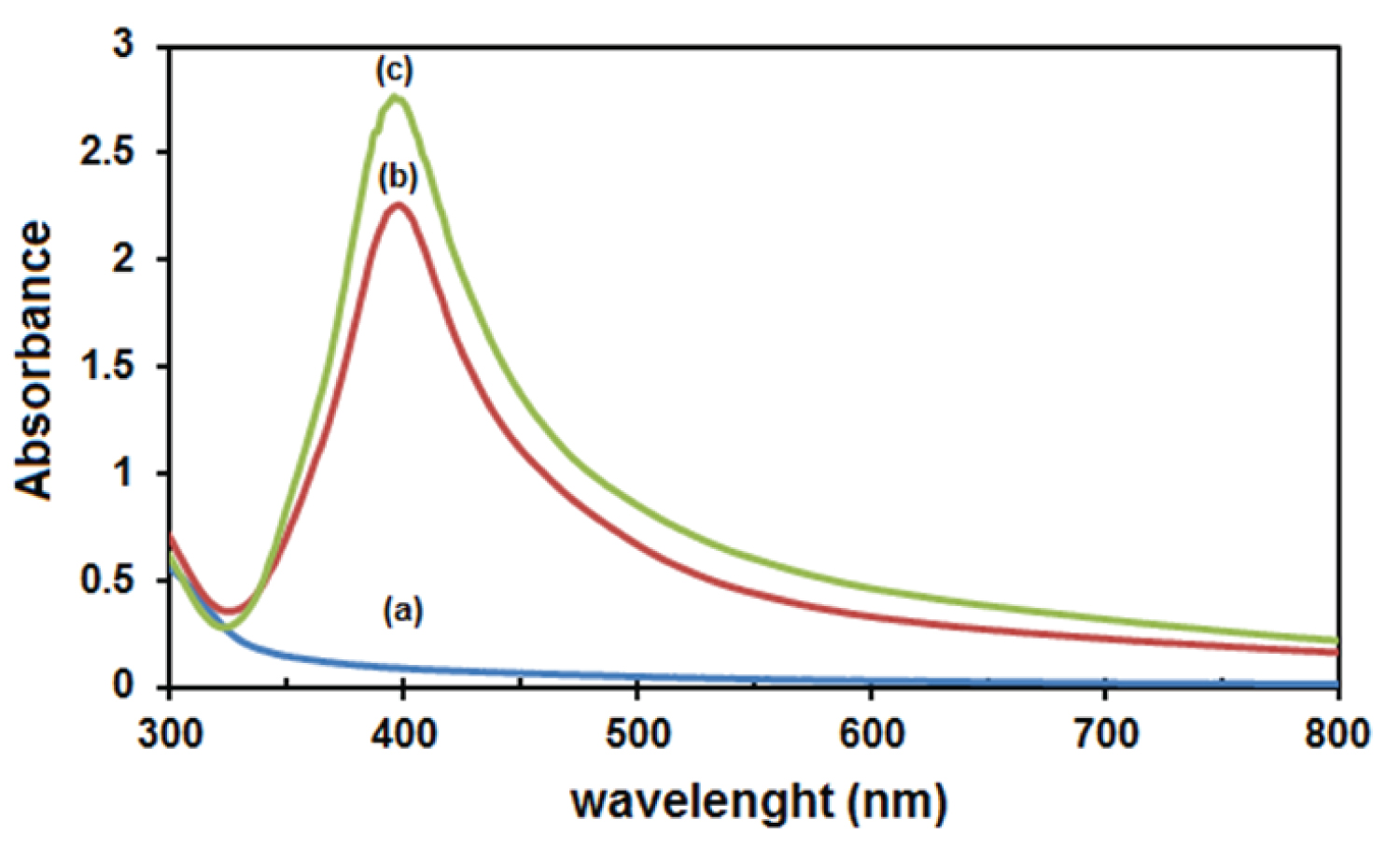
UV-Vis spectroscopy is one of the most widely used techniques to identify silver nanoparticles. Free electrons in the silver nanoparticles are evoked by absorbing visible light and transmitted to a higher energy level. But the electron is unstable in an excited state and returns to the base energy level, and then simultaneously a photon is emitted [26]. The characteristics of the absorption bands, related to the surface plasmon resonance (SPR) of metallic NPs, are influenced by the size, shape and distribution of particles, as well as the dielectric constant of the surrounding media [27]. The absorption spectrum (Figure 3a) of the Cynara cornigera -AgNO 3 showed no absorption band, which means there are no nanoparticles before starting the reaction. However, after stirring the mixture, an absorption band was identified around 400 nm, indicating the presence of Ag nanoparticles (Figure 3b) [28]. Furthermore, the absorption band appears at 400 nm with an absorbance increase after UV irradiation (Figure 3c). The increase in the absorbance refers to an increase in the formation of AgNPs with smaller size [6].
X-ray diffraction (XRD) analysis
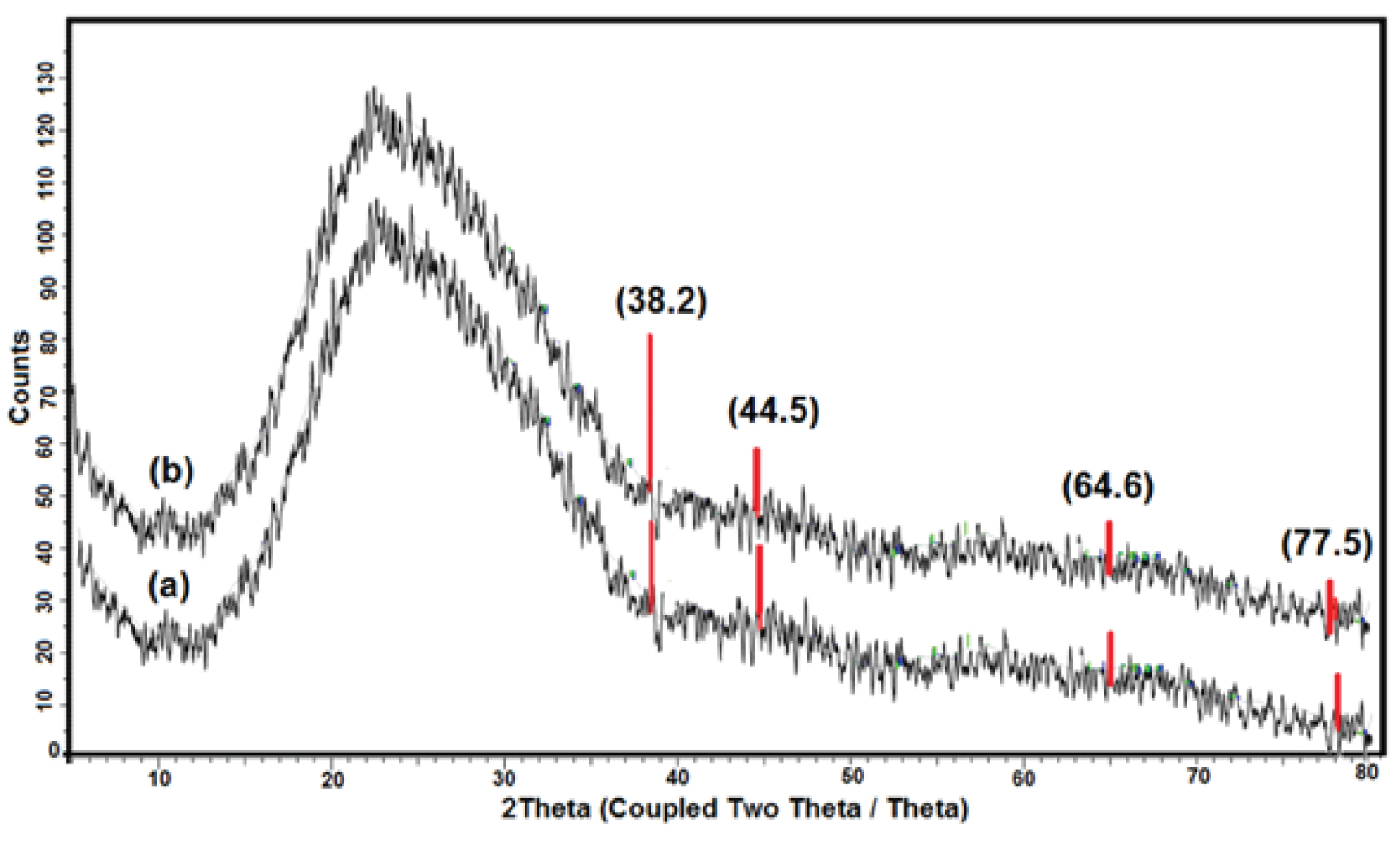
XRD studies were conducted to obtain information about the nanoparticles' pattern and nature. Figure 4a and Figure 4b show XRD patterns of AgNPs synthesized by stirring time and UV irradiation of Cynara cornigera -AgNO 3 , respectively. The two diffractogram patterns show diffraction peaks of the main four characteristics of the prepared AgNPs. They were observed at 2θ = 38.2°, 44.5°, 64.6°, and 77.5°, which correspond to the (111), (200), (220), and (311) planes, respectively. The peaks confirm the presence of AgNPs and suggest the face-centred cubic (FCC) structure in the two samples [11,29-31]. The unassigned peak (2θ around 24.5°) could refer to the biomolecule phase crystallization on the AgNPs surface, which acts as the capping and stabilizing agent [32,33].
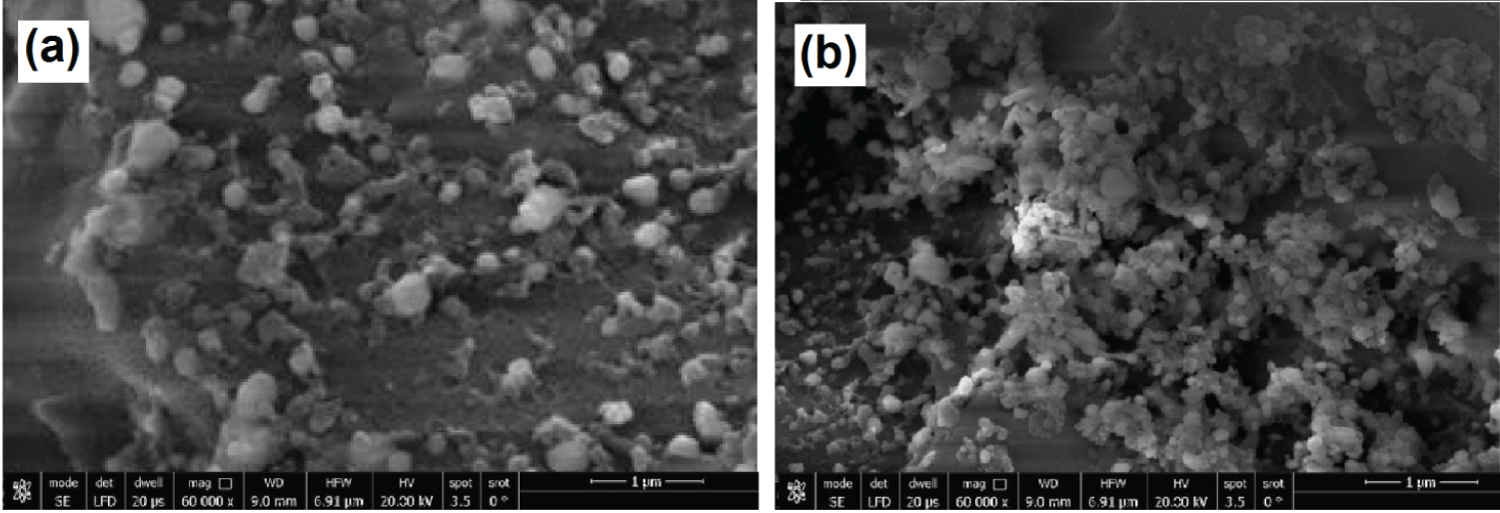
Morphology and size of AgNPs . Figure 5 shows the SEM images of the AgNPs and displays the change in the morphology. Figure 5a illustrates the AgNPs formation under stirring time at room temperature, and Figure 5b illustrates the formation of AgNPs under UV irradiation. From the SEM images (Figure 5a) can be found the formation of AgNPs with an average size of 16 ± 5 nm.
Moreover, the UV irradiation increases the yield of AgNPs with nearly homogeneous spherical shapes and smaller sizes with mean diameters about 10 ± 2 nm (Figure 5b). The results are in agreement with the data of the UV-vis analysis as well as data obtained from published research in the literature [11,13,31].
Conclusion
AgNPs with spherical shapes and an average size of 16 ± 5 nm and 10 ± 2 nm were synthesized using aqueous Cynara cornigera extract. The AgNPs were analyzed using a UV-Vis spectrophotometer, XRD, and SEM. Biosynthesis of AgNPs using the green resources of Cynara cornigera is a good alternative to chemical synthesis since this green synthesis is eco-friendly. UV irradiation improved the formation of AgNPs, From the results obtained in this research, one can affirm that Cynara cornigera can play an important role in the bioreduction and stabilization of silver ions to AgNPs.
References
- Bayda S, Adeel M, Tuccinardi T, et al. (2019) The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules 25: 112.
- Mitchell MJ, Billingsley MM, Haley RM, et al. (2021) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20: 101-124.
- Bruna T, Maldonado-Bravo F, Jara P, et al. (2021) Silver nanoparticles and their antibacterial applications. Int J Mol Sci 22: 7202.
- Guilger-Casagrande M, Lima RD (2019) Synthesis of silver nanoparticles mediated by fungi: A review. Front Bioeng Biotechnol 7: 287.
- Niculescu AG, Chircov C, Grumezescu AM (2022) Magnetite nanoparticles: Synthesis methods - A comparative review. Methods 199: 16-27.
- Guzmán MG, Dille J, Godet S (2009) Synthesis of silver nanoparticles by chemical reduction method and their antibacterial activity. Int J Chem Biomol Eng 2: 104-111.
- Shameli K, Ahmad M, Wan Yunus W, et al. (2010) Synthesis and characterization of silver/polylactide nanocomposites. Proc World Acad Sci Eng Technol 64: 28-32.
- Shameli K, Ahmad M, Wan Yunus W, et al. (2010) Synthesis of silver/montmorillonite nanocomposites using γ-irradiation. Int J Nanomedicine 5: 1067-1077.
- Shameli K, Ahmad M, Wan Yunus W, et al. (2010) Green synthesis of silver/montmorillonite/chitosan bionanocopmosites using the UV irradiation method and evaluation of antibacterial activity. Int J Nanomedicine 5: 875-887.
- Tsuji M, Hashimoto M, Nishizawa Y, et al. (2005) Microwave assisted synthesis of metallic nanostructures in solution. Chemistry 11: 440-452.
- Elsupikhe RF, Shameli K, Ahmad MB (2015) Effect of ultrasonic radiation’s times to the control size of silver nanoparticles in κ-carrageenan. Res Chem Intermediat 41: 8829-8838.
- Bhardwaj S, Lata S, Garg R (2022) Phyto-mediated green synthesis of silver nanoparticles using Acmella oleracea leaf extract: Antioxidant and catalytic activity. Pharmacogn Mag 18: 22-28.
- Nguyen HT, Nguyen TD, Nguyen DP, et al. (2022) Synthesis efficiency of silver nanoparticles by light-emitting diode and microwave irradiation using starch as a reducing agent. Nanotechnol Environ Eng 7: 297-306.
- Khodashenas B, Ghorbani HR (2019) Synthesis of silver nanoparticles with different shapes. Arab J Chem 12: 1823-1838.
- Noah NM, Ndangili PM (2022) Green synthesis of nanomaterials from sustainable materials for biosensors and drug delivery. Sens Int 3: 100166.
- Abdussalam-Mohammed W, Mohamed L, Abraheem MS, et al. (2023) Biofabrication of silver nanoparticles using teucrium apollinis extract: Characterization, stability, and their antibacterial activities. Chemistry 5: 54-64.
- Awwad AM, Salem NM (2012) Green synthesis of silver nanoparticles by Mulberry Leaves Extract. Nanosci Nanotechnol 2: 125-128.
- Ying S, Guan Z, Ofoegbu PC, et al. (2022) Green synthesis of nanoparticles: Current developments and limitations. Environ Technol Innov 26: 102336.
- Filip GA, Moldovan B, Baldea I, et al. (2019) UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their comparative effects in experimental inflammation. J Photochem Photobiol B 191: 26-37.
- Hasham YE, Garabulli FE, Shatshat SE (2020) Antimitotic and genotoxicity effects of wild Libyan artichoke cynara cornigera leaves aqueous extract. IJRD 5: 448-454.
- Yusra FE, Maraia FE, Fatma AA (2022) Phytochemical screening, total phenols and total flavonoids content of cynara cornigera roots. In: The libyan conference on chemistry and its applications (LCCA 2021), University of Benghazi, Benghazi, Libya, 102-105.
- Pandino G, Lombardo S, Mauromicale G, et al. (2011) Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild Cynara cardunculus L. genotypes. Food Chem 126: 417-422.
- Jain D, Daima HK, Kachhwaha S, et al. (2009) Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Dig J Nanomater Biostructures 4: 557-563.
- El-Shahaby O, El-Zayat M, Salih E, et al. (2013) Evaluation of antimicrobial activity of water infusion plant-mediated silver nanoparticles. J Nanomed Nanotechnol 4: 178.
- Bharadwaj KK, Rabha B, Pati S, et al. (2021) Green synthesis of silver nanoparticles using Diospyros malabarica fruit extract and assessments of their antimicrobial, anticancer and catalytic reduction of 4-nitrophenol (4-NP). Nanomaterials 11: 1999.
- Darroudi M, Ahmad MB, Zak AK, et al. (2011) Fabrication and characterization of gelatin stabilized silver nanoparticles under UV-light. Int J Mol Sci 12: 6346-6356.
- Behravan M, Panahi AH, Naghizadeh A, et al. (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124: 148-154.
- Soto KM, Quezada-Cervantes CT, Hernández-Iturriaga M, et al. (2019) Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT-Food Sci Technol 103: 293-300.
- Shameli K, Ahmad MB, Yunus WZW, et al. (2010) Synthesis and characterization of silver/talc nanocomposites using the wet chemical reduction method. Int J Nanomedicine 5: 743-751.
- Joseph S, Mathew B (2015) Facile synthesis of silver nanoparticles and their application in dye degradation. Mater Sci Eng B 195: 90-97.
- Khan MJ, Kumari S, Shameli K, et al. (2019) Green synthesis and characterization of pullulan mediated silver nanoparticles through ultraviolet irradiation. Materials 12: 2382.
- Khalir W, Azira W, Shameli K, et al. (2020) Biosynthesized silver nanoparticles by aqueous stem extract of Entada spiralis and screening of their biomedical activity. Front Chem 8: 620.
- Al Mashud M, Moinuzzaman M, Hossain M, et al. (2022) Green synthesis of silver nanoparticles using Cinnamomum tamala (Tejpata) leaf and their potential application to control multidrug resistant Pseudomonas aeruginosa isolated from hospital drainage water. Heliyon 8: 1-9.
Corresponding Author
RANDA F. Elsupikhe, Department of Chemistry, Faculty of Science, University of Benghazi, Benghazi, Libya.
Copyright
© 2025 Elsupikhe RF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.