Synthesis of a Silver Nanowire Array on Cu-BTC MOF Micropillars
Abstract
An array of Ag nanowires has been prepared from a facile, templated approach on Cu(BTC) (1,3,5-benzenetricarboxylic acid) metal organic framework (MOF) micropillars. The Ag-deposited scaffolding material may be used to prepare electronic or optoelectronic device for various applications.
Introduction
Silver (Ag) nanowires have been used to build a variety of nanoelectronics [1,2] and optoelectronic devices [3,4]. Most of those applications are due to the high electrical and thermal conductivity of the silver metal. Recently, Ag nanowires have also been used for chemical imaging, sensing [5], photonics [6], plasmon [7], surface-enhanced Raman scattering (SERS) [8] etc. Due to the high surface area of Ag nanowires, they are also promising materials for catalytic reactions and energy storage [9-11].
Over the past two decades, a variety of synthetic approaches have been developed for the synthesis of Ag nanowires. The major methods include:
1) reduction of Ag+ in an aqueous solution [12], which is called the "wet chemical synthesis". This method usually produces nanowires with a wide size distribution, and most of the time a "seed" is required for the nanowire growth
2) Electrochemical deposition in templates [13] as a "Vapor-liquid-solid" method [14]. This method generally produces a good morphology of the nanowires [15]
3) Microwave-assisted aerobic synthesis, which is a facile and rapid process to synthesize Ag nanowires [16]
4) "Successive multistep growth" method [4], which can be used to prepare long Ag nanowires by controlling the experimental condition to reduce AgNO3.
It is noteworthy that among these nanowires, the synthesis and applications of nanowire arrays is of special interest because of its unique structure. So far, the only method to prepare Ag a nanowire array is the templated method [17-19]. Porous materials, such as anodic aluminum oxide [20,21], provide a one-dimensional channel for Ag to grow within. Ag nanowires arrays can be used in fabricating unique devices, such as electrochemical sensor [18], SER substrate for biosensing [21,22] multicolor subwavelength imaging [20 ] etc.
In this short communication, we report our discovery that an array of Ag nanowires can be prepared from another templated approach on Cu(BTC) (1,3,5-benzenetricarboxylic acid) micropillars [22].
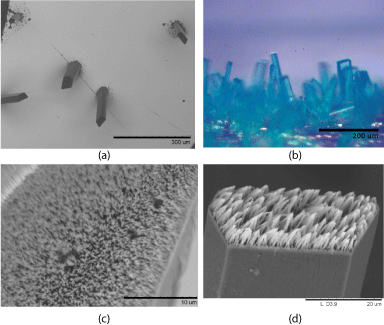
The Ag nanowire array was prepared using a two-part self-assembly process; first, the fabrication of Cu(BTC)∙3H2O metal organic framework (MOF) micropillar crystals on a Au surface and then photoreduction of Ag onto top facet of the MOF crystals. In typical experiments [23] 50 mg of BTC was partially dissolved in 10 mL of distilled water and stirred for 15 minutes at room temperature. The saturated solution was then filtered, and 8 mL of the filtrate was added to a clean 20 mL vial. To this, 8 mL of a 30 mM CuSO4•5H2O solution was mixed with the saturated/filtered BTC solution. A gold substrate 1 cm x 1cm was immersed into the solution for ~24 hours to product the Cu (BTC)∙3H2O MOF micropillars as cyan crystals oriented vertically from the surface. Density of the number of MOF crystals can be influenced by wet-etching (using aqua regia, HNO3: HCl, 1:3 v/v) or physical abrasion of the Ag surface, with the results published [23]. Scanning electron microscope, SEM, and digital images of Cu (BTC)∙3H2O MOF crystals can be seen in (Figure 1a, Figure 1b).
It has been previously demonstrated that Ag+ reduced onto HKUST-1 MOF crystals has a SERS effect [23]. We applied the same Ag reduction onto the previously studied Cu(BTC)∙3H2O nanopillar crystals. To deposit Ag onto the MOF micropillar crystals, a Au substrate containing Cu(BTC)∙3H2O crystals were immersed in a vial containing a 10 mL 0.1 M AgNO3 solution. The samples were irradiated in a UV reactor (Rayonet, sixteen 35 W 2537A° UV bulbs) operating at room temperature to promote to photoreduction of Ag. Silver wire nucleation can be achieved anywhere from 5 minutes to 2 hours, depending on desired Ag density. Substrates were then removed from the reaction vials and soaked in a distilled water bath for several minutes. Upon drying samples were analyzed using SEM to obtain surface morphologies.
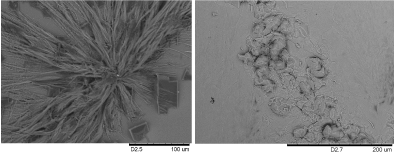
Ag nanowires typically collapse onto one another after drying (Figure 1d), most likely induced by capillary forces. This nano cohesion effect has been observed extensively by numerous authors [24-26]. Control experiments shows that Ag does not form an ordered nanowire array on silicon or glass surfaces under the same conditions, but random wires or irregular crystals (Figure 2).
In summary, we report that an array of silver nanowires can be scaffolded onto a Cu (BTC) MOF micropillar. The formation mechanism is under investigation. This Ag-deposited scaffolding material would be a cheap alternative to building an electronic or optoelectronic device for various applications, which is also under investigation in our group. Furthermore, we are investigating whether the Ag nanowire array can be prepared on a larger 2D MOF surface and the results will be publish in due course.
References
- Lazzara TD, Bourret GR, Lennox, et al. (2009) Polymer templated synthesis of AgCN and Ag Nanowires. Chem. Mater 21: 2020-2026.
- Valizadeh S, George J M, Leisner P, et al. (2002) Electrochemical synthesis of Ag/Co multilayered nanowires in porous polycarbonate membranes. Thin Solid Films 402: 262-271.
- Clayton DA, Benoist DM, Zhu Y, et al. (2010) Photoluminescence and spectro electrochemistry of single Ag nanowires. ACS Nano 4: 2363-2373.
- Lee J, Lee P, Lee H, et al. (2012) Very long Ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 4: 6408-6414.
- Chen J, Wiley BJ, Xia Y. et al. (2007) One-Dimensional nanostructures of metals: large-scale synthesis and some potential applications. Langmuir 23: 4120-4129.
- Gudiksen M S, Lauhon LJ, Wang J, et al. (2002) Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature 415: 617-620.
- Graff A, Wagner D, Ditlbacher H, et al. (2005) Silver nanowires. U Eur Phys. 34: 263-269.
- Peng M, Gao J, Zhang P, et al. (2011) Reductive self-assembling of Ag nanoparticles on germanium nanowires and their Application in ultrasensitive surface-enhanced raman spectroscopy. Chem Mater 23: 3296-3301.
- Zhou W, Thomas JM, Shephard DS, et al. (1998) Ordering of ruthenium cluster carbonyls in mesoporous silica. Science 280: 705-708.
- Kim SW, Son S U, Lee SI, et al. (2000) Cobalt on mesoporous Silica: The first heterogeneous pauson-khand catalyst. J. Am. Chem. Soc 122: 1550-1551.
- Hu LB, Choi J W, Yang Y, et al. (2009) Highly conductive paper for energy-storage devices. Proc Natl Acad Sci USA 106: 21490-21494.
- Carmona F, Barreau F, Delhaes P, et al. (1980) An experimental model for studying the effect of anisotropy on percolative conduction. J Phys Lett 41: 531-533.
- Sun Y, Yin Y, Mayers B T, et al. (2002) Uniform silver nanowires synthesis by reducing AgNO3 with ethylene glycol in the presence of seeds and poly (Vinyl Pyrrolidone). Chem Mater 14: 4736-4745.
- Ghoshal T, Biswas S, Kar S, et al. (2008) Synthesis of Ag/Si core/shell coaxial nanowire heterostructures by the vapor-liquid-solid technique. J Phys Chem C 112: 20138-20142.
- Law M, Greene LE, Radenovic A, et al. (2006) ZnO-Al2O3 and ZnO-TiO2 Core-Shell nanowire dye-sensitized solar cells. J Phys Chem B 110: 22652-22663.
- Gou L, Chipara M, Zaleski JM (2007) Convenient, rapid synthesis of Ag nanowires. Chem Mater 19: 1755-1760.
- Kurowska E, Brzózka A, Jarosz M, et al. (2013) Silver nanowire array sensor for sensitive and rapid detection of H2O2. Electrochimi Acta 104: 439-447.
- Ju W, Zhang X, Wu S (2005) Wet chemical synthesis of Ag nanowires array at room temperature. Chem Lett 34: 510-511.
- Huang JA, Zhao YQ, Zhang XJ, et al. (2013) Ordered Ag/Si nanowires array: wide-range surface-enhanced raman spectroscopy for reproducible biomolecule detection. Nano Lett 13: 5039-5045.
- Zhou Z K, Li M, Yang Z J, et al. (2010) Plasmon-mediated radiative energy transfer across a silver nanowire array via resonant transmission and subwavelength imaging. ACS Nano 28: 5003-5010.
- Xu W, Meng G, Huang Q, et al. (2013) Large-scale uniform Ag-NW tip array with enriched sub-10-nm gaps as SERS substrate for rapid determination of trace PCB77. Appl Surf Sci 271: 125-130.
- Kojtari A, Carroll P J, Ji H-F (2014) Metal organic framework (MOF) micro/nanopillars. Cryst Eng Comm 14: 2885-2888.
- Houk RJ, Jacobs B W, El Gabaly F, et al. (2009) Silver cluster formation, dynamics, and chemistry in metal-organic frameworks. Nano Lett 9: 3413-3418.
- Dawood M K, Zheng H, Kurniawan N A, et al. (2012) Modulation of surface wettability of superhydrophobic substrates using Si nanowire arrays and capillary-force-induced nano cohesion. Soft Matter 8: 3549-3557.
- Dawood M K, Zheng H, Liew T H, et al. (2011) Mimicking both petal and lotus effects on a single silicon substrate by tuning the wettability of nanostructured surfaces. Langmuir 27: 4126-4133.
- Duan H, Berggren KK (2010) Directed self-assembly at the 10 nm scale by using capillary force-induced nano cohesion. Nano Lett 10: 3710-3716.
Corresponding Author
Hai-Feng Ji, Department of Chemistry, Drexel University, Philadelphia, PA 19104, USA.
Copyright
© 2021 Qiao Z. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.