Are AKAP4 and proAKAP4 Found in Canine Semen? Preliminary Results
Contents
Canine sperm evaluation is routinely assessed by optic microscopy, spectrophotometer, computer assisted semen analysis and sometimes flow cytometry. These methods can be time-consuming and are not always available for canine reproduction professionals. Furthermore, it is possible that a dog shows bad fertility despite a promising sperm quality. This is why new tools are studied in order to predict the quality and the fertility of a semen. Proteomic research is actually trying to identify some protein markers related to the fertility in the semen. Among these spermatic proteins, it has been discovered the proAKAP4. It is the precursor of the AKAP4 which is part of the AKAPs (A-Kinase Anchoring Proteins). AKAPs are able to fix the regulatory sub-unit of the PKA (Protein Kinase A) and then confine it at a specific site of the cell. ProAKAP4 is synthetized at the round spermatid stage. It is incorporated in the fibrous sheath of the flagellum during its development. At the condensed spermatid stage, the proAKAP4 is cleaved and it releases the mature AKAP4. AKAP4 is related to good quality of the spermatogenesis, spermatozoa motility and also capacitation and fertility. The aim of this study is to identify this protein in the dog testicle and ejaculate. 4 testicles from 2 dogs and 2 ejaculates from 2 dogs were used. ProAKAP4 and AKAP4 have been identified only in the fibrous sheath of the spermatozoa extracted from the testicles and in the spermatic fraction of the ejaculate, nor in uretral or prostatic fractions.
Introduction
Canine sperm evaluation is assessed by analyzing spermatic motility, spermatic concentration and spermatozoa morphology using optic microscopy. Computer assisted analysis is also used: It permits to standardize motility results, to measure some parameters unmeasurable with optic microscopy and also to avoid the subjectivity due to the human operator. Flow cytometry brings some new information about spermatozoa morphology and sperm motility. Nevertheless, these techniques cannot precisely predict the fertility of a semen.
The spermatozoa is a mobile cell thanks its flagellar tridimensional and asymmetrical movements. The movement is initiated by the sliding of axonema microtubules one above the other. The attachable-detachable cycle of the dynein, arms on the microtubules are the fuel for the sliding. The sliding is first longitudinal and then transformed in a curved movement. Once the flagellum is curved, it creates a wave, which grows in intensity by getting closed to the end piece of the spermatozoa. At halfway flagellum, another wave is created on the opposite side. The first wave then switch in a second plane: A 180° rotation movement is then given to the flagellum. After the rotation, a new wave starts when the two previous ones ends their progression in a different plane. As a result, the flagellum has a tridimensional movement, and the head of the spermatozoa has a pseudo sinusoidal oscillation movement [1].
The flagellar movement begins in the epididymis: It is regulated by the AMPc (Adenosine Monophosphate Cyclic) concentration [1]. The ATP (Adenosine Triphosphate) is the energy carrier of the motility. It is produced by oxidative phosphorylation in mitochondrions and by a glytolytic alternative way. This alternative way is especially involved during capacitation hypermotility [2]. ATP is used by ATPase dyneins but also by adenylate cyclase for the production of AMPc [1].
Peri-axonemal structures, such a fibrous sheath, are in charge of regulating the curvature degree of the flagellum and promoting the rotation of the spermatozoa [1]. These structures are also in charge of integrating signalizing molecules in charge of the regulation of spermatic motility [3].
The interaction and the coordination between cytoskeleton proteins, phosphatases, kinases and phosphodiesterases creates a motility signalization way. Their coordination and activation is linked to AKAP4 (A-Kinase Anchoring Protein 4) which is in charge of a localized production of AMPc [4,5].
AKAP4 (A-Kinase Anchoring Protein 4) protein is involved in expression and regulation of the spermatozoa motility. It is an essential actor of the transduction signal of the PKA and yet promotes the sliding of the doblets of microtubules one above the other [6,7].
Nevertheless, capacitation occurs in the female genital tract: it gives to the spermatozoa the ability to fertilize. This event depends on an intracellular signalization mechanism including a calcium influx and phosphorylation of tyrosine residues on flagellar proteins. Theses phosphorylated proteins are involved in a signalization cascade including AMPc, PKA, tyrosine kinase and phosphatase [8,9]. ProAKAP4 (Pro A-Kinase Anchoring Protein 4) and AKAP4 are actively phosphorylated on their tyrosine and serin residues during capacitation. They seem to be a marker of the capacitation status [4,10,11].
AKAP4 is part of the AKAP. It is localized in the fibrous sheath of the spermatozoa. Its precursor is proAKAP4. The gene encoding for this protein in localized on the X chromosome [12]. ProAKAP4 is a 97 kDa protein synthetized during round spermatid stage, it is incorporated in the fibrous sheath during its development. At the condensed spermatid stage, proAKAP4 is cleaved and releases the mature AKAP4 [8,9]. AKAP4 represents 50% of the fibrous sheath proteins in the mouse [13]. AKAP4 is found on the entire principal piece of the flagellum, in the longitudinal columns and in transverse ribs [14]. It is also described in the fibrous sheath in spermatozoa from mouse [15], bull [16], man [12], stallion [17] and boar [18].
Knock out male mouse for AKAP4 (which are not synthetized AKAP4 in their sperm) produce a normal number of spermatozoa. Nevertherless, the absence of AKAP4 causes a flagellar disorganization: the fibrous sheath is not developed, the principal piece and the flagellum are shorter. These spermatozoa are immobile: So they are infertile. The altered structure of the fibrous sheath is no longer available to organize the spermatic motility signals [19].
It has been shown that the absence of expression of weak expression of AKAP4 and proAKAP4 is associated with poor spermatic motility and with DFS (dysplasia of the fibrous sheath) in the man [20,21].
In a study about men consulting for infertility, proAKAP4 and AKAP4 concentration were positively correlated with spermatic motility [22,23].
Sergeant, et al. [18] have shown that proAKAP4 concentration is correlated to progressive motility in normozoosperm human patients (unpublished data). In the stallion, proAKAP4 concentration in post-thawed semen was correlated to total and progressive motility [24].
The aim of this study is to identify proAKAP4 in dog testicle and analyze its expression in the ejaculate of this specie.
Material and Method
Animals
4 testicles from 2 dogs (beagle, chihuahua) aged from 6 months to 5-years old have been removed under general anesthesia at the Veterinary University Centre ONIRIS National Veterinary School Nantes. Testicles were descended into the scrotal sack. Once the spaying is done, testicles were frozen at -20 ℃ until the achievement of electronic microscopy at Inserm UMRS1172 (1 place de Verdun, Centre JPARC, 59045 Lille).
2 ejaculates from 2 Beagle dogs aged of 1 year and 2 months from the ONIRIS National Veterinary School kennel and 1 ejaculate from a boar (control) were collected. Ejaculates from dogs were collected by splitting the 3 parts of the ejaculate (urethral, spermatic, and prostatic part). Then, 100μL of each spermatic part was frozen at 20 ℃. Urethral and prostatic parts were melted and then frozen at - 20 ℃. Those samples were kept frozen until the achievement of the Western Blot at 4biodx, SPQI SAS in Inserm UMRS1172 (1 place de Verdun, Centre JPARC, 59045 Lille).
All animal handling meets the requirements of the Oniris ethics committee.
Methods
Electron microscopy
4 testicles were thawed at room temperature. Tissues were fixed in a 0.1 M phosphate buffer (pH = 7.4) with 2% paraformaldehyde and 0.05% glutaraldehyde during 1h at 4 ℃. They were then dehydrated in increasing concentration ethanol bathes and anchored in white resin (EMS, United States). Testicles micro-sections were then realized. Grids were incubated in TBS buffer with 1% goat antiserum and 1% bovine serum albumin during 30 min at room temperature. They were then incubated with monoclonal antibody anti-proAKAP4 and anti-AKAP4 clone 6F12 or 7E10 (4BioDx, 4BDX-1701 et 4BDX-1602, France) diluted at 1/50. Grid were washed three times in TBS buffer and incubated with colloidal gold 12 nm conjugated to an anti-mouse IgG (H (Heavy) + L (Light)) goat antibody diluted at 1/50 (Jackson Immunoresearch Laboratories, United States). Control grids were only incubated with colloidal gold conjugated to the antibody. After 3 washes in TBS, all grids were fixed with 2% uranyl acetate. Grids were observed with a Zeiss 900 transmission electron microscope.
Western Blot
Sperm samples were thawed at room temperature. 25 μL of each sample has been melted with 50 μL of LDS 2X buffer (LDS NuPAGE® Buffer, Life technologies, USA). Proteins from each sample have been separated by electrophoresis with a CRITERION gel and transferred on a 0.45 µm nitrocellulose membrane (G&E Healthcare, USA). Membranes were stained with a reversible red Ponceau in order to verify the quality and the homogeneity of the transfer. Membranes were then incubated with primary antibodies anti-AKAP4 clone 7E10 (4BioDx, 4BDX - 1602S, France) at 1 μg/mL and anti-proAKAP4 clone 6F12 (4BioDx, 4BDX-1701, France) at 1 μg/mL all night at 4 ℃. After three washes in TNT (Tris, NaCl, Tween 20) during 10 minutes, membranes were incubated with the detection antibody HRP Horse Anti-Mouse Antibody Peroxidase (Catalog number: PI-2000, 0.02 µg/mL, Vector Laboratories, Burlingame, CA USA). Immune complexes were revealed with ECL kit (ECL Western Blotting Detection Reagents, G&E Healthcare, USA) and LAS kit (LAS Amersham Imager 600, G&E Healthcare, USA). After a last wash in TNT, membranes were dryed and preserved.
Results
Electron microscopy
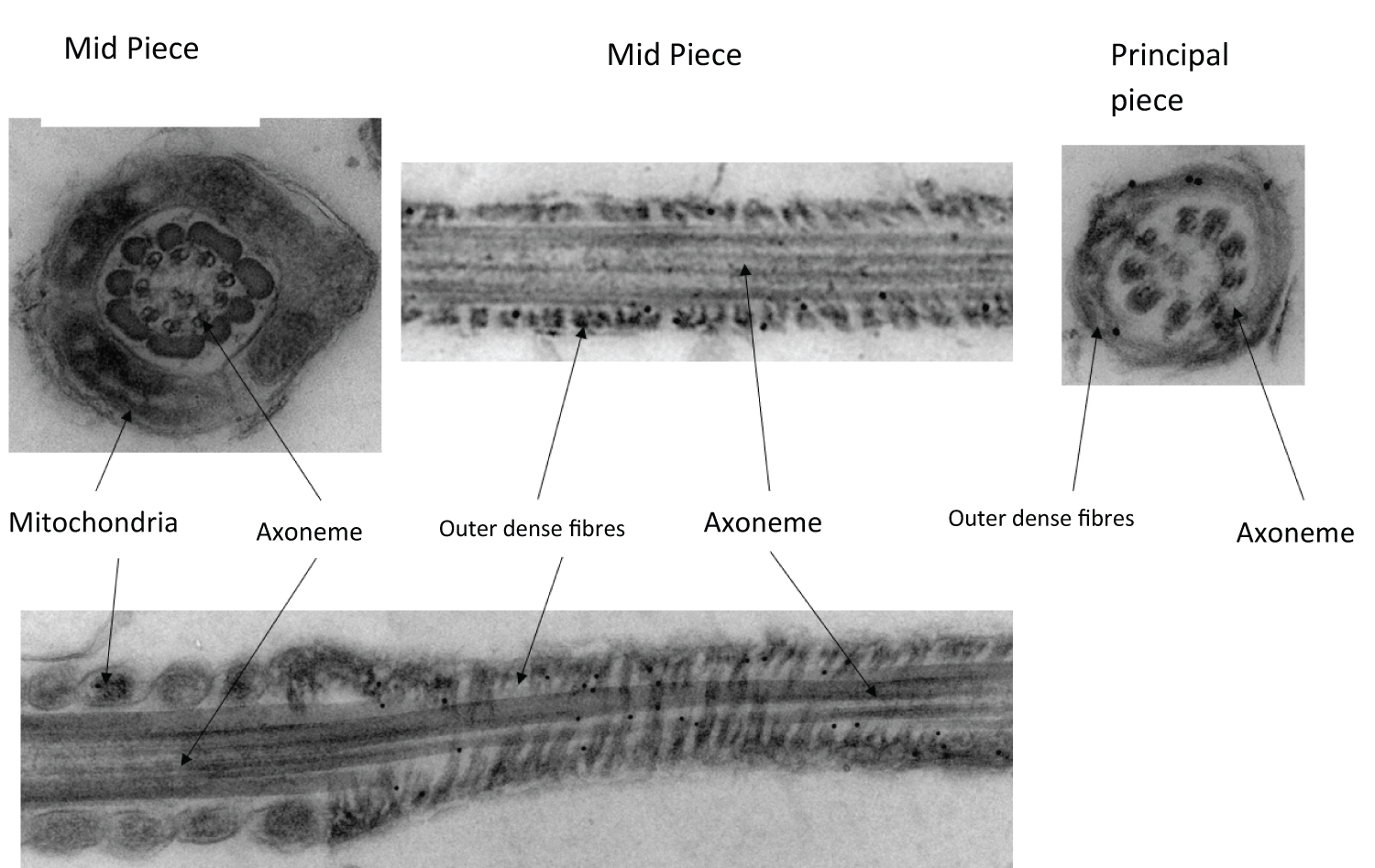
Two antibodies anti-proAKAP4 (clone 6F12) and anti-AKAP4 (clone 7E10) have been used to localize the proAKAP4 precursor and the mature AKAP4 in spermatozoids. AKAP4 and proAKAP4 are principal constituents of the spermatozoid flagellum. They are only expressed in the fibrous sheath of the flagellum principal piece and more specially in transverse ribs and longitudinal columns. Head and midpiece don't contain proAKAP4 or AKAP4 (Figure 1).
Western blot
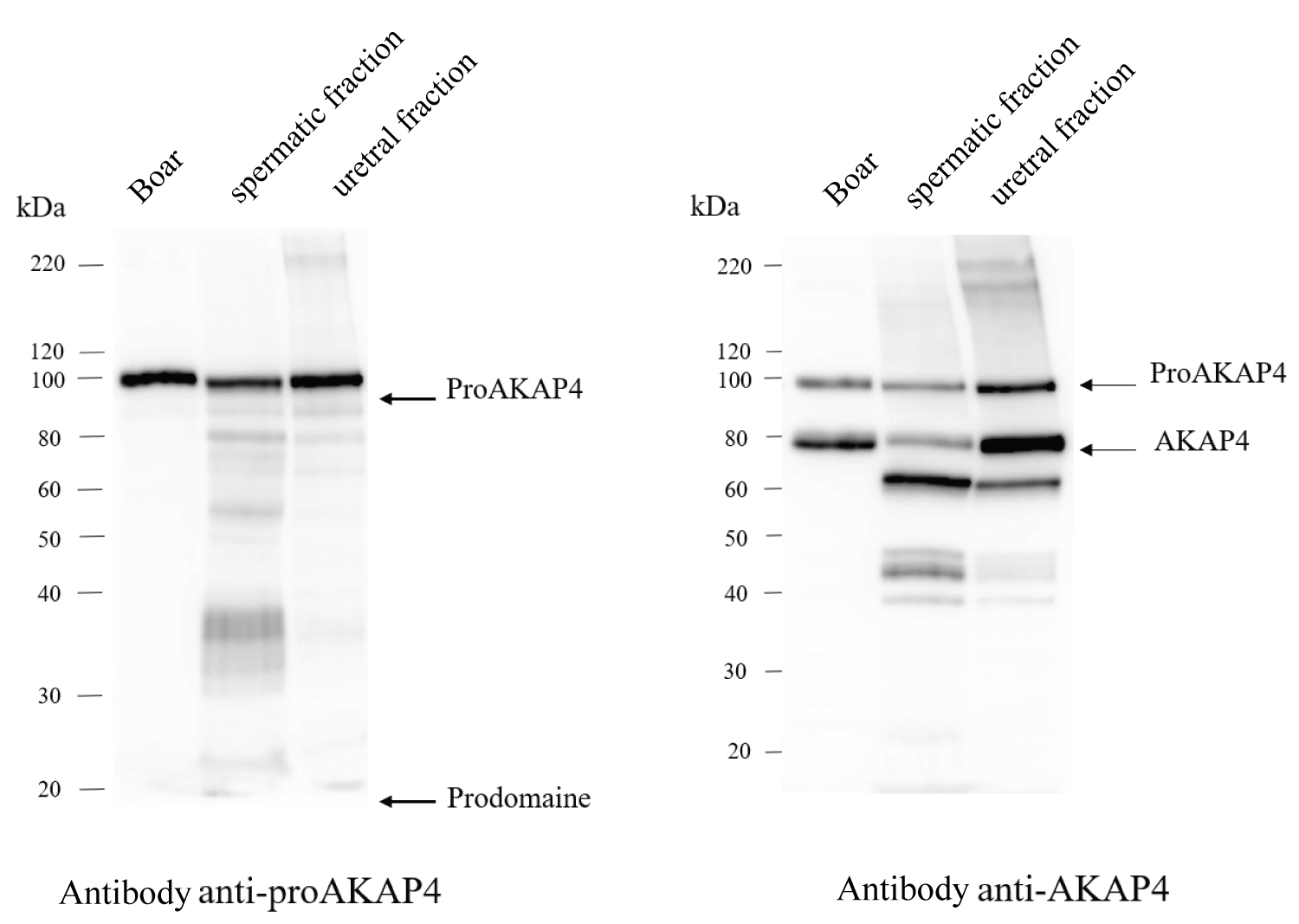
Immunolabelling of proAKAP4 with anti-proAKAP4 antibody (clone 6F12) revealed a 100 kDa protein in dog spermatic fraction and in boar ejaculate which served as a control. A second 20 kDa protein has been detected in those fractions: it corresponds to the prodomain of the proAKAP4. No immunolabelling was found in the mix of the urethral and prostatic parts. ProAKAP4 is only expressed in the spermatic part of the dog ejaculate (Figure 2).
Immunolabelling of AKAP4 with anti-AKAP4 antibody (clone 7E10) revealed 2 proteins: One at 100 kDa (corresponding to the precursor proAKAP4) and one at 82 kDa (corresponding to mature AKAP4 without the 182 amino acid prodomain). Those 2 proteins have been found in the dog spermatic fraction and in the boar ejaculate but not in the mix of the urethral and prostatic parts (Figure 2).
Discussion
ProAKAP4 and AKAP4 expression have never been studied in dog spermatozoid. This study is the first to highlight the presence of both proteins in spermatozoids and more specially in the fibrous sheath such as in mouse [15], bull [16], man [12], stallion [17] and boar [18]. They have not been found in another part of the spermatozoid.
Dog ejaculate can be separated in 3 fractions (urethral, spermatic and prostatic). The two proteins have been found in the spermatic part but not in the urethral and prostatic part. This is a consistent finding because these 2 fractions are not supposed to contain spermatozoid (or a few). Furthermore, we have just showed that the two proteins are expressed in the spermatozoid cells from testicles. Nevertheless, proAKAP4 and AKAP4 can originated from intact spermatozoid or from damaged spermatozoid which releases proteins in the solution. In fact, in the Western Blot experience, spermatozoids have been first exposed to a lysing solution in order to extract their proteins. In the bull, flow cytometry experiences have shown a positive correlation between proAKAP4 concentration and the percentage of spermatozoids expressing the protein [25]. It could be interesting to know if the same thing can be highlighted in the dog.
The sampling (4 testicles and 2 ejaculates) was weak but sufficient to show the presence of both AKAP4 and proAKAP4 in the fibrous sheath of the spermatozoid flagellum and in the spermatic part of the ejaculate.
Within the complex intracellular signal mechanisms of the spermatic motility, AKAP4 is an essential protein by interacting with AMPc and PKA [4,5]. AKAP4 is an essential actor of transduction signals governing the sliding of the microtubules doblets of the axonema which are the source of the spermatozoid motility [5,7]. ProAKAP4 is a functional reserve of spermatic motility: It reflects the number of viable spermatozoids that can reach the oocyte and fertilize it.
In the stallion, proAKAP4 concentration is correlated to total and progressive motility in post-thawed semen [24]. In the bull, proAKAP4 concentration is correlated to progressive motility in fresh semen [25]. In the human, proAKAP4 and AKAP4 amounts are correlated with spermatic motility [22,23]. Yet, progressive motility is correlated to the success rate of fertilization particularly in in vitro fertilization [26,27]. It could be interesting to check if a similar correlation exists in the dog given that progressive motility is routinely assessed to judge the quality of the semen in this specie.
In the boar, different levels of proAKAP4 concentration have been found from a animal to another. ProAKAP4 concentration is impacted by the age: proAKAP4 concentration is lower in reform boars with a significant decreasing spermatic motility [25]. ProAKAP4 concentration in the semen of a boar is a tool to monitor its breeding career.
Furthermore, proAKAP4 is associated with fertility quality of the semen. In fact, in the bull, we can notice higher fertility with semen containing high quantities of proAKAP4 [28]. In the man, proAKAP4 concentration is lower in non-fertile patients despite a normal spermogram [29]. In the boar, fertility index is also correlated with proAKAP4 spermatic concentration [18]. This link has not been investigated yet in the dog: it could be interesting to know if proAKAP4 is correlated to fertility in this specie in order to increase the chance of success in natural matings and artificial inseminations. It is essential for breeders to optimize the chance of success in artificial insemination particularly when partners are distant from the other or when high genetic values are at stake.
Finally, proAKAP4 is also associated with prolificity, particularly in the boar. In fact, proAKAP4 concentration is positively correlated to litter size: In a study, from a 45 ng/10.106 spermatozoid proAKAP4 concentration threshold, 2.05 piglets in addition are obtained in average (Sergeant, et al., 2019). Once again, it could be interesting to know if a similar correlation exists in the dog with a simultaneous monitor follicular development until the ovulation. In fact, it is more interesting for a dog breeder to have big litter size.
Conclusion
Proteomic research is actually giving us the opportunity to know more and more about proteins and their role in specialized cells such as the spermatozoid. Among spermatic proteins, proAKAP4 is the precursor of the AKAP4 which is a protein involved in the motility of this cell by permitting a cascade transduction signals. Coordination and activation of protein in charge of motility is linked to AKAP4 which permits a local production of AMPc. Spermatic motility is a sine qua none condition for the function of this cell: Fertilize the oocyte once in the genital female tract. This study is the first to show the localization of proAKAP4 and AKAP4 in the fibrous sheath of the dog spermatozoid such as the man, the mouse, the bull and the boar. This study also showed their expression in the spermatic part of the dog ejaculate. They are potential markers of the quality and the fertility of the semen. Once it is investigated in further studies, routine canine sperm evaluation can be supplemented with proAKAP4 sperm concentration.
Acknowledgements
The authors thank the team of INSERM, UMRS 1172, Lille France for performing electron microscopy.
Conflict of Interest Statement
None of the authors has any conflict of interest to declare.
Data Available on Request from the Authors
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Jouannet P, Serres C (1995) Mouvement normal et pathologique du spermatozoïdehumain. Médecine/sciences 11: 555-562.
- Fraser LR, Quinn PJ (1981) A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J Reprod Fertil 61: 25-35.
- Luconi M, Cantini G, Baldi E, et al. (2011) Role of a-kinase anchoring proteins (AKAPs) in reproduction. Front Biosci (Landmark Ed 16: 1315-1330.
- Ficarro S, Chertihin O, Westbrook VA, et al. (2003) Phosphoproteome analysis of capacitated human sperm. J Biol Chem 278: 11579-11589.
- Huang Z, Somanath PR, Chakrabarti R, et al. (2005) Changes in intracellular distribution and activity of protein phosphatase PP1?2 and its regulating proteins in spermatozoa lacking AKAP41. Biol Reprod 72: 384-392.
- Vijayaraghavan S, Goueli SA, Davey MP, et al. (1997) Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem 272: 4747-4752.
- Nipper RW, Chennothukuzhi V, Tutuncu L, et al. (2005) Differential RNA expression and polyribosome loading of alternative transcripts of the akap4 gene in murine spermatids. Mol Reprod Dev 70: 397-405.
- Marquez B, Suarez SS (2004) Different signalling pathways in bovine sperm regulate capacitation and hyperactivation. Biol Reprod 70: 1626-1633.
- Baldi E, Casano R, Falsetti C, et al. (1991) Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl 12: 323-330.
- JaganMohanarao G, Atreja SK (2011) Identification of capacitation associated tyrosine phosphoproteins in buffalo (Bubalus bubalis) and cattle spermatozoa. Anim Reprod Sci 123: 40-47.
- Turner RM, Eriksson RL, Gerton GL, et al. (1999) Relationship between sperm motility and the processing and tyrosine phosphorylation of two human sperm fibrous sheath proteins, pro-hAKAP82 and hAKAP82. Mol Hum Reprod 5: 816-824.
- Turner RMO, Johnson LR, Haig-ladewig L, et al. (1998) An X-linked gene encodes a major human sperm fibrous sheath protein, hAKAP82. J Biol Chem 273: 32135-32141.
- Eddy EM, Toshimori K, Brien DAO (2003) Fibrous sheath of mammalian spermatozoa. Microsc Res Tech 61: 103-115.
- Johnson LR, Foster JA, Haig-Ladewig L, et al. (1997) Assembly of AKAP82, a protein kinase aanchor protein, into the fibrous sheath of mouse sperm. Dev Biol 192: 340-350.
- Carrera A, Gerton GL, Moss SB (1994) The major fibrous sheath polypeptide of mouse sperm: Structural and functional similarities to the a-kinase anchoring proteins. Dev Biol 165: 272-284.
- Moss SB, Turner RMO, Burkert KL, et al. (1999) Conservation and function of a bovine sperm a-kinase anchor protein homologous to mouse AKAP82. Biol Reprod 61: 335-342.
- Blommaert D, Sergeant N, Delehedde M, et al. (2019) Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (proAKAP4) in equine semen: Promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 131: 52-60.
- Sergeant N, Briand-Amirat L, Bencharif D, et al. (2019) The sperm specific protein Proakap4 as an innovative marker to evaluate sperm quality and fertility. J Dairy Vet Sci 11: 1-7.
- Miki K, Willis WD, Brown PR, et al. (2002) Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol 248: 331-342.
- Moretti E, Scapigliati G, Pascarelli NA, et al. (2007) Localization of AKAP4 and tubulin proteins in sperm with reduced motility. Asian J Androl 9: 641-649.
- Baccetti B, Collodel G, Estenoz M, et al. (2005) Gene deletions in an infertile man with sperm fibrous sheath dysplasia. Hum Reprod 20: 2790-2794.
- Jumeau F, Sigala J, Fernandez-Gomez F-J, et al. (2018) Gel electrophoresis of human sperm: A simple method for evaluating sperm protein quality. Basic Clin Androl 28: 10.
- Jumeau F, Sigala J, Dossou-Gbete F, et al. (2018) A-kinase anchor protein 4 precursor (pro-AKAP4) in human spermatozoa. Andrology 6: 854-859.
- Bloomaert D, Sergeant N, Delehedde M, et al. (2018) Significant correlation between the proAKAP4 concentration and the total and progressive motility in stallion sperm after thawing. J Equine Vet Sci 66: 43.
- Delehedde M, Bloomaert D, Jouy N, et al. (2018) Concentration of proAKAP4 as a pertinent read-out of sperm quality in mammals. Anim Reprod Sci 194: 1-27.
- Araújo LFP, Filho EA, Fácio CL, et al. (2013) Efficacy of sperm motility after processing and incubation to predict pregnancy after intrauterine insemination in normospermic individuals. Reprod Biol Endocrinol 11: 101.
- Guan H-T, Zheng Y, Wang J-J, et al. (2016) Relationship between donor sperm parameters and pregnancy outcome after intrauterine insemination: Analysis of 2821 cycles in 1355 couples. Andrologia 48: 29-36.
- Peddinti D, Nanduri B, Kaya A, et al. (2008) Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst Biol 2: 19.
- Frapsauce C, Pionneau C, Bouley J, et al. (2014) Proteomic identification of target proteins in normal but nonfertilizing sperm. Fertil Steril 102: 372-380.
Corresponding Author
Dr Djemil Bencharif, Laboratory of Biotechnology and Pathology of Reproduction, ONIRIS: The National Veterinary, Food Agriculture, and Food Hygiene School of Loire Atlantique, BP 40706, 44307 Nantes, France Tel: +33-2-40687709, Fax: +33-2-40687748, E-mail: djemil.bencharif@oniris-nantes.fr
Copyright
© 2021 Couazer DL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.