Evaluation of the Potential Genotoxicity of Quantum Dots. A Review
Abstract
The Quantum Dots (QDs) are widely used in different biological and biomedical applications due to their unique optical, electronic, physicochemical properties. Different QDs recently have been used for tumour imagining, gene therapy, and drug delivery. They are developed for numerous applications. Although various studies concerning possible harmful effects of QDs have been conducted, there is need to consolidate evidence, concerning the genotoxic effects of semiconductor QDs. In this manuscript, recent in vivo and in vitro studies concerning the genotoxic effects of QDs are reviewed and potential mechanisms underlying their genotoxicity discussed. Genotoxicity tests have been used in many QD studies in the model systems. This review presents an overview of the genotoxicity assessment of QDs using different assay systems and discusses how genotoxicity of QDs is affected by experimental conditions, such as, light or dark conditions, in vivo/in vitro assays or cell types used, as well as the physicochemical properties of QDs (core, shell and coating materials, etc.). This review article is intended to promote the awareness of QD applications in biology, evaluate the potential genotoxicity of QDs, and present approaches to reveal the possible underlying mechanisms of this genotoxicity.
Keywords
Quantum dots (Qds), Cadmium, Nanomaterials, Nano-genotoxicology, Risk assessment
Introduction
The semiconductors quantum dots (QDs) constitute a generation of nanomaterials (NMs) characterized by their small size (1-10 nm), containing about 200-10,000 atoms, and having invaluable optical, chemical, electrical and magnetic properties [1]. Because of their photophysical properties and adjustable emission in different wavelengths, they are being developed for different biological and biomedical applications. QDs can connect covalently with the different molecules (such as antibody peptides, nucleic acids or small-molecule ligands) for use as targeted fluorescent probes. QDs have been used for in vitro diagnostic applications [2], fluorescently labelled DNA probes for gene mapping identification of chromosomal abnormalities and fluorescent in situ hybridisation-FISH [3], gene therapy [4], targeted imaging of membrane proteins and receptors [5], in vivo detection of cancer metastasis [6,7], targeted tumour imaging and therapy [8,9], drug delivery [10-12], photodynamic therapy [13], toxin detection [14] and magnetic resonance imaging [15].
Multicolour and multiplexing potentialities of QDs have been used for the detection of different protein biomarkers (CD15, CD30, CD45, and Pax5) of Hodgkin's lymphoma [16], imaging of respiratory syncytial virus [17], as FRET- and BRET-based sensors [18], as labels for detection of multiple mRNA targets using fluorescence in situ hybridisation (FISH) [19], detection of multiple proteins on Western blots [20] and pH probes [21]. QDs have also been used as a tool in neuroscience to visualize and track dynamic molecular processes and assess axon growth [22]. Although QDs shown great potential in imaging and biomedical applications, a huge number of studies suggested and clearly demonstrated that QDs may pose risks to human health and the environment under certain conditions [23]. The present review summarizes the available toxicological data for QDs.
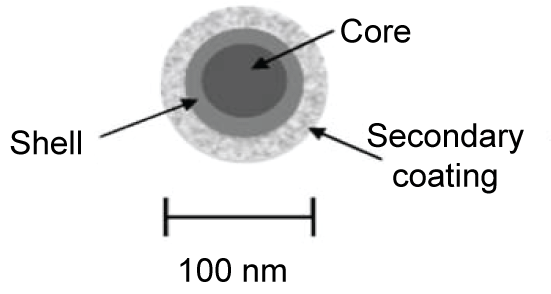
A first step in elucidating the potential toxicity and genotoxicity of QDs is to characterize their physicochemical properties. QDs are generally composed of groups III-V, II-VI, or IV-VI elements in periodic table, such as CdS, CdSe, CdTe, CdS/ZnS, CdSe/ZnS, and CdSeTe/ZnS. QDs exhibit physical dimensions smaller than the exaction Bohr radius according to their bulk form [24-26]. QD have a metalloid core, the wavelength of fluorescence emitted depends on the composition and size of this metal core. The nanocrystal core may be subject to photochemical degradation, which may cause toxic effects. Usually the core structure consists of cadmium selenide (CdSe), cadmium telluride (CdTe), indium phosphate (InP) or indium arsenate (InAs), etc. [27]. The QD core is surrounded by an external coating. This coating material is usually an amphiphilic polymer, which increases solubility in biologically compatible media. QDs may have an outer layer (or "shell") of zinc sulphide (ZnS) between the core and the coating, to reduce leaching of metals from the core and improve photo-stability. Figure 1 shows a diagram of the general structure of QDs [28].
Potentially, QDs could be used widely for many biological applications, because of their unique physicochemical properties. In spite of the beneficial properties of QDs, the potential harmful health effects may be a concern. These concerns are related to the fact that these materials contain heavy metals, such as Cd, As, Zn, Pb, etc. Assessing exposure routes and potential toxicity of QDs is not a simple matter. Furthermore, not all QDs are alike, and toxicity would depend on multiple physicochemical as well as environmental factors [23]. Many questions remain to be answered before QDs move into broader biomedical applications especially for in vitro or in vivo sensing/imaging. For safe application of QDs in a clinical context, understanding the response of humans to QDs is critical [29,30]. In the past years, several reviews have focused on the toxicity of QDs. However, none have assessed the possible genotoxic effects of QDs or discussed possible mechanisms involved in this genotoxicity, such as core material, size, coating, charge, etc.). The present review sets out to discuss whether QDs may elicit genotoxicity, with a special focus on the underlying possible mechanisms. Furthermore, this review will summarize the current knowledge of QD toxicity and genotoxicity and highlight areas where new information is critical, suggesting directions for future research. Focus will be placed on new strategies for genotoxicological research that need to be developed in order to resolve knowledge gaps. This review will also summarize how much genotoxicity of QDs can be affected by different experimental conditions, for example concentration of exposure, light or dark conditions, in vivo/in vitro experiments, cell types, etc., and physicochemical properties of QDs such as core, shell, coating, size, charge, etc. Though the number of genotoxicity studies on QDs is still limited, this endpoint warrants increased attention. This review will focus ontests most often employed to evaluate the genotoxicity of QDs i.e., the Ames test, Comet assay, micronucleus assay and other DNA damage and oxidative damage tests.
There are several studies about QDs toxicity in cellular systems, plants, and mammals. Navarro, et al. reported that QDs caused oxidative stress inplants determined as a decrease in the ratio of reduced glutathione levels (GSH) to the oxidized glutathione (GSSG) [31]. Mercaptosuccinic acid (2-sulfanyl butanedioic acid)-capped QDs, were reported to betoxicin vascular endothelial cells [32]. QD toxicity can be due to composition (coreorsurface coating), size or surface charge [33,34]. In addition, biological response is determined by absorption, metabolism, distribution and excretion. Kidneys and liver are major organs that are for responsible for clearing QDs from different organs after exposure to QDs containing heavy metals, such as cadmium (Cd) and selenium (Se) particles. The literature indicates that QDs accumulate in theliver and spleen with lesser quantities in kidneys, lungs, lymph nodes, bone marrow, while rarely entering the brain [35]. Ates, et al. reported that CdSe QDs accumulated in a time dependent manner in spleen and liver and to a lower extent in the kidney and intestine [36]. However, they reported that CdSe QDs did not accumulate in the brain, heart, and lungs in CD-1 mice. Furthermore, intravenous (i.v.) exposure to CdSe QDs induced oxidative stress measured as an increase in malondialdehyde (MDA) levels [36].
Genotoxicity can be described as damage to the genetic material namely DNA. Further, genotoxicity occurs when different agents damage the genetic information within a cell causing mutations, which may lead to cancer. When DNA integrity is compromised by a damaging agent, a number of scenarios are possible. These depend upon the type of damage, its extent and persistence [37]. Primary genotoxicity is induced in the absence of an inflammatory response, while secondary genotoxicity is mediated through the activation of inflammatory cells, for example neutrophils and macrophages, which can generate substantial amounts of reactive species [38]. Furthermore, while direct genotoxicity can result from physical interactions of the particulate material with DNA, indirect genotoxicity can result from increased ROS generation upon interaction with other cellular components (e.g. mitochondria, cell membrane, etc.), resulting indepletion of intracellular antioxidants [39]. In this manner, ROS, produced by physiological cellular pathways, may accumulate and cause DNA damage. The alteration can have direct or indirect effects on DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. Various types of DNA mutations are known. Mutations of key loci within the genetic code are the molecular hallmark of cancer. These key genes can be divided into proto-oncogenes that stimulate cellular growth and proliferation (e.g. K-ras), and tumour suppressor genes that inhibit proliferation or are involved in DNA repair (e.g. p53). In addition to their role in carcinogenesis, DNA mutations can cause various pathologies and can modulate susceptibility to disease. Mutations can involve relatively small sequences, involving single genes, or can occur on a larger scale. Examples of small-scale mutations are point mutations (i.e. transition or transversion), in which one nucleotide is replaced by another; insertions, which add nucleotides to the genetic code; and deletions, which remove nucleotides from DNA. Mutations can also affect chromosomal structure another endpoint of genotoxicity. Chromosomal aberrations are caused by clastogenic chemicals. Chromosome aberrations can either be structural (clastogenic) or numerical (an eugenic). The micronucleus (MN) assay is considered a sensitive tool to determine chromosomal damage induced by clastogenic (DNA breakage) or aneugenic (abnormal segregation) processes, while the alkaline Comet assay enables the identification of DNA strand breaks (single and double) [40,41]. DNA damage, such as double-strand breaks (DSBs), threatens the integrity of chromosomes and viability of cells. Unrepaired or misrepaired DSBs can lead to mutations, chromosome rearrangements, cell death and cancer [42-45].
In vitro genotoxicity of Qds
Quantum dots (QDs) have many potential clinical and biological applications because of their advantages over traditional fluorescent dyes. However, the genotoxicity potential of QDs still remains unclear. Assessment of genotoxicity is considered a useful tool for understanding of the potential carcinogenic risk of QDs. In addition, mutations in somatic cells are not only involved in the carcinogenesis process but also play a role in the pathogenesis of artery diseases, such as atherosclerosis and heart diseases, which are the leading causes of death in the human population [46-48]. Results of in vitro and in vivo studies of the genotoxicity of QDs are summarized in Table 1 and Table 2. Data indicate that QDs, having different surface coatings and sizes, may elicite different effects in various organisms and cells. In the next section, different studies of QD-induced genotoxicity involving various in vitro and in vivo systems are presented.
DNA damage
The Comet assay or "single-cell gel electrophoresis" is an assay which measures DNA damage in a single eukaryotic cell. Recently, genotoxicity of QDs was evaluated using the Comet assay. Nagy, et al. performed a study in primary normal human bronchial epithelial cells using negatively or positively charged QDs coated with mercaptopropionic acid (MPA) or cysteamine (CYST) [49]. Results showed that CYST-CdSe QDs induced cytotoxic effects accompanied by DNA strand breakage. On the other hand, MPA-CdSe QDs induced a high number of DNA strand breaks but did not induce reactive oxygen species (ROS) production and cytotoxicity. This DNA damage was accompanied by the presence of p53 binding protein 1 (53BP1) in the nuclei of exposed cells. Aye, et al. reported the genotoxic effects using the Comet assay on Chinese hamster ovary cells (CHO-K1) [50]. Results showed that QDs, in dark conditions, were genotoxic in the Comet assay.
In sunlight conditions, QDs exhibited genotoxic effects using the Comet assay. This DNA-damaging activity was suppressed partially by l-ergothioneine. Polyethylene glycol (PEG) modified and uncoated CdSe/ZnS QDs were evaluated in terms of cytotoxicity and genotoxicity as well as oxidative stress for 24 hrs in human skin epithelial cells (HSF-42) [51]. The results showed that the uncoated CdSe/ZnS QDs induced DNA damage, with ROS at least partially involved in this process, while CdSe/ZnS QDs with PEG coating exhibited no genotoxicity in the Comet assay. This study concluded that the genotoxicity and ROS generation induced by of QDs could be significantly decreased following proper surface modification, such as PEG encapsulation.
Another study by Pathakoti, et al. evaluated the effects of CdSe/ZnS QDs with different surface coatings or functional groups in the immortalized human keratinocyte HaCaT cell line [52]. Amine-polyethylene glycol and amphiphilic polymer coated QDs did not induce cytotoxicity but polyethylenimine coated CdSe/ZnS QDs were highly cytotoxicity. Furthermore, these QDs exhibited genotoxicity in HaCaT cells with the DNA damage being highest with the polyethylenimine coated CdSe/ZnS QD (620 nm wavelength) at 10 nmol/L concentration. Polyethylenimine coated CdSe/ZnS QDs (530-580 nm wavelength) also showed significant genotoxicity based on the increase in tail moment, percent DNA, and tail length at 1 and 0.2 nmol/L concentrations. Conversely, DNA damage was insignificant as shown by the tail length at 1 nmol/L concentration of polyethylenimine coating CdSe/ZnS QDs (580 nm) and 5 nmol/L concentrations of polyethylenimine coating CdSe/ZnS QDs (530 and 620 nm wavelength). In summary, this study showed DNA damage in the HaCaT cells after exposure to polyethylenimine coating CdSe/ZnS QDs. This damage may be attributed to increase in ROS production and a subsequent decrease in mitocondrial membrane potentials [52]. CdS QDs and silver sulphide (Ag2S) coated with methyl polyethylene glycol (M-PEG) were studied in a rainbow trout cell line (RTG-2) [53]. CdS QD exhibited significant genotoxicity with a concentration response in the sub-toxic concentration range (0.01-1 μg/mL) after 24 hrs. However, no genotoxicity was observed in CdS QDs exposed RTG-2 cells after 48 hrs. This finding may be associated with the well-known pro-apoptotic effect of Cd [54]. Indeed, apoptotic low-molecular-weight DNA fragments are not detected by the alkaline Comet assay, since highly damaged cells are lost from the gel during electrophoresis [55]. However, DNA repair cannot be ruled out as responsible for restoring DNA integrity [53]. Katsumiti, et al. reported that CdS QDs as well as bulk CdS and ionic Cd increased DNA damage in mussel haemocytes and gill cells Mytilus galloprovincialis [56]. These results are in agreement with those of other researchers on the effects of CdTe QDs in freshwater mussels [57]. Comparing the genotoxic effects observed in the two cell types, hemocytes were slightly more sensitive than gill cells in the Comet assay. In both hemocytes and gill cells, CdS QDs were less genotoxic than ionic Cd but produced more DNA damage at lower concentrations than with bulk CdS exposure. Privezentsev, et al. suggested that Cd genotoxicity occurs mainly by single strand breaks in DNA through direct cadmium-DNA interactions as well as by the action of incision nucleases and/or DNA-glycosylase during DNA repair [58]. The increase in ROS produced by CdS QDs exposure could also contribute to the DNA damage, since ROS generated in the mitochondria and from other sources, can cause serious damage to lipids, proteins and DNA [59,60]. CdS QDs have also been shown to deplete antioxidants and protein-bound sulfhydryl groups [61-63]. Thus, it was concluded that the toxicity of CdS QDs in mussel hemocytes and gill cells may involve ROS production [56].
Generally, chromosomal damage is assessed using the chromosomal aberration assay or by the micronucleus test (MN). These assays evaluate structural chromosomal alterations using a microscope or the formation of micronuclei, which can be formed when a part of a chromosome is broken. Aye, et al. reported the clastogenic effects of a new QD nanoplatform (QDsN), consisting of CdSe/ZnS core-shell QDs encapsulated by a natural fusogenic lipid (DOPC) and functionalized by DOTAU [50]. The micronucleus test on CHO-K1 cells was conducted in bothdark and sunlight conditions. Results showed that QDs in a dark condition were mutagenic in MN, but not in sunlight conditions. The uptake and toxicity of negatively and positively charged CdSe: ZnS QDs of the same core size but with different surface chemistries (carboxyl or amine polymer coatings) were investigated in full and reduced serum containing media following 1 and 3 cell cycles. Gross chromosomal damage was quantified with the cytokinesis-blocked micronucleus (CBMN) assay in three different cell lines namely, epithelial (BEAS-2B), fibroblast (HFF-1), and lymphoblastoid (TK6) cells [64]. These cells represented the 3 main portals of NP exposure: pulmonary, dermal, and circulatory. Results showed that only the carboxyl-QD induced chromosomal damage in full serum containing media. The carboxyl-QDs resulted in a significant increase in MN frequency at several exposure concentrations in both TK6 and HFF-1 cells after 1 cell cycle exposures. HFF-1 cells showed a concentration dependent increase in MN following exposure to amine-QDs up to 10 nM in media with reduced serum. No MN was detected in BEAS-2 cells exposed to either of the QDs. The mechanisms underlying the cytotoxic and genotoxic effects of the QDs were subsequently examined, focusing on the nature of the DNA damage, the effect of oxidative stress, and secondary mechanisms. Results revealed that TK6 cells seem to be the most sensitive, especially given the lower level of cell-associated QDs, indicating the cells were unable to tolerate even low levels of internalized QDs. Carboxyl-QDs induced genotoxicity, oxidative stress, and mitochondrial damage especially in high concentrations. The carboxyl-QDs did not impart any chromosomal damage in the HFF-1 cells, while amine-QDs induced a significant induction of MN after 1 cell cycle, which was absent following 3 cell cycles. This difference in NP toxicity points out the importance of physicochemical characteristics as well as other factors (for example exposure media composition and serum content, plus the exposure duration) in different studies [64].
The Ames Salmonella/microsome mutagenicity assay (Salmonella test or Ames test) is a short-term bacterial reverse mutation assay specifically designed toevaluate whether a wide range of chemical substances can produce genetic damage that leads to gene mutations. Studies to evaluate the mutagenic effects of QDs by the Ames test arethus far limited in number. Aye, et al. reported no mutagenic effects of a QD nanoplatform (QDsN) in Salmonella assay, consisting of CdSe/ZnS core-shell QDs encapsulated by a natural fusogenic lipid (1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and functionalized with a nucleolipid N-[5'-(2',3'-di-oleoyl)uridine]-N',N',N'-trimethylammonium tosylate (DOTAU) [50].
In addition, PCR analyses revealed upregulation of DNA damage and response genes, as well as, several proinflammatory cytokine genes. Furthermore, transcriptome sequencing showed that up-regulation of the metallothionein family of genes in cells exposed to MPA-CdSe QDs but not CYST-CdSe QDs [49].
In conclusion, from the genotoxicological point of view, it was reported that QDs exposure may produce some adverse effects, including DNA and chromosomal damage in the Comet, MN, and chromosomal aberration assays.
Other assays
The intracellular uptake of QDs could disturb the oxidative balance of the cell and cause oxidative stress. The production of ROS, such as superoxide, hydroxyl radicals, peroxide radicals, hydrogen peroxide, and singlet oxygen, can adversely affect cellular functions. Oxidative stress may cause mitochondrial malfunctions, as well as apoptosis. Furthermore high ROS could cause DNA fragmentation, breakage of the DNA double strand, and suppression of DNA functions, such as replication and transcription [39].
Expression of p53 is a good indicator of DNA damage. P53 is a tumour suppressor that regulates the cell cycle and is described as a "guardian of the genome". It prevents the conversion of damaged DNA to genome mutation [65]. Choi, et al. reported that uncoated CdTe QD activated the p53 genotoxic stress pathways and resulted in the up-regulated transcription of Puma (p53-upregulated modifier of apoptosis) and Noxa (NADPH oxidase activator 1), which are involved in apoptosis in human breast adenocarcinoma cells (MCF-7) [66]. Further results showed that chromatin reorganization was observed in CdTe QD-treated MCF-7cells, and chromatin condensation was associated with changes in the epigenome after QD insult. Global hypoacetylation of histones was observed in cells treated with CdTe QDs for 24 hrs. This was associated with decreased gene transcription, and suggests a global decrease in transcription, which could include anti-apoptotic genes. In this study, the most marked reduction of mRNA was found for GPx (glutathione peroxidase), which has a critical role in cell protection from CdTe QD-induced oxidative stress [66]. Anas, et al. and Green and Howman used the plasmid nicking assay to demonstrate that ZnS-coated QDs can also cause DNA breakdown and nucleobase damage due to the generation of free radicals both photogenerated and surface oxide generated [67,68].
In vivo genotoxicity of Qds
Reports indicated that different QDs display a range of oxidative damage and DNA effects on diverse organisms such as fruit fly and mice. QDs effects may vary depending on the test organism, evaluation parameter, particle characteristics, application duration, and environmental conditions. These in vivo genotoxicity results are summarized in Table 2.
DNA damage
Genotoxicity was evaluated by Aye, et al. in several organs of rats (brain, liver, kidneys, lungs and testicles) after intraperitoneal (I.P.) injection of CdSe/ZnS with DOPC/DOTAU (5.5 × 10-1, 5 × 10-2 and 5 × 10-3 g/kg concentrations) [69]. DNA damage in the different organs was measured by the Comet assay 24 hrs after the QD injection. Genotoxic effects were observed in the brain and liver, andonly at the highest QD concentrationin testicles. CdSe/ZnS with DOPC/DOTAU caused clastogenic effects, increasing the frequency of micronucleation in rat blood reticulocytes (MNRET) 24 hrs post exposure. No genotoxic effects were seen in the kidney or lungs of rats.
Another in vivo study [70], male mice were exposed to CdSe modified with mercaptoacetic acid (MAA) with or without doping with 1% cobalt ions. Mice were exposed intraperitoneally for two and seven days at concentrations of 500, 1000, and 2000 mg/kg, and genotoxic effects evaluated by MN. Results show that following two days exposure the high dose of doped MAA-QDs induced genotoxicity,with both doped and undoped MAA-QDs increasing DNA damage.
Alaraby, et al. showed in Drosophila, using the Comet assay, that both Cd QDs and CdCl2 showed a significant dose-dependent increase in DNA strand breaks regardless of their chemical composition, which suggested that the observed effects were related to the presence of Cd2+ ions [71]. Alaraby, et al. found that CdSe QDs were able to penetrate the intestinal barrier of the D. melanogaster larvae, reaching the hemolymph, interacting with hemocytes, and inducing concentration/time dependent significant genotoxic effects using the Comet assay [71]. Although a direct relationship between DNA damage and Cd2+ was observed, the underlying mechanism was not determined.
In digestive glands and gills of fresh water mussels (Elliption complanata), cadmium-telluride (CdTe) QDs, reduced DNA strand breaks in gills and digestive glands [57]. A transient but marginal increase in DNA strand breaks was noted at the lowest concentration of CdTe. Similarly, CdTe functionalized with carboxyl groups (-COOH) (10 μg/L) showed genotoxicity (with the micronucleus test and nuclear abnormalities assay) after a 14 days exposure in marine mussel Mytilus galloprovincialis [72]. The Comet assay indicated that soluble Cd was thecytotoxic and cytogenotoxic agent on Mytilus hemocytes.
In different model species, cytotoxicity and genotoxicity of QDs were shown to be dependent on route of exposure and duration of QDs.
Drosophila model
D. melanogaster as an in vivo organism has proved to be a useful model for detecting potential harmful effects of NMs [73-78]. Drosophila has already been successfully used to detect the toxic effect of CdSe/ZnS QDs [79]. Furthermore, Alaraby, et al. used D. melanogaster to detect toxic and genotoxic effects associated with CdSe QD exposure [71]. Additionally, indirect genotoxic effects of CdSe/ZnS QDs in Drosophila showed positive effects of CdCl2 in midgut cells by the tunnel assay [79,80].
Other assays
Gagné, et al. investigated the genotoxic effect (DNA strands breaks) and oxidative stress (lipid peroxidation) effects of cadmium-telluride (CdTe) QDs (0, 1.6, 4 and 8 mg/L) on freshwater mussels, Elliption complanata, in digestive glands and gills [57]. Results showed that lipid peroxidation was significantly increased at a threshold concentration of 5.6 mg/L in gills and significantly reduced in digestive glands at a threshold concentration < 1.6 mg/L CdTe. DNA strand breaks were significantly reduced in gills at < 1.6 mg/L CdTe. In digestive glands, a transient but marginal increase in DNA strand breaks occurred at the lowest concentration and dropped significantly at the higher concentrations [57]. On the other hand, immunotoxicity was revealed by a significant decrease in hemocyte viability.
Galeone, et al. studied CdSe-ZnS QDs with different surface coatings, namely mercaptoundecanoic acid (QD-MUA), polymer coating with poly-maleic anhydride octadecene (QD-PC) and polymer and polyethylene glycol (PEG) coating (QD-PC-PEG) in Drosophila hemocytes using the TUNEL assay (to highlight DNA damage) and Annexin V/PI assay (to analyze the presence of apoptotic and/or necrotic cells) after a 7-day feeding exposure [79]. They reported genotoxic effects and an increase in a apoptotic hemocytes. CdSe-ZnS QDs with different surface coatings affected the lifespan of treated Drosophila populations and induced a marked increase in reactive oxygen species (ROS). The authors suggested that genotoxic and toxic effects were the result of release of Cd2+ ions, and that coating the QDs can modulate their bioaccumulation in the organism, partly but not completely decreasing their overall toxicity.
An in vivo study was conducted with male mice using CdSe modified with mercaptoacetic acid (MAA) with or without doping with 1% cobalt ions. Mice were exposed for two and seven days at concentration of 500, 1000 and 2000 mg/kg and genotoxic effects evaluated by measurement of DNA oxidant damage (8-hydroxy-2-deoxyguanosine, 8-OHdG). Results show that following two days exposure the high dose of doped MAA-QDs induced genotoxicity. Also both doped and undoped MAA-QDs increased DNA damage for the highest concentration exposure. Investigators indicated that pure or doped MAA-QDs or MAA-QDs may cause genotoxicity in vivo by free radical-induced oxidative stress process [70].
To address the molecular response to QD exposure, Alaraby, et al. have monitored changes of expressionof different types of genes related to general stress, antioxidant protection and DNA damage response [71]. Previous studies with Drosophila demonstrated changes in gene expression in response to NPs exposure [81-83] and QDs [83]. Up-regulation of Hsp genes is important to counteract proteotoxic effects via chaperoning proteins during synthesis, folding, assembly and degradation and give preliminary information on the potential effects of exposure to foreign substances. Chang, et al. postulated that CdSe-core QD suppressed Hsp 90 in human cells and, accordingly, they have observed similar results related to expression of Hsp 70 and Hsp 83 in Drosophila [84]. Antioxidant capacity of cells is the first line of defence line to maintain oxidant/antioxidant balance. Drosophila showed an antioxidant response to the exposure to Cd QDs by significantly increasing expression of SOD mRNA, especially at the highest dose. This would agree with results obtained with CdSe QDs in solution [85] and in the epithelial A549 (human lung carcinoma) and the neuronal SH SY5Y (human neuroblastoma) cell lines [83]. In addition, Alaraby, et al. reported that CdCl2 induceded significant expression of catalase (CAT) at all doses of CdSe QDs. In contrast, SOD expression was inhibited [71]. The inhibition effect of cadmium on SOD might be due to protein conformation changes interfering with the enzyme [86].
Mechanisms of QD genotoxicity
Water-soluble, cysteine-capped CdSe (15 nm) DNA damage was observed at a concentration of 200 μg/mL of CdSe QDs [87]. Furthermore, these CdSe QDs induced aggregation of blood platelets in a dose dependent manner. Mercaptopropionic acid (MPA) or cysteamine (CYST) CdSe QDs were tested in primary normal human bronchial epithelial cells [49]. MPA-CdSe QDs induced a high number of DNA strand breaks but did not induced ROS production or cytotoxicity. In contrast, both cytotoxicity and genotoxicity of CdTe QDs in human umbilical vein endothelial cells (HUVECs) was associated with ROS production [88]. Nagy, et al. [49], assumed that DNA damage was driven largely by expression of pro-inflammation factors that promote DNA damage, while Wang, et al. [88] showed that CdTe-induced DNA damage was reduced, in the presence of an antioxidant molecule, namely N-acetyl-cysteine (NAC). They concluded that CdTe QD-induced cytotoxic and genotoxic effects were due to ROS generation. On the other hand, some NPs and QDs can bind to DNA strongly with high affinity, inhibit DNA replication, and cause genotoxic effects [89].
A study by Tang, et al. revealed the direct chemico-biological interactions between DNA and mercaptoacetic acid-coated CdSe core QDs (MAA-QDs) [90]. They used the plasmid transformation assay as a functional tool to determine the genotoxic damage caused by nano-engineered materials. After exposure with different concentrations of MAA-QDs (0.043, 0.13, 0.4, 1.2, and 3.6 μmol/L) in dark conditions, DNA conversion of the covalently closed circular (CCC) DNA to the open circular (OC) DNA was significantly enhanced (from 13.9% ± 2.2 to 59.9% ± 12.8), while the residual transformation activity of plasmid DNA was greatly decreased (from 80.7% ± 12.8 to 13.6% ± 0.8) in the plasmid transformation assay. Results indicate that the DNA structure and biological activities were affected by MAA-QDs in a concentration-dependent manner. Circular dichroism spectroscopy and transformation assay results suggested that the Cd-MAA complex may interact with DNA through the groove-binding mode and preferred binding to DNA fragments with high adenine and thymine content. According to the Tang, et al. changes in DNA configuration and biological activities were detected via agarose gel electrophoresis [90]. Genetic transformation and the in vitro interaction of the Cd-MAA complex with DNA was investigated by a quantitative analysis method. Tang, et al. reported that the Cd-MAA complex formed in the solution of MAA-coated CdSe QDs has an innate tendency to damage plasmids with a high AT content or an AT-rich region through a groove-binding mode, and therefore affect the biological activity of DNA [90].
According to Katsumiti, et al. in mussel hemocytes and gill cells, both ROS production and catalase (CAT) activity increased at lower Cd concentrations in three types of Cd (ionic Cd, bulk, and CdS QDs-5 nm) [56]. The lower dissolution of Cd from both CdS forms (lower availability of Cd ions) may result in a lower potency to promote oxidative stress. Results showed that CdS QDs and bulk CdS led to an increase in CAT activity in hemocytes but not in gill cells. This could be related to the fact that maximum ROS production occurred earlier in hemocytes than in gill cells or there is different antioxidant capacity in the two cell types, since it is well known that hemocytes play a vital role in the detoxification of metals through the sequestration and accumulation in the endolysosomal system [91,92]. Bulk CdS could affect both ROS production and CAT activity through the extracellular release of free Cd. Given the small size of QDs tested (5 nm), CdS QDs could affect ROS production and CAT activity not only by releasing extracellular Cd ions, but also by entering the cells through endocytic pathways and then interacting with different cell organelles (e.g. mitochondria and lysosomes) and/or by releasing intracellular free Cd ions. Indeed, Katsumiti, et al. showed that CdS QDs were found in the endolysosomal system of hemocytes [56].
Surface charge and coating material change the QDs effects. For example, the uptake and toxicity of negatively and positively charged CdSe/ZnS QDs of the same core size but with different surface chemistries (carboxyl or amine polymer coatings) were investigated in full and reduced serum containing media following 1 and 3 cell cycles and in three different cell lines namely, epithelial (BEAS-2B), fibroblast (HFF-1), and lymphoblastoid (TK6) cells [64]. Only minimal cytoplasmic ROS was detected, mainly in HFF-1 cells exposed to the carboxyl-QD and TK6 cells exposed to amine-QDs. No observable mitochondrial membrane permeability effects were seen in HFF-1 or TK6 cells exposed to any of the QDs in full serum conditions. In contrast, a significant and concentration related increase in MMP was found in TK6 and HFF-1 cells treated with carboxyl-QDs in reduced serum containing media. In this study, the potential inflammatory effects of the QDs were also evaluated by determining the release of either IL-8 or TNF-a by TK6, BEAS-2B, or HFF-1 cells exposed to amine- or carboxyl-QDs. The cells were exposed to the QDs for 24 hrs over a broad concentration range (0, 2.5, 5, 7.5, 10, or 15 nM), but no increase in IL-8 or TNF-a release could be observed for any QD type at any concentration. In the light of all these findings, researchers proposed that differences in cellular response to QD exposure could partly be due to the mechanisms by which they impart cellular stress [93,94]. However, Manshian, et al. showed that no significant induction of ROS was detected in any of the treatments of QDs [64]. These observations differ from those of Soenen, et al. where ROS induction was found after exposure to the same commercially available carboxyl-QDs [94]. This difference could be due to the very different cell types used (HUVEC, PC12, and C17.2 cells), possessing important differences in anti-oxidative capacity [94]. This differencescould also be due to differences in the assay used to determine ROS. It is well known that quantitative analysis of ROS can be affected by the intracellular presence of high levels of thiyl or sulfinyl radicals formed by glutathione, which can scavenge ROS [95]. Although genotoxic effects were seen in the TK6 cells following exposure to QDs, the limited induction of ROS in TK6 cells correlates with the fact that largely aneugenic responses were detected (as oxidative stress typically induces clastogenicity) [96]. Thus, the intrinsic homeostatic differences in different cell types could be of particular importance in understanding the potential toxicity imparted by specific NMs. On the other hand different factors, such as charge, exposure media, cell lines etc., could also be responsible for the different mechanistic processes underlying cellular damage. For instance, carboxyl-QDs induced a significant concentration-dependent increase in MMP, while amine-QDs did not appear to cause such a change. These effects were only detected in reduced serum condition in both HFF-1 and TK6 cells. Consequently, this could suggest a role of the protein corona, which might influence the uptake mechanics of these NPs [64]. Ju, et al. used 2',7'-dichlorofluorescin diacetate (DCFH-DA) to determine the role of ROS in the induction of DNA damage [51]. Cells were first incubated with a ROS scavenger, N-acetylcysteine (NAC), for 2 hrs followed by CdSe/ZnS QDs. Uncoated QDs caused significant γH2AX foci formation in a time and dose dependent manner and ROS generation initially increased following high dose treatment. According to this study, the lack of observed toxic effects from PEG-coated QDs may be due to the fact that PEG-coating can reduce ROS generation.
ROS, degradation of metal core, coatings and/or functionalization
According to Hardman [23], the toxicity of QDs is affect by their physicochemical properties and environmental conditions: charge, size, concentration, coating materials, stability, etc. For example, some QDs exhibit cytotoxic effectsafter degradation of their core material, while other QDs exhibittoxic effects due to their coating materials [23]. Biological applications of QDs are limited because they are insoluble in water. To overcome this problem, surface functionalizationis often employed to improve water solubility [97]. Water-soluble functional molecules (e.g. dithiothreitol, mercaptocarbonic acids, 2-aminoethanethiol, dihydrolipoic acid, oligomeric phosphines, peptides, and cross-linked dendrons) have been used as a coating material for improved water solubility by a ligand exchange reaction or encapsulation [98]. After solubilization, QDs may be functionalization via conjugation with different biological molecules (e.g.avidin, biotin, oligonucleotides, peptides, antibodies, DNA and albumin) to recognise specific biological targets [99]. Some functional groups can also be added to QDs to make them water soluble. Thus, the toxic and genotoxic effects of QDs can be altered by such coatings. For example, polyethilene glycol (PEG) tends to decrease the toxicity of QDs. However, some functional groups or coating layers may also be cytotoxic or genotoxic. Thus careful consideration should be given when selecting these modifications. In addition, purification of the material is an important factor in its toxicity, as crude QDs coated with carboxyl groups prepared only by membrane filtration caused greater DNA damage than purified QD-COOH [100].
The toxicity and genotoxicity of QDs may derive from their intrinsic properties, such as size and surface chemistry. More importantly, since QDs are efficient energy donors [101,102], they can transfer energy to nearby oxygen molecules, and induce the generation of ROS, which in turn leads to cell damage or death [103]. QD induction of ROS has also been frequently reported in different studies. ROS have been shown to damage cells by peroxidizing lipids, altering proteins, disrupting DNA, interfering with signalling functions, and modulating gene transcription [104,105]. Mechanisms proposed for QD-induced ROS include the reactivity of surface-located transition metals [106], leaching of free Cd2+ ions [107], and direct interaction of QDs with mitochondria [106,108]. Release of Cd2+ ions is generally considered to be a main cause of QD-induced cellular toxicity. Therefore, the use of stabilizing coatings, passivating shell layers, and the development of cadmium-free QDs are all being investigated in order to reduce Cd2+ release and thereby promote "safer by design" QDs. On the other hand, QDs can be activatedby lighttogenerate ROS, which can cause cytotoxicity due to photo-oxidative processes. For instance, Tang, et al. found that unmodified CdSe QDs increased ROS production, which led to elevated cytoplasmic calcium levels [109]. Another study showed that cadmium selenide (CdSe) QDs generates singlet oxygen in vitro [110]. The mechanisms by which ROS may be generated by QDs can be summarized by analogy with the well-known Type 1 and 2 classifications of organic photosensitizers [111,112]. In Type 1 photo-oxidative or photo-reductive pathways, photo-induced electron transfer reactions take place which generate intermediates, including ROS such as superoxide radical anions. In Type 2 reactions, energy transfer takes place directly with molecular oxygen generating highly reactive singlet oxygen (1O2) [113,114]. Photo-induced formation of ROS by QDs in solutions and phototoxic effects on cells, have been reported using a range of techniques, although contrasting results have been observed. Ipe, et al. carried out studies on free radical generation in aqueous solutions by photo-irradiation of CdS, CdSe and CdSe/ZnS QDs [85]. Using electron paramagnetic resonance (EPR) spectroscopy and a fluorometric assay, they showed that photo-activated CdS and CdSe QDs stabilized with mercaptoacetic acid ligands generated hydroxyl and superoxide radicals, whereas under the same conditions ROS generation using CdSe/ZnS QDs was not detectable. Green and Howman reported that nicking of double-stranded DNA in the presence of CdSe/ZnS QDs after illumination induced the generation of free radicals, as evidenced by electron spin resonance (ESR) [68]. However, the authors failed to assign the observed ESR signals to a specific radical species. Clarke, et al. reported that UV irradiation of QD-dopamine conjugates (QDs: CdSe/ZnS) resulted in DNA damage of cells due to electron transfer from QDs to dopamine followed by oxidation of dopamine [115]. Similarly, Liang, et al. demonstrated calf thymus DNA (ctDNA) damage with water soluble CdSe QDs [116], and Rajendran, et al. showed that protein-conjugated CdS QDs under UV illumination generated significant amounts of hydrogen peroxide [117]. Anas, et al. described photo-sensitized damage of plasmid DNA using CdSe/ZnS QDs with various capping layers [67]. Cho, et al. showed that exposure of MCF-7 (breast adenocarcinoma) cells to CdTe QDs decreased cellular metabolic activity due to the presence of Cd2+ ions and resultant generation of ROS [118]. Some studies showed phototoxic effects on cells treated with mercaptopropionic acid-capped CdSe/ZnS QDs and InP QDs [119,120]. In a systematic study using a range of techniques including ESR, they found evidence for superoxide generation but not singlet oxygen (1O2). Using CdSe/ZnS QDs conjugated with dopamine, enhanced ROS generation was observed. In addition, the potential of CdSe/ZnS QDs to participate in Type 1 photochemical processesand generate superoxide was shown by oxygen consumption [121]. QD toxicity can be altered by surface modifications [100,122], suggesting that reactive surfaces may play a role in QD-induced cytotoxicity. PEG-coated CdSe/ZnS QDs are widely used in biomedical applications for in vitro and in vivo labeling, since PEG confers water solubility and minimizes uptake by organs of the reticulo endothelial system. PEG coating tend to decrease QD-induced ROS production. Some reports show report survival of cells loaded with QDs for weeks without alteration of cell growth and division [123]. However, other studies indicate that high concentrations of QDs can impair embryonic development [124]. It has been shown that exposure of the CdSe core to oxidative environments causes the decomposition and release of Cd2+ [33,122], a well known toxic ion [34]. Qu, et al. demonstrated that QDs could be readily engulfed by macrophages, resulting in intracellular ROS generation. PEG-NH2 coated QDs had a greater capability to enter the J774A.1 cells and decrease cell proliferation [125]. In addition, polymer coated QDs have been shown to accumulate in mouse bone marrow, spleen, and liver for at least 4 months after systemic administration [126]. To further search for the mechanism responsible for the cytotoxicity caused by QD-PEG-NH2 particles, Qu and colleagues examined the intracellular localization of QDs [125].
Drosophila hemocytes have been used as amodel to demonstrate oxidative stress induction [76], as well as innate immune response and DNA damage in vivo [127-129]. For this reason, hemocytes have been used to evaluate the effects of different chemicals in many studies. Alaraby, et al. showed that Cd-containing QDs increased ROS production in Drosophila hemocytes although to a lesser extent than after exposure to CdCl2 [71]. It has been proposed that changes in intracellular ROS levels may be due to the semiconducting properties of CdSe used and involvegap and valence/conduction band energy levels [130]. Further, the numbers of atomson the QD surface increases rapidlywith decreasing particle size, leading to an increase of dangling bonds that are prone to combine with other atoms to become saturated and stable; hence, the surface of QDscan behighlychemical reactive [131]. Generally, the toxic effects of QDs in Drosophila have beenrelated to an elevationof ROS [79]. ROS induction by QDs in human cultured cells has been related to the nature of the included ligands and the presence of free Cd2+ [132]. The proposal that ROS is free Cd2+ dependent is supported by several studies [133-135]. Generation of excessive ROS would overwhelm the antioxidant defense system and shift the redox balance of the cell. The resultant oxidation could damage cellular biomolecules, such as DNA, leading to heritable mutations [136]. For instance, the chemical modification of histones (or binding proteins that support the supercoiled structure of DNA) opens the coiled DNA and allows its alteration [137], indirectly resulting in genetic damage.
Many QD preparations produced characteristic signs of Cd toxicity that were weakly correlated with metallothionein (MT) expression, suggesting that QDs were slightly degraded in vivo. QDs are based on heavy metals, such as cadmium, zinc, and lead [101]. The toxic effects of QDs are known to be related to the release of Cd ions from the QDs [138-140]. Cd2+ ions have been shown to induce toxicity in zebrafish larvae. Indeed, using MT gene induction as an indicator of Cd2+ release, these studies were able to detect breakdown of QDs after absorption by the larvae. Zhang, et al. reported the effects of joint exposure of zebrafish embryos and larvae to cadmium selenide (CdSe) QDs and copper ion (Cu2+) [141]. Their findings revealed that QDs facilitated the accumulation of copper ions in zebrafish. QDs caused higher mortality, lower hatch rate, and more malformations. In addition,embryo cell apoptosis occurred in the head and tail region and indicated a synergistic effect of joint exposure to CdSe QDs and copper ions. Cd QDs continually biodegrade and, as consequence, free Cd2+ is continuously released and the size of the QDs becomes smaller during the degradation process [142,143]. As consequence the fluorescence emission shifts from red to blue and the excitation fluorescence peak becomes broader [144].These results were verified by Alaraby, et al. who reported aslight shift from blue to red and broadening of the fluorescence peak upon internalization of Cd QDs, indicating their biodegradation [71]. QDs may be degraded after several days within living cells [28]. This degradation maybe due to low pH or oxidation of QD surface [70]. In a study, where QDs were administered orally to Drosophila larvae, transit through the low pH conditions of the gastric tract can contribute to QD degradation [71].
QDs effects on gene products and other cellular structures
High doses of Cd can affect the repair of oxidatively damaged DNA by SOD-dependentdown-regulation of p53 activity [86]. Studies by Son, et al. and Xu, et al. indicate that exposure of neuronal and epithelial cells to Cd increased the levels of ROS and SOD [134,137]. Alaraby, et al. later found that CdSe QDs had a similar effect in vivo [71]. P53 plays a key role in cellular genotoxic stress response by acting as a transcription factor to elicit cellular functions of DNA repair, cell cycle arrest, and apoptosis. P53 is normally accumulated in the nucleus and converted into an active DNA-binding form to control several sets of genes, which prevent the proliferation of DNA-damaged cells [145]. Over-expression of p53 has been observed after Drosophila larva exposure to Cd QDs [71,83]. In contrast Cd2+ exposure of human breast MCF-7 cancer cellsresulted in impaired p53 functions, such as conformational changes, loss of DNA binding activity, down regulation of transcriptional activity, and inhibition of gamma radiation induced responses [146]. It appears that expression of p53 might depend on the level of free Cd2+, which would differ between Cd QDs and CdCl2 exposure. On the other Alaraby, et al. reported in Drosophila a significant dose-dependentincrease in DNA strand breaks related to the presence of Cd2+ ions [71]. However, the underlying mechanism was not clear. Cadmium has been demonstrated to amplify the intensity of damage by interfering with the DNA repair NER pathway [147]. Further, single strand breaks in DNA may involvedirect cadmium-DNA interactions, as well as the action of incision nucleases and/or DNA-glycosylase during DNA repair [56]. As noted in earlier sections, although genotoxic effects were seen in the TK6 cells following exposure to QDs, the limited induction of ROS in TK6 cells correlates with the fact that largely aneugenic responses were detected (as oxidative stress typically induces clastogenicity) [96]. So the intrinsic homeostatic differences in variouscell types could be of particular importance in understanding the possible toxicity imparted by specific NMs. Other factors that may play a role in QD induced toxicity in this study include QD surface charge, exposure media composition, serum content, and exposure duration. For example, HFF-1 cells demonstrated no cytotoxicity following exposure to amine QDs; yet significant cell death was observed when the cells were exposed to high concentrations of carboxyl-QDs for 3 cell cycles [64].
Agglomeration
QDs may agglomerate or aggregate, when they are injected into the body. In general, agglomeration indicates more loosely bound QDs and aggregation suggests tightly bound QDs (typically occurring during encapsulation of QDs within a polymer micelle). QDs might agglomerate due to the high ionic strength of blood, which shields the repulsion between the charges of QDs. The QD community has not devoted much attention to agglomeration of QDs in vivo, even though agglomeration would be expected to affect nanotoxicity by changing the size, surface area, and sedimentation properties of the QDs. Many QD formulations may agglomerate in the body before they reach their desired targets, but this has not been clearly demonstrated [30]. Carboxyl- and amine-QDs with a similar core size demonstrated different degrees of agglomeration according to their surface chemistry. Amine-QDs were found to agglomerate into large structures, whereas the carboxyl-QD agglomerates were smaller. The degree of agglomeration also depended largely on the nature of the cell culture media and the amount of serum present. These differences in agglomeration appear to lead to asignificant change in the resultant cellular interactions. The high internalization of carboxyl-QDs also resulted in greater induction of genotoxicity, oxidative stress, and mitochondrial damage [64].
In conclusion, QD-induced cytotoxicity and genotoxicity are strongly affected by a multitude of parameters for example: differences in cell type or organisms, the nature of the QD surface chemistry orproperties including, shell, core and coating material features, the degree of QD agglomeration in media of various compositions, andtime of exposure. In the literature, these factors influence the degree of agglomeration and sedimentation of the QDs in media or biological system andinfluenceinteraction with the target cells. The latter translates itself in differences in cytotoxicity and genotoxicity that do not always directly correlate with the quantity of internalized material, but are also strongly influenced by the intrinsic cellular capacity for handling internalized foreign material, which is cell type dependent. To make the QDs water-soluble different coating materials have been used. This sometimes makes QDs more or less toxic and genotoxic [e.g.reduced QDs toxicity involved coating their surface with biocompatible molecules such as polyethylene glycol (PEG)]. Also the size of QDs can determine the biological fate [148]. Studies with different sizes of QDs have shownthat thecytotoxicity of QDs was reduced by increasing particle size [34]. There are many factors that influence the distribution of QDs in tissue/organ, such as QD size, QD core-shell materials, and the bioactivity of conjugated or coated functional groups.
Future Perspectives
Over time, more information has become available regarding the toxicity and genotoxicity of QDs. However, knowledge gaps remain and additional studies are still required to truly advance our knowledge in this area, especially in the case of in vivo applications where further research is required to critically evaluate risk. Each type of QD has its own unique physicochemical properties, which determine potential toxicity. Along with composition, the physical and surface characteristics of the QDs play a major role in determining toxicity. These characteristics include size, shape, surface charge, and surface coverage (coatings, chemically conjugated molecules). For this reason detailed characterization of QDs must be conducted. A toxicity study performed with larger-size semiconductor particles cannot be used to assess the toxicity of QDs, since size likely affects the toxicity of QDs. Similarly, the surface charge of the QDs has a pronounced impact on toxicity. Furthermore, some QDs have been found to cytotoxic only after oxidative and/or photolytic degradation of their core [149]. QD dosages and concentrations reported in the literature vary in their units (e.g., mg/mL, M, mg/kg, or number of QDs per cell), and correlating dosage across current studies is usually difficult and sometimes impossible. As mentioned above, the majority of the mass of a QD formulation is often made up of the organic molecules encapsulating the inorganic nanocrystal. However, dosages and concentrations are often reported in terms of the total mass, including the organic component [30].
Another, important field involves the ecological effects of different QDs. For instance, Werlin, et al. revealed that QDs can be transferred from prey to predator in a typical food chain [150]. Furthermore, the QDs were shown to be bioaccumulated resulting in higher concentrations within organisms then in the environment. CdSe core, ZnS shell, and polymer layer (COO-) QDs accumulate in organisms and may be delivered to other organisms via the food chain. Therefore, QDs may be released to the environment and have the potential to cause risks to humans and the ecosystem. A study using Astasia longa (protozoa), Moina macrocopa (cladoceran), and Danio rerio (fish) as model organisms, representing an aquatic food chain, showed a three-level trophic transfer of QDs in an aquatic environment [151]. In another study, Zhang, et al. reported the effects of joint exposure to Cd-Se QDs and copper ion (Cu2+) on zebrafish embryo and larvae [141]. Results show toxic effects. Different QDs are emitted into the environment and they can interact with different metals or chemical compounds. For this reason in the future, different combinations of the QDs and different metals or chemicals should be evaluated to obtain more realistic exposure schemes for QDs in the environment and determine their effects on different species.
Mammalian exposures to QDs should include cardiovascular (eg. blood pressure, heart rate and electro cardiography), respiratory and other organ system measurements. Various route of exposure and fate within the animal model should be investigated. For example, Yong, et al. injected QDs into monkeys and reported neurotoxicity [30]. Also, some researchers suggest that specific immune histochemistry assays can be used to assess hepatotoxicity even though hepatic histopathological evaluation may not observe an inflammatory response. Genetic analysis, such as real-time polymerase chain reaction (q-RTPCR), can be performed on the tissue samples to investigate sub-clinical alterations in inflammatory gene regulation. The QD research community should investigate the impact of QD formulations and exposure route on translocation of QDs to various tissues. The translocation-induced toxicity can occur through different mechanisms. An example is the translocation of QDs into the brain. If QDs with appropriate ligands on their surface are introduced into blood circulation by i.v injection, they may cross the brain blood barrier (BBB) and enter the brain. The ability to cross the BBB can be due tothe small size or PEG coatingof QDs, or due BBB leakage resulting from interaction with QDs. Neuronal cells in the central nervous system are sensitive to stresses, because of their extensive, very thin, and fragile extensions, and they are also very sensitive to oxidant stress. Any inflammatory response developed by the QDs can cause neurological disorders. On the other hand, pulmonary toxicity by will be most pronounced when the NPs are introduced by instillation or inhalation within the respiratory tract. This may be more important for workplace exposures resulting from preparation of large quantities of NMs as powders that can be aerosolized during handling [30]. For these reasons, animal studies should evaluate the deposition, translocation, and clearance of QDs following various routes of exposure. Effects of physicochemical properties of the QDs and coatings should be evaluated.
To date the mechanistic links between elevated ROS levels and toxicity or genotoxicity are not fully defined. It is important to further investigate endpoints and signaling pathways influenced by ROS induction. Parameters, such as mitochondrial metabolism (using fluorescent probes such as JC-1), lipid or protein peroxidation, cytoplasmic calcium levels, cytoplasmic redox state (by measuring glutathione levels), DNA defects with different assays, or signaling pathways for control of proliferation or apoptosis should be studied to shed light on possible mechanisms inducing toxic and genotoxic effects of QDs [152].
Results suggest that the genotoxic potential of QDs is dependent upon their physicochemical characteristics, such as core size, shell or cap material size and properties. Effort should be made to relate the genotoxic potency of QDs to specific physicochemical properties by careful modifying a given property at a time. Effort should also be made to systematically evaluate how test conditions, such as serum content of media, cell type, or agglomeration state affect in vitro results [64].
With in vivo studies, it is important to determine differences in QD deposition, fate, clearance, and effect after exposure by different routes, such as i.v, i.p, inhalation, or oral. To what degree do responses in Drosophila inform results of rats or mice?
In summary, it is therefore important that future studies focus on investigating the genotoxicity of a range of QDs in parallel, assessing several DNA damage endpoints to determine the true impact of specific physicochemical features and elucidate the mechanisms of genotoxicity of QDs. To date most studies have been based on acute in vitro and in vivo genotoxicity assays.There is a need to conduct extended in vivo carcinogenicity investigations in the future. It is known that substances which induce genotoxicity are possible carcinogens, but this needs to be confirmed in appropriate studies for QDs. Thus, carcinogenicity assessments are now required to determine if their genotoxic impact is sufficient to initiate tumorigenesis and to establish the long-term health effects following exposure to QDs.
In conclusion, there is no doubt about the benefits that the use of quantum dots provides to medical and pharmaceutical sciences, which can be described as revolutionary. However, since these new materials contain heavy metals, a deep knowledge of their potential harmful effects is urgently required to allow the safe application of these unique particles.
Declaration of Interest Statement
The authors declare that there is no conflict of interest.
References
- Niemeyer CM (2001) Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science. Angew Chem Int Ed 40: 4128-4158.
- Weng J, Ren J (2006) Luminescent quantum dots: a very attractive and promising tool in biomedicine. Curr Med Chem 13: 897-909.
- Rizvi SB, Ghaderi S, Keshtgar M, et al. (2010) Semiconductor quantum dots as fluorescent probes for in vitro and in vivo bio-molecular and cellular imaging. Nano Rev 1: 5161-5176.
- Jiang Z, Li R, Todd NW, et al. (2007) Detecting genomic aberrations by fluorescence in situ hybridization with quantum dots-labeled probes. J Nanosci Nanotechnol 7: 4254-4259.
- Pinaud F, Michalet X, Iyer G, et al. (2009) Dynamic partitioning of a glycosylphosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking. Traffic 10: 691-712.
- Kim S, Lim YT, Soltesz EG, et al. (2004) Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol 22: 93-97.
- Kim J, Park Y, Yoon TH, et al. (2010) Phototoxicity of CdSe/ ZnSe quantum dots with surface coatings of 3-mercaptopropionic acid or tri-n-octylphosphine oxide/gum arabic in Daphnia magna under environmentally relevant UV-B light. Aquat Toxicol 97: 116-124.
- Akerman ME, Chan WC, Laakkonen P, et al. (2002) Nanocrystal targeting in vivo. Proc Natl Acad Sci U S A 99: 12617-12621.
- Chen Z, Chen H, Meng H, et al. (2008) Biodistribution and metabolic paths of silica coated CdSeS quantum dots. Toxicol Appl Pharmacol 230: 364-371.
- Cuenca AG, Jiang H, Hochwald SN, et al. (2006) Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer 107: 459-466.
- Iga AM, Robertson JH, Winslet MC, et al. (2007) Clinical potential of quantum dots. J Biomed Biotechnol 2007: 76087.
- Hild WA, Breunig M, Goepferich A (2008) Quantum dots-nano-sized probes for the exploration of cellular and intracellular targeting. Eur J Pharm Biopharm 68: 153-168.
- Juzenas P, Chen W, Sun YP, et al. (2008) Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev 60: 1600-1614.
- Cui D, Pan B, Zhang H, et al. (2008) Self-assembly of quantum dots and carbon nanotubes for ultrasensitive DNA and antigen detection. Anal Chem 80: 7996-8001.
- Oostendorp M, Douma K, Hackeng TM, et al. (2010) Gadolinium-labeled quantum dots for molecular magnetic resonance imaging: R1 versus R2 mapping. Magn Reson Med 64: 291-298.
- Ray S, Reddy PJ, Choudhary S, et al. (2011) Emerging nanoproteomics approaches for disease biomarker detection: A current perspective. J Proteomics 74: 2660-2681.
- Tallury P, Malhotra A, Byrne LM, et al. (2010) Nanobioimaging and sensing of infectious diseases. Adv Drug Deliv Rev 62: 424-437.
- Zhang CY, Yeh HC, Kuroki MT, et al. (2005) Single-quantum-dot-based DNA nanosensor. Nat Mater 4: 826-831.
- Chan P, Yuen T, Ruf F, et al. (2005) Method for multiplex cellular detection of mRNAs using quantum dot fluorescent in situ hybridization. Nucleic Acids Res 33: e161.
- Bruchez MP (2005) Turning all the lights on: quantum dots in cellular assays. Curr Opin Chem Biol 9: 533-537.
- Wu D, Chen Z (2012) ZnS quantum dots as pH probes for study of enzyme reaction kinetics. Enzyme Microb Technol 51: 47-52.
- Pathak S, Cao E, Davidson MC, et al. (2006) Quantum dot applications to neuroscience: new tools for probing neurons and glia. J Neurosci 26: 1893-1895.
- Hardman R (2006) A Toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ Health Perspect 114: 165-172.
- Bruchez M Jr, Moronne M, Gin P, et al. (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281: 2013-2016.
- Chan WC, Maxwell DJ, Gao X, et al. (2002) Luminescent quantum dots for multiplexed biological detection and imaging. Curr Opin Biotechnol 13: 40-46.
- Zhou M, Ghosh I (2007) Quantum dots and peptides: a bright future together. Biopolymers 88: 325-339.
- Jamieson T, Bakhshi R, Petrova D, et al. (2007) Biological applications of quantum dots. Biomaterials 28: 4717-4732.
- Aillon KL, Xie Y, El-Gendy N, et al. (2009) Effects of nanomaterial physicochemical properties on In vivo toxicity. Adv Drug Deliv Rev 61: 457-466.
- Rzigalinski BA, Strobl JS (2009) Cadmium-containing nanoparticles: perspectives on pharmacology and toxicology of quantum dots. Toxicol Appl Pharmacol 238: 280-288.
- Yong KT, Law WC, Hu R, et al. (2013) Nanotoxicity assessment of quantum dots: from cellular to primate studies. Chem Soc Rev 42: 1236-1250.
- Navarro DA, Bisson MA, Aga DS (2012) Investigating uptake of water-dispersible CdSe/ZnS quantum dot nanoparticles by Arabidopsis thaliana plants. J Hazard Mater 211-212: 427-435.
- Yan M, Zhang Y, Xu K, et al. (2011) An in vitro study of vascular endothelial toxicity of CdTe quantum dots. Toxicology 282: 94-103.
- Kirchner C, Liedl T, Kudera S, et al. (2005) Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett 5: 331-338.
- Lovrić J, Bazzi, HS, Cuie Y, et al. (2005) Differences in subcellular distribution and toxicity of green and red emitting CdTe quantum dots. J Mol Med 83: 377-385.
- Yang RS, Chang LW, Wu JP, et al. (2007) Persistent tissue kinetics and redistribution of nanoparticles, quantum dot 705, in mice: ICP-MS quantitative assessment. Environ Health Perspect 115: 1339-1343.
- Ates M, Arslan Z, Farah I (2011) Assessment of accumulation and toxicity of cadmium selenide nanoparticles (CdSe NPs) on mice in vivo. Eighth International Symposium on Recent Advances in Environmental Health Research, USA,12.
- Schins RP, Hei TK (2006) Genotoxic Effects of Particles. In: Donaldson K and Borm P, Particle toxicology. CRC Press/Taylor & Francis Group, USA, 285-298.
- Schins RP, Knaapen AM (2007) Genotoxicity of poorly soluble particles. Inhal Toxicol 19: 189-198.
- Akhavan O, Hashemi E, Zare H, et al. (2016) Influence of heavy nanocrystals on spermatozoa and fertility of mammals. Mater Sci Eng C Mater Biol Appl 69: 52-59.
- Almeida C, Pereira C, Gomes T, et al. (2011) DNA damage as abiomarker of genotoxic contamination in Mytilus galloprovincialis from the south coast of Portugal. J Environ Monit 13: 2559-2567.
- Bolognesi C, Fenech M (2012) Mussel micronucleus cytome assay. Nat Protoc 7: 1125-1137.
- Mirsalis JC, Monforte JA, Winegar RA (1995) Transgenic animal models for detecting of in vivo mutations. Annu Rev Pharmacol Toxicol 35: 145-164.
- Dronkert ML, Beverloo HB, Johnson RD, et al. (2000) Mouse RAD54 affects DNA double-strand break repair and sister chromatid exchange. Mol Cell Biol 20: 3147-3156.
- Dudaš A, Chovanec M (2004) DNA double-strand break repair by homologous recombination. Mutat Res 566: 131-167.
- Wesoly J, Agarwal S, Sigurdsson S, et al. (2006) Differential contributions of mammalian Rad54 paralogs to recombination, DNA damage repair, and meiosis. Mol Cell Biol 26: 976-989.
- De Flora S, Izzotti A (2007) Mutagenesis and cardiovascular diseases Molecular mechanisms, risk factors, and protective factors. Mutat Res 621: 5-17.
- Da Silva RMG, De Sousa NC, Graf U, et al. (2008) Antigenotoxic effects of Mandevilla veluntina (Gentianales, Apocynaceae) crude extract on cyclophosphamide-induced micronuclei in Swiss mice and urethane-induced somatic mutation and recombination in Drosophila melanogaster. Genet Mol Biol 31: 751-758.
- Nagarathna PKM, Johnson Wesley M, Sriram Reddy P, et al. (2013) Review on Genotoxicity, its Molecular Mechanisms and Prevention. Int J Pharm Sci Rev Res 22: 236-243.
- Nagy A, Hollingsworth JA, Hu B, et al. (2013) Functionalization-dependent induction of cellular survival pathways by CdSe quantum dots in primary normal human bronchial epithelial cells. ACS Nano 7: 8397-8411.
- Aye M, Di Giorgio C, Berque-Bestel I, et al. (2013) Genotoxic and mutagenic effects of lipid-coated CdSe/ZnS quantum dots. Mutat Res 750: 129-138.
- Ju L, Zhang G, Zhang C, et al. (2013) Quantum dot-related genotoxicity perturbation can be attenuated by PEG encapsulation. Mutat Res 753: 54-64.
- Pathakoti K, Hwang HM, Xu H, et al. (2013) In vitro cytotoxicity of CdSe/ZnS quantum dots with different surface coatings to human keratinocytes HaCaT cells. J Environ Sci 25: 163-171.
- Munari M, Sturve J, Frenzilli G, et al. (2014) Genotoxic effects of CdS quantum dots and Ag2S nanoparticles in fish cell lines (RTG-2). Mutat Res Genet Toxicol Environ Mutagen 775-776: 89-93.
- Kondoh M, Ogasawara S, Araragi S, et al. (2001) Cytochrome c release from mitochondria induced by cadmium. J Health Sci 47: 78-82.
- Tice RR, Agurell E, Anderson D, et al. (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35: 206-221.
- Katsumiti A, Gilliland D, Arostegui I, et al. (2014) Cytotoxicity and cellularmechanisms involved in the toxicity of CdS quantum dots in hemocytes and gillcells of the mussel Mytilus galloprovincialis. Aquat Toxicol 153: 39-52.
- Gagné F, Auclair J, Turcotte P, et al. (2008) Ecotoxicity of CdTe quantum dots to freshwater mussels: Impacts on immune system, oxidative stress and genotoxicity. Aquat Toxicol 86: 333-340.
- Privezentsev KV, Sirota NP, Gaziev AI (1996) The genotoxic effects of cadmium studied in vivo. Tsitol Genet 30: 45-51.
- Choksi KB, Boylston WH, Rabek JP, et al. (2004) Oxidatively damaged proteins of heart mitochondrial electron transport com-plexes. Biochimica et Biophysica Acta 1688: 95-101.
- Murphy MP (2006) Induction of mitochondrial ROS production by electrophiliclipids: a new pathway of redox signaling. Am J Physiol Heart Circ Physiol 290: 1754-1755.
- Hart BA, Lee CH, Shukla GS, et al. (1999) Characterization of cadmium-induced apoptosis in rat lung epithelial cells: evidence for the participation of oxidant stress. Toxicology 133: 43-58.
- Rikans LE, Yamano T (2000) Mechanisms of cadmium-mediated acute hepatotoxicity. J Biochem Mol Toxicol 14: 110-117.
- Stohs SJ, Bagchi D, Hassoun E, et al. (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Oncol 19: 201-213.
- Manshian BB, Soenen SJ, Al-Ali A, et al. (2015) Cell type-dependent changes in CdSe/ZnS quantum dot uptake and toxic endpoints. Toxicol Sci 144: 246-258.
- Lane DP (1992) Cancer. p53, guardian of the genome. Nature 358: 15-16.
- Choi AO, Brown SE, Szyf M, et al. (2008) Quantum dot-induced epigenetic and genotoxic changes in human breast cancer cells. J Mol Med 86: 291-302.
- Anas A, Akita H, Harashima H, et al. (2008) Photosensitized breakage and damage of DNA by CdSe-ZnS quantum dots. J Phys Chem B 112: 10005-10011.
- Green M, Howman E (2005) Semiconductor quantum dots and free radical induced DNA nicking. Chem Commun 7: 121-123.
- Aye M, Di Giorgio C, Mekaouche M, et al. (2013) Genotoxicity of intraperitoneal injection of lipoamphiphile CdSe/ZnS quantum dots in rats. Mutat Res 758: 48-55.
- Khalil WK, Girgis E, Emam AN, et al. (2011) Genotoxicity evaluation of nanomaterials: Dna damage, micronuclei, and 8-hyroxy-2deoxyguanosine induced by magnetic doped CdSe quantum dots in male mice. Chem Res Toxicol 24: 640-650.
- Alaraby M, Demir E, Hernández A, et al. (2015) Assessing potential harmful effects of CdSe quantum dots by using Drosophila melanogaster as in vivo model. Sci Total Environ 530-531: 66-75.
- Rocha TL, Gomes T, Cardoso C, et al. (2014) Immunocytotoxicity, cytogenotoxicity and genotoxicity of cadmium-based quantum dots in the marine mussel Mytilus galloprovincialis. Mar Environ Res 101: 29-37.
- Pompa PP, Vecchio G, Galeone A, et al. (2011) Physical assessment of toxicology at nanoscale: nano dose-metrics and toxicity factor. Nanoscale 3: 2889-2897.
- Pompa PP, Vecchio G, Galeone A, et al. (2011) In vivo toxicity assessment of gold nanoparticles in Drosophila melanogaster. Nano Research 4: 405-413.
- Demir E, Turna F, Vales G, et al. (2013) In vivo genotoxicity assessment of titanium, zirconium and aluminium nanoparticles, and their microparticulated forms, in Drosophila. Chemosphere 93: 2304-2310.
- Alaraby M, Hernández A, Annangi B, et al. (2015) Antioxidant and antigenotoxic properties of CeO2 NPs and cerium sulphate: studies with Drosophila melanogaster as a promising in vivo model. Nanotoxicology 9: 749-759.
- Ong C, Yun LY, Cai Y, et al. (2015) Drosophila melanogaster as a model organism to study nanotoxicity. Nanotoxicology 9: 396-403.
- Vecchio G (2015) A fruit fly in the nanoworld: once again Drosophila contributes to environment and human health. Nanotoxicology 9: 135-137.
- Galeone A, Vecchio G, Malvindi MA, et al. (2012) In vivo assessment of CdSe-ZnS quantum dots: coating dependent bioaccumulation and genotoxicity. Nanoscale 4: 6401-6407.
- Shukla AK, Pragya P, Chowdhuri DK (2011) A modified alkaline comet assay for in vivo detection of oxidative DNA damage in Drosophila melanogaster. Mutat Res 726: 222-226.
- Ahamed M, Posgai R, Gore TJ, et al. (2010) Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol Appl Pharmacol 242: 263-269.
- Siddique YH, Khan W, Khanam S (2014) Toxic potential of synthesized graphene zinc oxide nanocomposite in the third instar larvae of transgenic Drosophila melanogaster (hsp70-lacZ) Bg9. Biomed Res Int 2014: 382124.
- Brunetti V, Chibli H, Fiammengo R, et al. (2013) InP/ZnS as a safer alternative to CdSe/ZnS core/shell quantum dots: in vitro and in vivo toxicity assessment. Nanoscale 5: 307-317.
- Chang E, Thekkek N, Yu WW, et al. (2006) Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small 2: 1412-1417.
- Ipe BI, Lehnig M, Niemeyer CM (2005) On the generation of free radical species from quantum dots. Small 1: 706-709.
- Casalino E, Calzaretti G, Sblano C, et al. (2002) Molecular inhibitory mechanismsof antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 179: 37-50.
- Dunpall R, Nejo AA, Pullabhotla VS, et al. (2012) An in vitro assessment of the interaction of cadmium selenide quantum dots with DNA, iron, and blood platelets. IUBMB Life 64: 995-1002.
- Wang L, Zhang J, Zheng Y, et al. (2010) Bioeffects of CdTe quantum dots on human umbilical vein endothelial cells. J Nanosci Nanotechnol 10: 8591-8596.
- Li K, Zhao X, K Hammer B, et al. (2013) Nanoparticles inhibit DNA replication by binding to DNA: Modeling and experimental validations. ACS Nano 7: 9664-9674.
- Tang W, Fan J, He Y, et al. (2012) The cadmium-mercaptoacetic acid complex contributes to the genotoxicity of mercaptoacetic acid-coated CdSe-core quantum dots. Int J Nanomedicine 7: 2631-2640.
- Marigómez I, Soto M, Cajaraville MP (1995) Morphofunctional patterns of cell and tissue system involved in metal handling and metabolism. In: Cajaraville MP, Cell Biology in Environmental Toxicology. University of the Basque Country Press, Bilbao, 89-134.
- Marigómez I, Soto M, Cajaraville MP, et al. (2002) Cellular and subcellular distribution of metals in molluscs. Microsc Res Tech 56: 358-392.
- Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4: 26-49.
- Soenen SJ, Demeester J, De Smedt SC, et al. (2012) The cytotoxic effects of polymer-coated quantum dots and restrictions for live cell applications. Biomaterials 33: 4882-4888.
- Cossarizza A, Ferraresi R, Troiano L, et al. (2009) Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat Protoc 4: 1790-1797.
- Emerit I, Serejo F, Filipe P (2000) Clastogenic factors as biomarkers of oxidative stress in chronic hepatitis C. Digestion 62: 200-207.
- Kim S, Bawendi MG (2003) Oligomeric ligands for luminescent and stable nanocrystal quantum dots. J Am Chem Soc 125: 14652-14653.
- Rogach AL (2000) Optical Properties of Colloidal Synthesized II-VI Semiconductor Nanocrystals. In: Sadowski ML, Potemski M and Grynberg M, Optical Properties of Semiconductor Nanostructure. Kluwer Academic publishers, The Netherlands, 379-393.
- Alivisatos AP, Gu W, Larabell C (2005) Quantum dots as cellular probes. Annu Rev Biomed Eng 7: 55-76.
- Hoshino A, Fujioka K, Oku T, et al. (2004) Physicochemical properties and cellular toxicity of nanocrystal quantum dots depend on their surface modification. Nano Lett 4: 2163-2169.
- Clapp AR, Medintz IL, Mauro JM, et al. (2004) Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J Am Chem Soc 126: 301-310.
- Medintz IL, Trammell SA, Mattoussi H, et al. (2004) Reversible modulation of quantum dot photoluminescence using a protein- bound photochromic fluorescence resonance energy transfer acceptor. J Am Chem Soc 126: 30-31.
- Bakalova R, Ohba H, Zhelev Z, et al. (2004) Quantum dots as photosensitizers? Nat Biotechnol 22: 1360-1361.
- Brown DM, Donaldson K, Borm PJ, et al. (2004) Calcium and ROS-mediated activation of transcription factors and TNF- alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol 286: 344-353.
- Buzea C, Pacheco II, Robbie K (2007) Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2: 17-71.
- Choi AO, Cho SJ, Desbarats J, et al. (2007) Quantum dot-induced cell death involves Fas upregulation and lipid peroxidation in human neuroblastoma cells. J Nanobiotechnology 5: 1.
- Li KG, Chen JT, Bai SS, et al. (2009) Intracellular oxidative stress and cadmium ions release induce cytotoxicity of unmodified cadmium sulfide quantum dots. Toxicol In Vitro 23: 1007-1013.
- Chan WH, Shiao NH, Lu PZ (2006) CdSe quantum dots induce apoptosis in human neuroblastoma cells via mitochondrial-dependent pathways and inhibition of survival signals. Toxicol Lett 167: 191-200.
- Tang M, Wang M, Xing T, et al. (2008) Mechanisms of unmodified CdSe quantum dot induced elevation of cytoplasmic calcium levels in primary cultures of rat hippocampal neurons. Biomaterials 29: 4383-4391.
- Samia AC, Chen X, Burda C (2003) Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc 125: 15736-15737.
- Macdonald IJ, Dougherty TJ (2001) Basic principles of photodynamic therapy. J Porphyr Phthalocya 5: 105-129.
- Dolmans DE, Fukumura D, Jain RK (2003) Photodynamic therapy for cancer. Nat Rev Cancer 3: 380-387.
- Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther 1: 279-293.
- Castano AP, Demidova TN, Hamblin MR (2005) Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn Ther 2: 1-23.
- Clarke SJ, Hollmann CA, Zhang Z, et al. (2006) Photophysics of Dopamine Modified Quantum Dots and Effects on Biological Systems. Nat Mater 5: 409-417.
- Liang J, He Z, Zhang S, et al. (2007) Study on DNA damage induced by CdSe quantum dots using nucleic acid molecular "light switche" as probe. Talanta 71: 1675-1678.
- Rajendran V, König A, Rabe KS, et al. (2010) Photocatalytic activity of protein-conjugated CdS nanoparticles. Small 6: 2035-2040.
- Cho SJ, Maysinger D, Jain M, et al. (2007) Long-term exposure to CdTe quantum dots causes functional impairments in live cells. Langmuir 23: 1974-1980.
- Cooper DR, Dimitrijevic NM, Nadeau JL (2010). Photosensitization of CdSe/ZnS QDs and reliability of assays for reactive oxygen species production. Nanoscale 2: 114-121.
- Chibli H, Carlini L, Park S, et al. (2011) Cytotoxicity of InP/ZnS quantum dots related to reactive oxygen species generation. Nanoscale 3: 2552-2559.
- Yaghini E, Pirker KF, Kay CW, et al. (2014) Quantification of reactive oxygen species generation photoexcitation of PEGylated quantum dots. Small 10: 5106-5115.
- Derfus AM, Chan WC, Bhatia SN (2004) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett 4: 11-18.
- Jaiswal JK, Mattoussi H, Mauro JM (2003) Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol 21: 47-51.
- Dubertret B, Skourides P, Norris DJ, et al. (2002) In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298: 1759-1762.
- Qu G, Wang X, Wang Z, et al. (2013) Cytotoxicity of quantum dots and graphene oxide to erythroid cells and macrophages. Nanoscale Res Lett 8: 198.
- Ballou B, Lagerholm BC, Ernst LA, et al. (2004) Noninvasive imaging of quantum dots in mice. Bioconjug Chem 15: 79-86.
- Irving P, Ubeda JM, Doucet D (2005) New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7: 335-350.
- Cherry S, Silverman N (2006) Host-pathogen interactions in Drosophila: newtricks from an old friend. Nat Immunol 7: 911-917.
- Carmona ER, Guecheva T, Creus A, et al. (2011) Proposal of an in vivo comet assay using haemocytes of Drosophila melanogaster. Environ Mol Mutagen 52: 165-169.
- Ekimov AI, Onushchenko AA (1984) Size quantization of the electron energy spectrum in a microscopic semiconductor crystal. JETP Letters 40: 337-340.
- Zhan Q, Tang M (2014) Research advances on apoptosis caused by quantum dots. Biol Trace Elem Res 161: 3-12.
- Nagy A, Steinbrück A, Gao J, et al. (2012) Comprehensive analysis of the effects of CdSe quantum dot size, surface charge, and functionalization on primary human lung cells. ACS Nano 6: 4748-4762.
- Wang SH, Shih YL, Kuo TC, et al. (2009) Cadmium toxicity toward autophagy through ROS-activated GSK-3 in mesangial cells. Toxicol Sci 108: 124-131.
- Xu B, Chen S, Luo Y, et al. (2011) Calcium signaling is involved in cadmium-induced neuronal apoptosis via induction of reactive oxygen species and activation of MAPK/mTOR network. PLoS One 6: e19052.
- Son YO, Wang X, Hitron JA, et al. (2011) Cadmium induces autophagy through ROS-dependent activation of the LKB1-AMPK signaling in skin epidermal cells. Toxicol Appl Pharmacol 255: 287-296.
- Risom L, Møller P, Loft S (2005) Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res 592: 119-137.
- Peters A, Veronesi B, Calderón-Garcidueñas L, et al. (2006) Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol 3: 13.
- Kim BY, Rutka JT, Chan WC (2010) Nanomedicine. New Engl J Med 363: 2434-2443.
- Pace HE, Lesher EK, Ranville JF (2010) Influence of stability on the acute toxicity of CdSe/ZnS nanocrystals to Daphnia magna. Environ Toxicol Chem 29: 1338-1344.
- Zolotarev KV, Kashirtseva VN, Mishin AV, et al. (2012) Assessment of Toxicity of Cdse/Cds/Zns/S,S-Dihydrolipoic Acid/Polyacrylic Acid Quantum Dots at Danio rerio Embryos and Larvae. ISRN Nanotechnology 2012: 914636.
- Zhang W, Sun X, Chen L, et al. (2012) Toxicological effect of joint cadmium selenium quantum dots and copper ion exposure on zebrafish. Environ Toxicol Chem 31: 2117-2123.
- Kwon D, Kim MJ, Park C, et al. (2012) In vivo biodegradation of colloidal quantum dots by a freshwater invertebrate, Daphnia magna. Aquat Toxicol 114-115: 217-222.
- Sabella S, Carney RP, Brunetti V, et al. (2014) A general mechanism for intracellular toxicity of metal-containing nanoparticles. Nanoscale 6: 7052-7061.
- Tsoi KM, Dai Q, Alman BA, et al. (2013) Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc Chem Res 46: 662-671.
- Inoue T, Wu L, Stuart J, et al. (2005) Control of p53 nuclear accumulation in stressed cells. FEBS Lett 579: 4978-4984.
- Méplan C, Mann K, Hainaut P (1999) Cadmium induces conformational modifications of wild-type p53 and suppresses p53 response to DNA damage in cultured cells. J Biol Chem 274: 31663-31670.
- Candéias S, Pons B, Viau M, et al. (2010) Direct inhibition of excision/synthesis DNA repair activities by cadmium: analysis on dedicated biochips. Mutat Res 694: 53-59.
- Zimmer JP, Kim SW, Ohnishi S, et al. (2006) Size series of small indium arsenide-zinc selenide core-shell nanocrystals and their application to in vivo imaging. J Am Chem Soc 128: 2526-2527.
- Kobayashi H, Hama Y, Koyama Y, et al. (2007) Simultaneous multicolor imaging of five different lymphatic basins using quantum dots. Nano Lett 7: 1711-1716.
- Werlin R, Priester JH, Meilke RE, et al. (2011) Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain. Nat Nanotechnol 6: 65-71.
- Lee WM, An YJ (2015) Evidence of three-level trophic transfer of quantum dots in an aquatic food chain by using bioimaging. Nanotoxicology 9: 407-412.
- Soenen SJ, Rivera-Gil P, Montenegro JS, et al. (2011) Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today 6: 446-465.
Corresponding Author
Eşref Demir, Department of Genetics and Bioengineering, Faculty of Engineering, Giresun University, 28200 Güre, Giresun, Turkey, Tel: +90-454-310-1740, Fax: +90-454-310-1749;
Vincent Castranova, Department of Pharmaceutical Sciences, School of Pharmacy, RC Byrd Health Sciences Center, West Virginia University, Morgantown, WV 26506, USA, Tel: +1-304-293-8236.
Copyright
© 2017 Demir E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.