Polymorphism, Crystallinity and Hydrophilic-Lipophilic Balance (HLB) of Cetearyl Alcohol and Cetyl Alcohol as Raw Materials for Solid Lipid Nanoparticles (SLN)
Abstract
The use of Solid Lipid Nanoparticles (SLN) has become a very popular approach in the development of innovative pharmaceutical drug delivery systems. The first step is the assessment the lipids' suitability. This paper is focused on the physicochemical characterization of lipids, namely cetyl alcohol and cetearyl alcohol, for the production of SLN. The bulk lipids were analyzed by Differential Scanning Calorimetry (DSC) and Wide Angle X-ray Diffraction (WAXD) without any treatment, after tempering the raw materials (bulk lipid) for 1 hour at 80 ℃, and after their spray-drying process. Hydrophilic-Lipophilic Balance (HLB) values were determined using a combination of surfactants (polysorbate 20 and trioleate sorbitan) for the production of the hot o/w emulsion. Results of DSC and WAXD showed that after the thermal stress applied to the bulk lipids, both the melting point and the intensity of the refractogram peaks have decreased. Cetyl alcohol and cetearyl alcohol crystallized in more unstable polymorphic forms, which anticipate the suitability of these lipids for the production of SLN. The best HLB values obtained for the produced emulsions were 15.5, 16.0, and 16.7, combining accepted surfactants. The results showed that both cetearyl alcohol and cetyl alcohol are adequate lipids for the development of stable SLN.
Keywords
Cetearyl alcohol, Cetyl alcohol, Lipid polymorphism, Hydrophilic lipophilic balance, Solid lipid nanoparticles
Introduction
Solid Lipid Nanoparticles (SLN) are receiving great attention as pharmaceutical and cosmetic delivery systems. This fact is due to the production facilities and scale-up, regulatory acceptance of the raw materials [1-3], and marketing potential [4]. SLN may increase the bioavailability of drugs, reduce the risk of toxic effects, and promote sustained release [2,3,5].
The use of SLN as carriers for drugs for topical administration has been highlighted due to advantages over chemical degradation protection of the formulation of the active, hydration (occlusion of the stratum corneum) and enhanced penetration of drugs in the different layers, and promote the sustained release with reduced systemic absorption and possess properties of photoprotection against UV light [6-10].
When applied onto the skin, SLN adhere to the skin' surface, therefore are expected to prolong the contact time of the drug with the skin and enhance drug's permeation [11]. SLN can be produced from lipids as triglycerides and their mixtures, fatty acids, and waxes [12-14].
Polymorphism is a phenomenon that can be demonstrated in lipids after the fusion process, recrystallization or in the production process [15]. It can lead to changes in pharmaceuticals compromising the drug stability, effectiveness and bioavailability [16]. The polymorphic changes can result in the impairment of the effect in vivo, defined as the presence of the same substance in different crystalline forms. Polymorphic characteristics influence the physicochemical properties. Under defined conditions of temperature and pressure, only one of the polymorphic is stable, other forms called metastable [17].
Polymorphism and crystallinity of the lipid raw-materials influence the drug's encapsulation efficiency in SLN, as well as the drug retention in the lipid matrix during storage time [18]. Indeed, the drug may be expelled from the particle upon polymorphic transition from the form α (less stable) to the β form (more stable), an event that can easily take place during shelf-life of drug-loaded SLN [19]. When developing SLN, it is desirable to obtain particles with a less perfect matrix, to be able to load the drug molecules between lipid chains and imperfections of crystals [16,20]. However, the long-term stability of these less perfect matrices may be compromised if recrystallization occurs unpredictably. Depending on the type of lipids and production method, super-cooled melts, which are similar to o/w emulsions, may be obtained when the lipids remain in the liquid state dispersed in the aqueous phase, creating heterogeneous systems. These systems result from delayed recrystallization of the lipid [21].
The aim of this study was to evaluate the polymorphism and crystallinity of cetyl and cetearyl alcohol after melting the raw materials by tempering for 1 h at 80 ℃, as well as their analysis after spray-drying process. The Hydrophilic-Lipophilic Balance (HLB) value for lipids was determined to reach the best combination of surfactants (trioleate sorbitan and polysorbate 20) to obtain a stable emulsion during SLN production.
Materials and Methods
Materials
Polysorbate 20 (Tween® 20) and ethanol were purchased from Synth (Diadema/SP, Brazil). Trioleate sorbitan (Span® 85), cetearyl alcohol (Crodacol® CS90) and cetyl alcohol (Crodacol® C90) were donated by Croda (Campinas/SP, Brazil). Double distilled water was used after filtration in a Millipore system (home supplied).
Methods
Thermal treatment of lipid materials
Lipids were subject to melting. The melting consisted of heating the sample up to 80 ℃ in a thermostatic water bath (Marconi, model MA 127/BD, Piracicaba/Brazil), following tempering by incubating the sample in the same bath for 1 h at 80 ℃. Samples were then kept at room temperature until complete cooling and solidification, to check for the creation of polymorphic forms [18]. Then, the lipids were analyzed by Differential Scanning Calorimetry (DSC) and Wide Angle X-ray Diffraction (WAXD).
Spray-drying process
Lipids were solubilized in pure ethanol (1% m/v) at 30 ℃ under magnetic stirring (Tecnal-80 TE, Piracicaba/Brazil). A spray-dryer LM MSD 1.0 (Ribeirão Preto/Brazil) was used applying the following parameters: Feed rate of 14.1 mL/min, air flow 15 l/min, nozzle atomizer 1 mm air inlet temperature of 90 ± 5 ℃; and temperature air outlet 50 ± 5 ℃ [22]. After the process, the samples were analyzed by DSC and WAXS.
Differential Scanning Calorimetry (DSC)
Thermal behavior of lipid matrices was assessed by DSC (Shimadzu, DSC-60/60, Japan). A volume of sample containing approximately 5-10 mg of lipid mass was weighted in an aluminum pan and sealed hermetically, under inert atmosphere (N2), 45 ml/min. The analysis was performed at a heating and cooling rate of 5 K/min, using an empty pan as reference. The samples were heated from 10 ℃ up to 100 ℃, following cooling down to 10 ℃. Through the product software, the initial melting and crystallization temperatures (onset temperatures), the temperatures of endothermic and exothermic peaks, the final melting temperatures and crystallization temperatures (end set temperatures) and phase transition enthalpies (ΔH J.g-1) have been obtained [18].
Wide Angle X-ray diffraction (WAXD)
To study the polymorphism and crystalline properties of the lipids, WAXD was carried out in a diffractometer X-ray (Philips, model Xˇıpert, Pennsylvania, USA), using copper anode. WAXD measurements were taken from 5º to 40º in 0.015º steps (1 s per step). The interlayer spacing's were calculated from the reflections using the Bragg's equation:
where λ (λ = 0.154056 nm) is the wavelength of the incident X-ray beam and θ is the scattering angle. The parameter d, otherwise called the interlayer spacing, is the separation between a particular set of planes of the crystal lattice structure. Data of the scattered radiation were recorded with a blend local-sensible detector using an anode voltage of 40 kV, a current of 25 mA and a scan rate of 0.5° per min. The samples were mounted on a thin glass capillary being fastened to a brass pin without any previous sample treatment [18].
Assessment of Hydrophilic-Lipophilic Balance (HLB)
For the assessment of the Hydrophilic-Lipophilic Balance (HLB), a series of 5 emulsions employing a combination of surfactant and co-surfactant with known HLB, to originate distinct HLB values [23] (Table 1). The aqueous phase consisted of water (95%, v/v) and surfactant (5% w/v). Trioleate sorbitan oleate (HLB = 1.8) and polysorbate 20 (HLB = 16.7) were used as surfactants. The oil phase consisted of cetearyl alcohol or cetyl alcohol (10% m/v). Then, the aqueous phase and oil phase were heated separately up to 80 ℃ using a thermostatic water bath (Marconi, model MA 127/BD, Piracicaba/Brazil). Aqueous phase was added to the oil phase under stirring (Ultra-Turrax® T25, USA), 10,000 rpm for 15 min. Then, the emulsions were cooled down to room temperature during 15 min under mechanical stirring (Tecnal TE 039/1, Piracicaba/Brazil). The emulsions were stored for 24 h at room temperature for further visual and optical microscopic analyses (Reichert-Jung, Series 150, Austria). The physical stability of the emulsions was evaluated by visual examination by phase separation or creamming.
Results
Cetyl and cetearyl alcohol were characterized before and after tempering and spray-drying treatment. Tempering was performed to mimic the production process carried out for SLN production using heat process [18]. Then, the spray dried was used to study the effect on their polymorphic and crystallization properties of lipid [24,25]. The characterization was done by DSC and WAXD [18,26-29]. DSC and WAXD were applied to the study of lipid matrices because of their complementarity data provision.
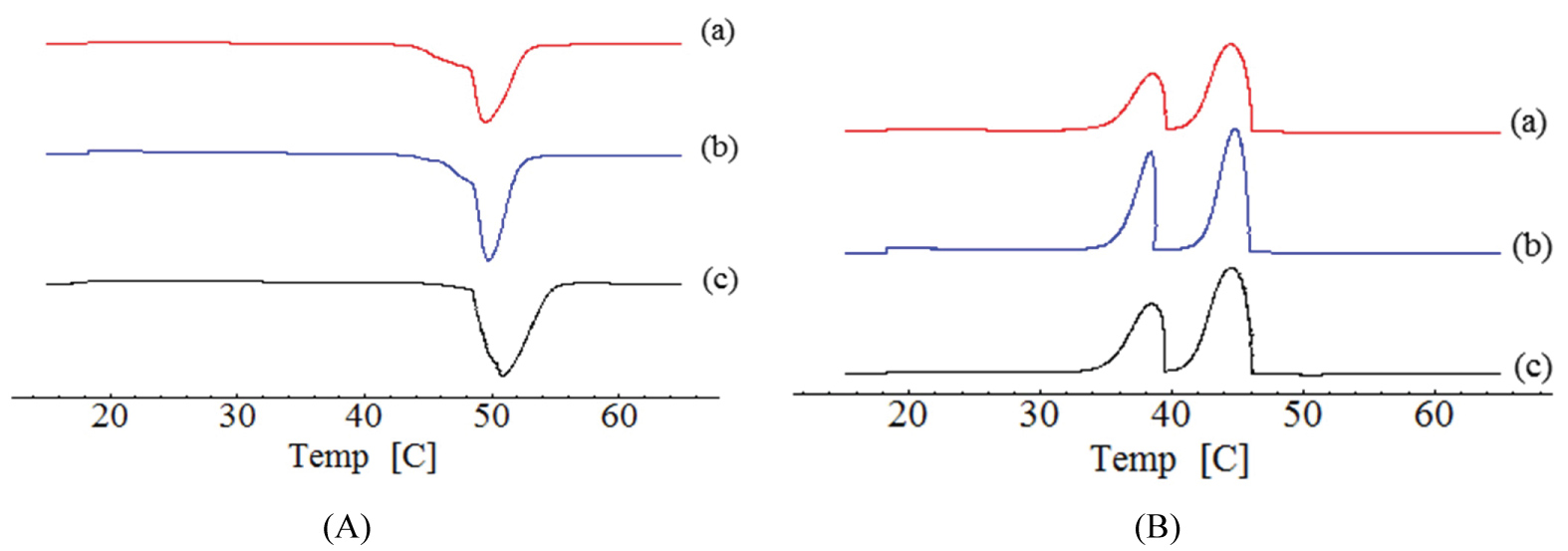
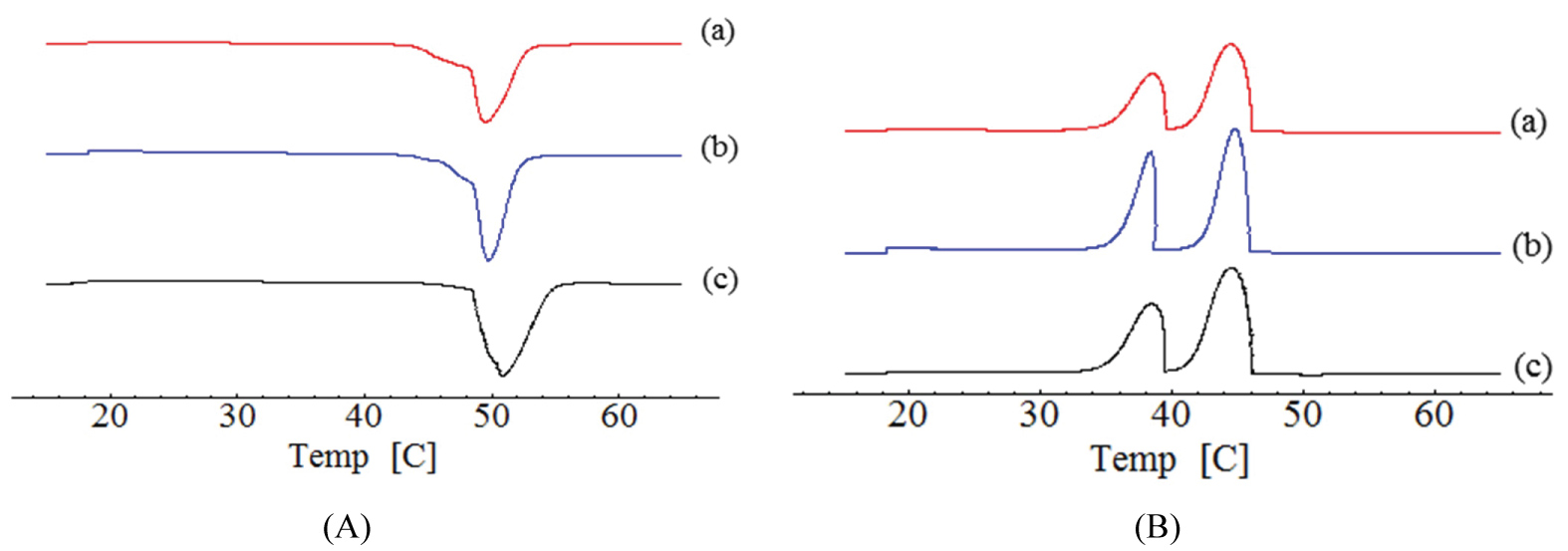
Figure 1 and Figure 2 show the thermograms of cetyl alcohol and cetearyl alcohol lipids after and before treatment. The melting temperature of cetyl alcohol untreatment was recorded at 50.81 ℃, the onset and endset temperatures were 45.79 ℃ and 56.44 ℃, respectively, and the heat capacity -255.41 J.g-1. Similar results have been reported elsewhere [30,31]. During the cooling process, 2 peaks were present with melting temperature at 38.49 ℃ and 44.51 ℃, respectively. The presence of two peaks during cooling demonstrated the presence of two polymorphs of the lipid.
Cetearyl alcohol untreatment showed 2 melting peaks, the first started at 34.54 ℃ and finished at 48.58 ℃, the melting point was 43.76 ℃, and the heat capacity was -54.84 J.g-1. The second peak started at 49.72 ℃ and finished at 58.24 ℃, the melting point was 53.89 ℃ and the heat capacity was -115.83 J.g-1. The cooling peak occurred in the crystallization temperature 47.95 ℃ and 26.96 ℃. After the tempering, cetyl alcohol showed a melting point at 49.49 ℃, with the onset at 41.42 ℃ and the endset at 49.49 ℃. The heat flow was -204.42 J.g-1. In the cooling curve two peaks were recorded at 44.45 ℃ and 38.34 ℃, respectively. The first peak started at 29.99 ℃ and finished at 42.71 ℃ with melting point in 37.71 ℃ and heat flow in -56.25 J.g-1. The second peak started at 45.40 ℃ and finished at 56.74 ℃. The melting point was 52.98 ℃ and the flow rate was -118.04 J.g-1. The 2 cooling peaks occurred at the crystallization temperatures of 48.08 ℃ and 26.16 ºC, respectively.
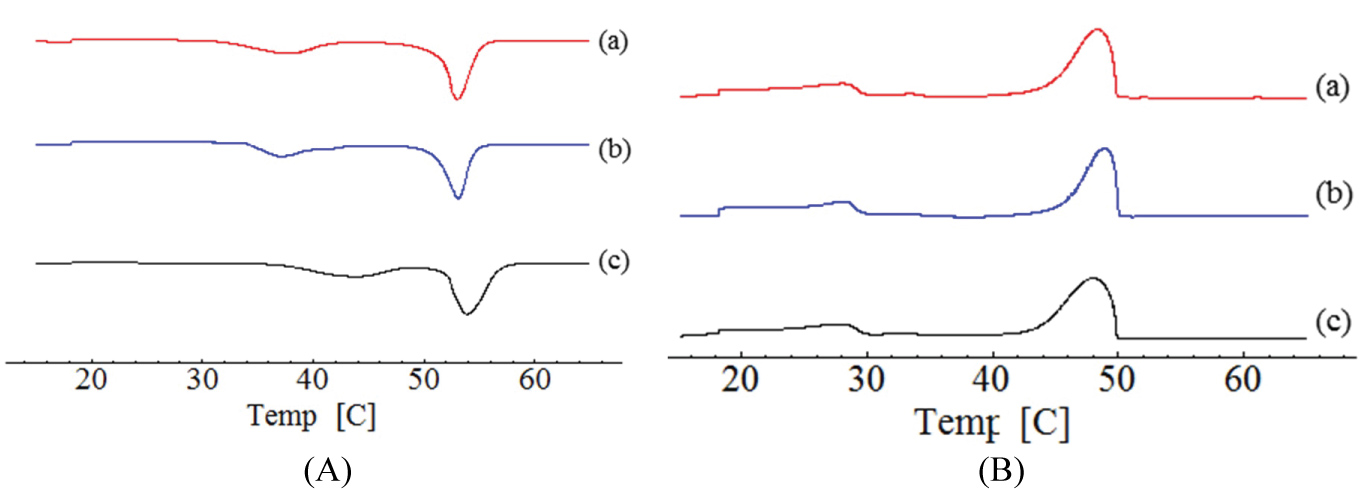
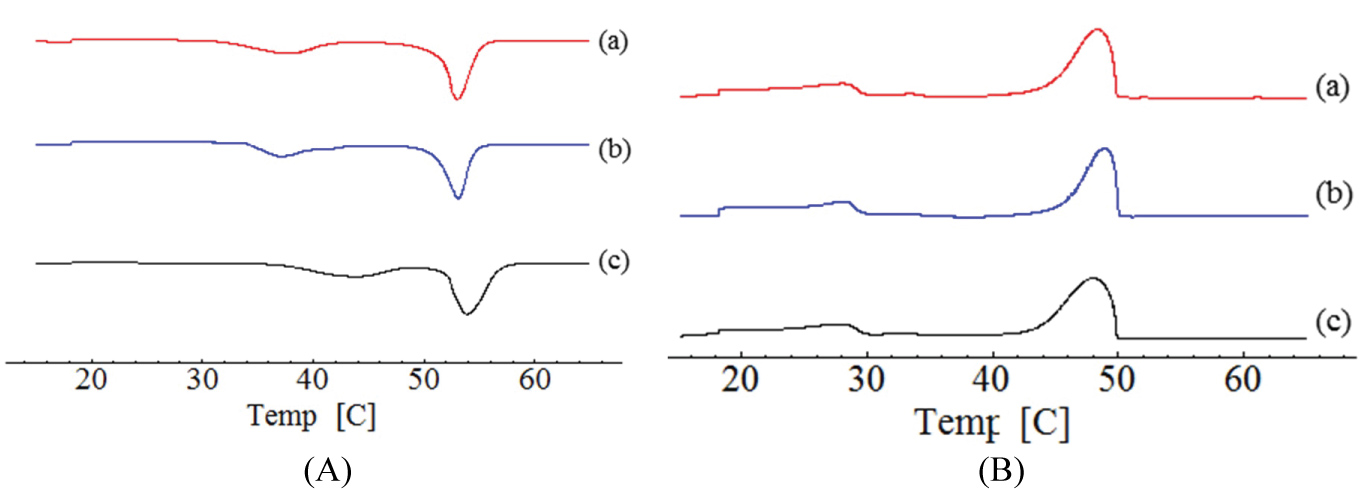
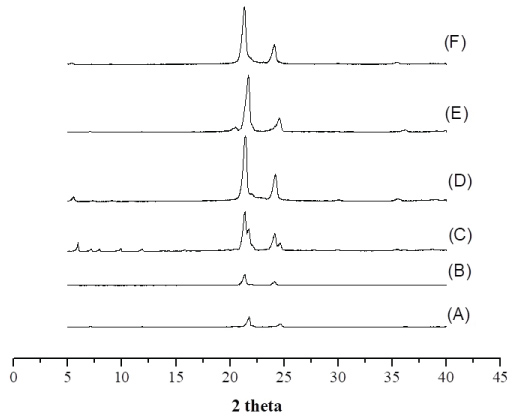
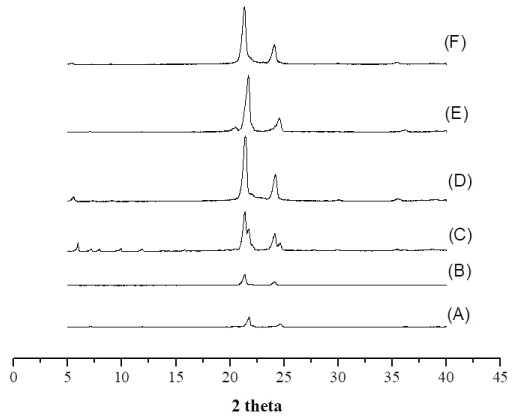
The WAXD features the crystal structure and/or polymorphic materials, identifying the interactions between the incident x-ray beam and the electrons of the component atoms in the sample to be analyzed by detecting the diffracted photons. Cetyl alcohol and cetearyl alcohol were performed by WAXD analysis (Figure 3).
The WAXD patterns show that after tempering cetyl alcohol and cetearyl alcohol, the intensity of the peaks was reduced. No defined width was observed after treatment. From the WAXD analysis, cetyl alcohol depicted two distinct polymorphic forms, whereas only one form was observed by DSC analysis, giving the similar melting points of both polymorphs.
The small difference of diffractograms is observed in interlayer spacings. Cetyl alcohol showed 21.75 (2θ) i.e. d = 0.223 nm, and 24.68 (2θ) i.e. d = 1.31 nm without process; 21.36 (2θ) i.e. d = 0 227 nm and 24.17 (2θ) i.e. d = 0.2062 nm before tempering. Cetearyl alcohol showed 21.33 (2θ) i.e. d = 0.227 nm, and 24.12 (2θ) i.e. d = 0.206 nm untreatment; 21.41 (2θ) i.e. d = 0.226 nm and 24.17 (2θ) i.e. d = 0.2062 nm before tempering. In WAXD analysis, the peak was kept but with decreased intensity, results that are in agreement with those obtained for DSC. The stable form β decreased after spray drying treatment.
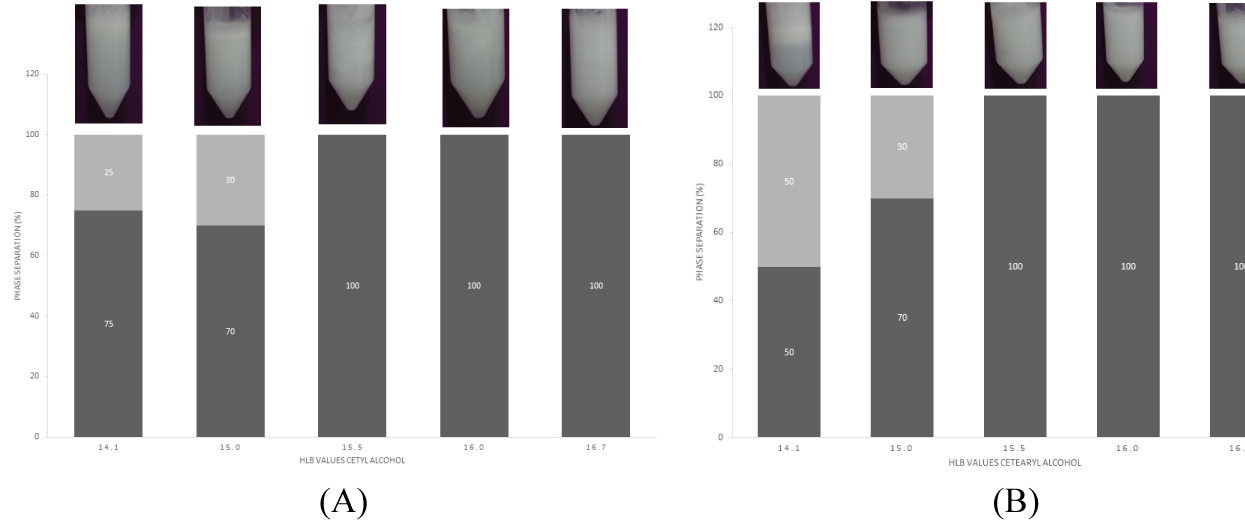
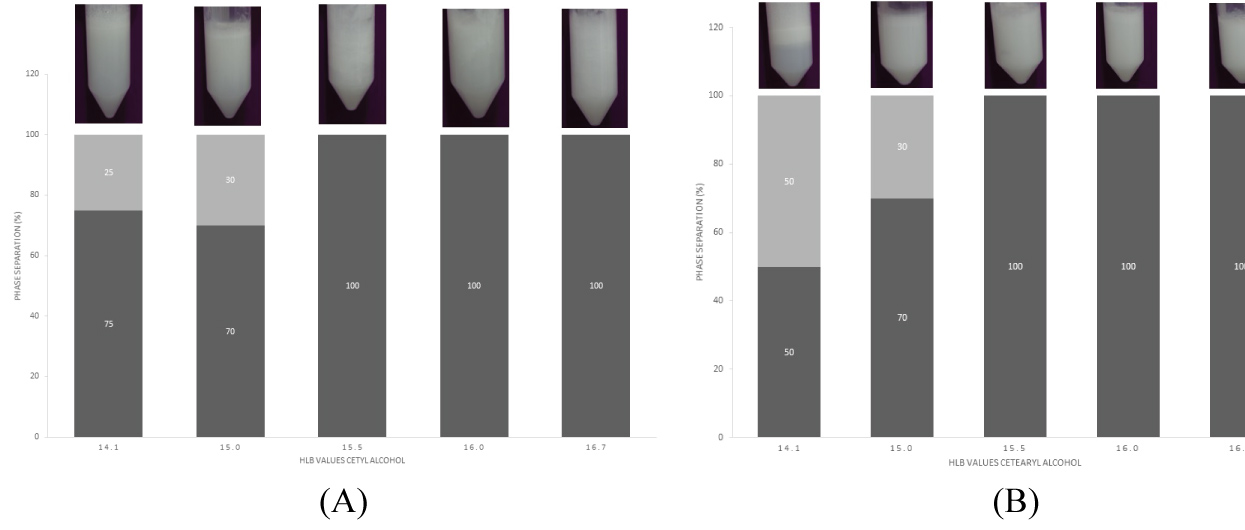
Figure 4 shows the HLB values obtained for stable emulsions produced from cetyl alcohol and cetearyl alcohol.
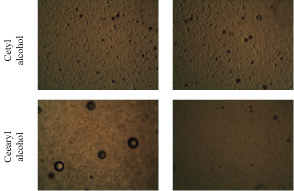
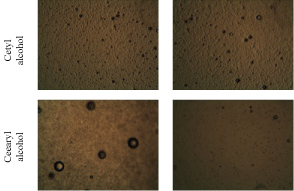
Figure 5 shows the results of the optical microscopic analyses (100-fold increase) from cetyl alcohol and cetearyl alcohol emulsions with HLB 15.5. A smooth and homogeneous structure was observed.
Discussion
A variety of production methods are used to develop SLN. The main method is the High-Pressure Homogenization because it is easy to scale up and it has been used for a long time to produce emulsions for parenteral nutrition [32]. With this methodology, it is possible to control the mean diameter and the polydispersity index of SLN, avoiding the use of organic solvents [33]. The employment of high temperatures in this method can modify the crystal structures of lipids, causing differences in formulation after solidification compared to the material previously studied [34].
The International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use, composed of representatives of the European Union, Japan, the United States and representatives of the pharmaceutical industry, establishes the protocols for quality control of pharmaceutical ingredients, published as guides or laws in several countries. The guide published by the American agency Food and Drug Administration (FDA), establishes the criteria for the characterization of the polymorphic form and level of criticality on the final product. The techniques listed this characterization are WAXD, DSC, microscopy and spectroscopy [35].
Lipids suffer polymorphism, thereby it has ability to solidify in different crystalline forms, with significant physical and chemical changes in form, solubility, melting point and crystallization. Amorphous matrix showed disordered arrangements of molecules, favoring the high loading of the drug. In the crystalline matrix, the crystals form organized arrangements of molecules with fixed repeating array built of unit cells [34]. Standard WAXD may identify polymorphs. Long spaces provide information about the distance between the crystal planes (chain length), while the short spacing's provide information about the structure of the sub-cell (distance between chains). Lipids widely used in the production of SLN and NLC are typical examples of molecules that commonly present three distinct and important polymorphic forms, α and β' or α, β' and β respectively, according to the structure of the subcell. The α form (hexagonal) is the least stable, with lower melting point and latent heat. The form β (tricyclic) is more stable with higher melting point and higher latent heat. The transformation of α to β' (orthorhombic) and β this to occur in this order and are irreversible [36]. It is fundamental to know the physical form of the crystal habitat of lipids to produce stable SLN.
Cetyl alcohol and cetearyl alcohol are biocompatible lipids and of Generally Regarded as Safe (GRAS) status. They have been used as raw materials for the production of SLN [31,37], o/w emulsions [38] and microparticles [39]. Cetyl alcohol is produced from vegetable oils coconut or palm kernel. It is characterized as an alcohol with functional group OH (hydrophilic) and long chain with 16 carbons (lipophilic). Cetearyl alcohol consisting predominantly of cetyl and stearyl alcohols mixture, and it is classified as a fatty alcohol composed CH3(CH2)nOH; n = variable, typically 15-17.
The differences between the melting and the onset temperatures of cetyl alcohol before (10.65 ℃) and after tempering (8.07 ℃) are slower after the tempering process. These values suggesting changes from a more unstable polymorphic form. According to the chemical nature of the lipid, there may be modifications in the fractions of the polymorphs present in the composition of the nanoparticles, which leads to the reduction, displacement or change in the melting temperature of the system.
For cetearyl alcohol, the first peak was recorded at 14.04 ℃ and 8.52 ℃ before and after tempering, respectively, while the second peak was obtained at 8.52 ℃ and 11.34 ℃ before and after tempering, respectively. It is known that the melting peak of the lipid occurs between the onset and endset temperatures, and the greater the difference between onset and endset, the greater the disorder of the crystals. Cetyl alcohol depicted a short difference between onset and endset temperatures, therefore suggesting a decrease of the crystalline arrangements. Cetearyl alcohol showed the presence of two peaks, where the decrease of the first peak and increase of the second peak was attributed to the mixture of cetyl and stearyl alcohols existing in this lipid arranged in different form [40].
After tempering, the onset temperature decreased for both lipids. These informations suggest the presence of β/βi caused by the thermal stress in the lipid [16].
The tempering process of cetyl alcohol decreased the melting enthalpy in 20% (255.41 to 204.42 J.g-1), indicating significant disruptions in the microstructure. After tempering, the energy was lower, and the lipid changed from a more stable to a more unstable polymorphic form. Cetaryl alcohol did not show a significant difference on the enthalpy. However, in both lipids, the peaks were maintained after tempering, but the intensity of peaks was lower after tempering. This fact suggests lower crystallization when compared without treatment. Tempering affected the recrystallization of lipids. However, these results are agreement with polymorphism existing in the lipids [41].
Crystallinity is a property of lipids, and this has been studied before and after tempering the bulk materials for 1 h at 80 ℃. The peaks observed in lipids are in β' form, identified by the spacings between 0.42-0.43 nm and 0.37-0.40 nm. Comparing the results obtained by DSC and WAXD it is possible to see that after tempering, the decrease of the melting point and intensity of WAXD peaks demonstrated the presence of a less stable crystalline lipid. These demonstrates agreement between the DSC and WAXD results, confirming that after tempering the peak of β'/β increases.
It is important to realize that the three-dimensional organization that acquires lipid matrix during solidification depends on the cooling rate of lipids and their composition. It is a very important factor for the loading of the drug molecules in SLN. When the cooling rate is slow, the hydrocarbon chains of lipids can be rearranged in a more ordered and stable form. This study was carried out at temperature. On the contrary, when the cooling rate is high, the solidification of the matrix also occurs rapidly, rearranging into a more unstable polymorphic form. Thus, the preparation of SLN by hot High-pressure homogenization follow high cooling rate, the newly formed particles acquire the polymorphic form β preferentially. Concerning the lipid composition, the matrices having a high content of diglycerides crystallize in the metastable polymorphic form β' [12,36].
Studies have used the spray drying technique as a scalable method in the bulk preparation of a dried lipid for secondary liposome production [22]. Spray drying technique was used to turn the lipid more amorphous i.e. less crystalline to produce stable SLN with more capacity to load drug molecules.
Spray drying process was used to evaluate the ability to maintain amorphous lipid matrix and possibly be able to provide a disorganized matrix to encapsulate high quantity of drug. The amorphous process upon thermal stress of cetyl alcohol and cetearyl alcohol, showing the decreasing of the difference between onset and endset. Before spray-drying, for cetyl alcohol one peak has been recorded at 10.65 ℃, whereas for cetearyl alcohol two peaks have been recorded at 14.04 ℃ and 11.34 ℃, respectively. After spray drying, for cetyl alcohol the peak was at 6.56 ℃, and for cetearyl alcohol the two peaks were shifted to 10.28 ℃ and 8.60 ℃ suggesting the formation of an amorphous arrangements of molecules.
The thermodynamic stability of the SLN and the degree of lipid packing increases, while the loading of drug molecules decreases in the following order: polymorphic form α, β' and β [42]. In general, the lipid molecules have a higher mobility when in the most thermodynamically unstable configurations such as, for example, the polymorph α. For this reason, these configurations have a lower degree of lipid packing, the greater the ability to load drug molecules. Therefore, for the preparation of SLN with high load capacity, in particular, for hydrophilic drugs, the lipid will crystallize preferentially the more unstable polymorphic form, i.e. in the α form, which must be maintained during storage [43-45].
In the crystalline matrix, the crystals form organized arrangements of molecules with fixed repeating array built of unit cells [41]. Standard WAXD may identify These polymorphs. The WAXS profile for cetyl alcohol are shown in Figure 3A, Figure 3C and Figure 3E and correspond to untreated lipid, after tempering, and after spray-drying, respectively.
The diffraction patterns of sample (A) display non-equidistant reflections (peaks X and Y) in comparison to sample (A) and (E). The scattering angle ranging between 5.0º and 18º (2θ) and these peaks are indicative of a non-periodic lamellar arrangement within the lipid structure suggesting tempering process influence polymorphism of lipid. Therefore, in all samples of cetyl alcohol a difference in width and intensity of peaks was observed. Sample (A) shows one reflection at Bragg spacing values of 0.585 nm, 21.72 (2θ). After tempering (C) and spray-drying (E) Bragg's spacing values of 0.280 nm and 0.424 nm were observed, which is indicative of the presence of β' polymorphic modification with the characteristic spacings between 0.42-0.43 nm and 0.37-0.40 nm.
Results also showed that samples B, D and F, prepared with Cetearyl alcohol without tempering, after tempering, and after dried spried process, respectively, different width of the peaks and the intensity. It is observed that presence of characteristic peaks for all, however there is a significant increase of intensity when compared with the untreated sample.
The WAXS profile for cetearyl alcohol are shown in Figure 3B, Figure 3D and Figure 3F and correspond to untreated lipid, after tempering, and after spray-drying, respectively. Differences in the peaks width and intensity have been observed between the samples. Characteristic peaks occurring in the same temperature have been recorded but with a significant increase of the intensity when compared to the sample without treatment.
In all samples without treatment, a minor intensity peak was recorded, which suggests the formation of more crystallinity polymorphs. This condition translates a higher thermodynamic stability of the system.
Long-term stability of emulsions, surfactants are adsorbed at the interface o/w to form a film, which decreases the surface tension [46]. The stability of the pre-emulsions after 24 hours were obtained with HLB of 15.5; 16.0 e 16.7 assessed for the two lipids. These values were consistent with the data obtained by manufacturers. The formulations with HLB de 14.1 and 15.0 showed separated phase. It is therefore desirable to operate with a surfactant composition of polysorbate 20 and trioleate sorbitan of HLB around 15.5 for the production of stable and homogeneous SLN.
The numbers corresponding to the HLB of some products are obtained from the literature. Lipids shows HLB between 6 and 17. The development of stable emulsion systems significantly because it is important and a useful, easy and practical methodology to forecast a stable SLN production.
Conclusions
DSC and WAXD are valuable tools to assess the presence of polymorphs in fatty alcohol crystals. Cetyl and cetearyl alcohol suffer polymorphic modification with tempering and spray dried process. Polymorphic structure of lipid suggesting a more unstable form being good options for loading drugs in SLN. Indeed, the high amount of β and β' decreased after tempering and spray drying process, changing the thermodynamic stability and favoring the loading of drugs in SLN. Also, the HLB allowed the selection of the best combination of surfactants, for the development of homogeneous and stable emulsions. The stable emulsion was obtained with a suitable mixture of polysorbato 20 and trioleate sorbitan with the best HLB values were 15.5, 16.0, and 16.7. The results showed that both cetearyl alcohol and cetyl alcohol promising to development SLN stable for drug delivery because produced a disorganized matrix able to be loading high amount of drug.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors wish to acknowledge the sponsorship received from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, #Processo 2011/20801-2 and #2012/21219-5) for financial support and from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, #443238/2014-6, #470388/2014-5). Fundação para a Ciência e Tecnologia do Ministério da Ciência e Tecnologia (FCT, Portugal) and Fundos Comunitários do Programa PRODER/COMPETE are also acknowledged under the research projects UID/QUI/50006/2013 and M-ERA-NET/0004/2015, co-financed by FEDER, under the Partnership Agreement PT2020.
References
- Doktorovova S, Kovacevic AB, Garcia ML, et al. (2016) Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm 108: 235-252.
- Doktorovova S, Souto EB, Silva AM (2014) Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers - a systematic review of in vitro data. Eur J Pharm Biopharm 87: 1-18.
- Doktorovova S, Silva AM, Gaivao I, et al. (2014) Comet assay reveals no genotoxicity risk of cationic solid lipid nanoparticles. J Appl Toxicol 34: 395-403.
- Souto EB, Severino P, Santana MHA (2011) Nanopartículas De Lipídios Sólidos: Métodos Clássicos De Produção Laboratorial. Quim Nova 34: 1762-1769.
- Padhye SG, Nagarsenker MS (2013) Simvastatin solid lipid nanoparticles for oral delivery: Formulation development and in vivo evaluation. Indian J Pharm Sci 75: 591-598.
- Battaglia L, Gallarate M (2012) Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin Drug Deliv 9: 497-508.
- Pawar KR, Babu RJ (2010) Polymeric and lipid-based materials for topical nanoparticle delivery systems. Crit Rev Ther Drug Carrier Syst 27: 419-459.
- Müller RH, Radtke M, Wissing SA (2002) Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54: S131-S155.
- Sanchez-Lopez E, Espina M, Doktorovova S, et al. (2017) Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye - Part I - Barriers and determining factors in ocular delivery. Eur J Pharm Biopharm 110: 70-75.
- Sanchez-Lopez E, Espina M, Doktorovova S, et al. (2017) Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye - Part II - Ocular drug-loaded lipid nanoparticles. Eur J Pharm Biopharm 110: 58-69.
- Korkm E, Gokce EH, Ozer O (2013) Development and evaluation of coenzyme Q10 loaded solid lipid nanoparticle hydrogel for enhanced dermal delivery. Acta Pharm 63: 517-529.
- Souto EB, Severino P, Santana M, et al. (2011) Solid lipid nanoparticles: Classical methods of lab production. Quím Nova 34: 1762-1769.
- Akbari J, Saeedi M, Morteza-Semnani K, et al. (2016) The design of naproxen solid lipid nanoparticles to target skin layers. Colloids Surf B Biointerfaces 145: 626-633.
- Gonçalves VSS, Matias A, Rodríguez-Rojo S, et al. (2015) Supercritical fluid precipitation of ketoprofen in novel structured lipid carriers for enhanced mucosal delivery-a comparison with solid lipid particles. Int J Pharm 495: 302-311.
- El-Salamouni NS, Farid RM, El-Kamel AH, et al. (2015) Effect of sterilization on the physical stability of brimonidine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Int J Pharm 496: 976-983.
- Severino P, Pinho SC, Souto EB, et al. (2011) Polymorphism, crystallinity and hydrophilic-lipophilic balance of stearic acid and stearic acid-capric/caprylic triglyceride matrices for production of stable nanoparticles. Colloids Surf B Biointerfaces 86: 125-130.
- Schoenitz M, Joseph S, Nitz A, et al. (2014) Controlled polymorphic transformation of continuously crystallized solid lipid nanoparticles in a microstructured device: A feasibility study. Eur J Pharm Biopharm 86: 324-331.
- Severino P, Pinho SC, Souto EB, et al. (2011) Crystallinity of Dynasan®114 and Dynasan®118 matrices for the production of Stable Miglyol®-loaded nanoparticles. Journal of Thermal Analysis and Calorimetry, 108: 101-108.
- Souto EB, Mehnert W, RH M (2006) Polymorphic behaviour of Compritol888 ATO as bulk lipid and as SLN and NLC. J Microencapsul 23: 417-433.
- Severino P, Chaud MV, Shimojo A, et al. (2015) Sodium alginate-cross-linked polymyxin B sulphate-loaded solid lipid nanoparticles: Antibiotic resistance tests and HaCat and NIH/3T3 cell viability studies. Colloids Surf B Biointerfaces 129: 191-197.
- Bunjes H, Koch MH, Westesen K (2003) Influence of emulsifiers on the crystallization of solid lipid nanoparticles. J Pharm Sci 92: 1509-1520.
- Alves GP, Santana MHA (2004) Phospholipid dry powders produced by spray drying processing: Structural, thermodynamic and physical properties. Powder Technology 145: 139-148.
- Griffin WC (1954) Calculation of Hlb Values of Nonionic Surfactants. J Soc Cosmet Chem 5: 249-256.
- Xia D, Shrestha N, Van De Streek J, et al. (2016) Spray drying of fenofibrate loaded nanostructured lipid carriers. Asian Journal of Pharmaceutical Sciences 11: 507-515.
- Chawla V, Saraf SA (2011) Glyceryl behenate and its suitability for production of Aceclofenac solid lipid nanoparticles. J Am Oil Chem Soc 88: 119-126.
- Kasongo KW, Pardeike J, Müller RH, et al. (2011) Selection and characterization of suitable lipid excipients for use in the manufacture of didanosine‐loaded solid lipid nanoparticles and nanostructured lipid carriers. J Pharm Sci 100: 5185-5196.
- Moghimipour E, Ramezani Z, Handali S (2013) Solid lipid nanoparticles as a delivery system for Zataria multiflora essential oil: Formulation and characterization. Curr Drug Deliv 10: 151-157.
- Yang R, Gao R, Li F, et al. (2011) The influence of lipid characteristics on the formation, in vitro release, and in vivo absorption of protein-loaded SLN prepared by the double emulsion process. Drug Dev Ind Pharm 37: 139-148.
- Shah RM, Malherbe F, Eldridge D, et al. (2014) Physicochemical characterization of solid lipid nanoparticles (SLNs) prepared by a novel microemulsion technique. Journal of Colloid and Interface Science 428: 286-294.
- Maheshwari M, Ketkar AR, Chauhan B, et al. (2003) Preparation and characterization of ibuprofen-cetyl alcohol beads by melt solidification technique: Effect of variables. Int J Pharm 261: 57-67.
- Wehrung D, Geldenhuys WJ, Oyewumi MO (2012) Effects of gelucire content on stability, macrophage interaction and blood circulation of nanoparticles engineered from nanoemulsions. Colloids Surf B Biointerfaces 94: 259-265.
- Muller RH, Maeder K, Sven Gohla (2000) Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. European Journal of Pharmaceutics and Biopharmaceutics 50: 161-177.
- Severino P, Santana MH, Souto EB (2012) Optimizing SLN and NLC by 2(2) full factorial design: Effect of homogenization technique. Mater Sci Eng C Mater Biol Appl 32: 1375-1379.
- Westesen K, Wehler T (1993) Investigation of the particle size distribution of a model intravenous emulsion. J Pharm Sci 82: 1237-1244.
- https://www.ich.org/home.html.
- Attama AA, Schicke BC, Müller-Goymann CC (2006) Further characterization of theobroma oil-beeswax admixtures as lipid matrices for improved drug delivery systems. Eur J Pharm Biopharm 64: 294-306.
- Pattarino F, Giovannelli L, Bellomi S (2007) Effect of poloxamers on nifedipine microparticles prepared by hot air coating technique. Eur J Pharm Biopharm 65: 198-203.
- Djuris J, Vasiljevic D, Jokic S, et al. (2013) Application of D-optimal experimental design method to optimize the formulation of O/W cosmetic emulsions. Int J Cosmet Sci 36: 79-87.
- Passerini N, Perissutti B, Albertini B, et al. (2003) Controlled release of verapamil hydrochloride from waxy microparticles prepared by spray congealing. J Control Release 88: 263-275.
- Abdel-Mottaleb MM, Neumann D, Lamprecht A (2010) In vitro drug release mechanism from lipid nanocapsules (LNC). Int J Pharm 390: 208-213.
- Anthony A Attama, Mumuni A Momoh, Philip F Builders (2012) Lipid nanoparticulate drug delivery systems: A revolution in dosage form design and development. In: Sezer AD, Recent advances in novel drug carrier systems. IntechOpen.
- Jenning V, Thunemann AF, Gohla SH (2000) Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm 199: 167-177.
- Bummer PM (2004) Physical chemical considerations of lipid-based oral drug delivery-solid lipid nanoparticles. Crit Rev Ther Drug Carrier Syst 21: 1-20.
- Muller RH, Keck CM (2004) Challenges and solutions for the delivery of biotech drugs - a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol 113: 151-170.
- Pandita D, Kumar S, Poonia N, et al. (2014) Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Research International 62: 1165-1174.
- Keck CM, Baisaeng N, Durand P, et al. (2014) Oil-enriched, ultra-small nanostructured lipid carriers (usNLC): A novel delivery system based on flip-flop structure. Int J Pharm 477: 227-235.
Corresponding Author
Severino, Program in Industrial Biotechnology, Laboratory of Nanotechnology and Nanomedicine (LNMED), Institute of Technology and Research (ITP), Tiradentes University (UNIT), Av. Murilo Dantas, 300, Farolândia, Aracaju, Sergipe, CEP 49.032-490, Brazil, Tel: +55-(79)-3218-2190 (R-2599), Fax: +55-(19)-999988910;
Eliana B Souto, Department of Pharmaceutical Technology, Faculty of Pharmacy, Pólo das Ciências da Saúde, University of Coimbra (FFUC), Azinhaga de Santa Comba, 3000-548, Coimbra, Portugal, Tel: +351-239-488-400, Fax: +351-239-488-503.
Copyright
© 2018 Diniz F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.