Seqex Therapy in Osteonecrosis of the Femoral Head: A Case Report
Abstract
The cause of non-traumatic Avascular Necrosis of the femoral head (AVN) is unknown in a large proportion of cases. Unless diagnosed early and treated appropriately, it can progress to collapse of the femoral head leading to early degenerative arthrosis. With the advent of modern imaging techniques, AVN can be detected early. Accurate diagnosis of AVN leads to appropriate selection of effective joint-preserving treatment. Techniques aimed at preserving the femoral head, including core decompression, and vascularized/nonvascularized bone graft which are often advised for early necrosis, have shown varying clinical outcomes. For these reasons, further investigations to better understand the effectiveness of conservative treatments are warranted. Bone marrow-derived mesenchymal stromal cells, hyperbaric oxygen, extracorporeal shock wave therapy and pulsed magnetic fields therapy are some of the technique proposal for AVN treatment. We utilized a new Seqex therapy with total resolution of AVN in early stages.
Keywords
Pulsed magnetic field, Avascular necrosis of femoral head, Seqex, Therapy
Introduction
Avascular necrosis of femoral head is characterized by cellular death in subchondral bone due to a deficiency in blood supply.
Although the exact pathological mechanisms of Avascular Necrosis of femoral head (AVN) are not fully understood, both non-traumatic (Including excessive consumption of alcohol, corticosteroid overdose, Caisson disease, hemoglobin disorder (s) [e.g., sickle cell anemia], Gaucher disease, and radiation [e.g., chemoradiation therapy] [1-3]) and traumatic causes (e.g., femoral neck fracture, dislocation of the hip) have been known to cause blood circulation disorders, there by changing normal cellular physiology and eventually resulting in necrosis [4,5].
In addition, AVN may also occur abruptly in young adults [6]. Necrosis of subchondral bone usually evolves toward articular cartilage damage, eventually leading to secondary osteoarthritis requiring major surgery, including total hip arthroplasty.
Management of AVN of the femoral head can be grouped into three categories: Non-surgical management, head preserving surgeries and prosthetic hip replacement.
Given the high efficacy of conservative treatment (s) for AVN, it is recommended that surgical interventions follow image diagnosis and/or when classified as necrosis with symptoms [7-9].
Some diseases are associated with hip symptoms and/or imaging findings that resemble those of early stages of AVN. Characteristic Magnetic Resonance Imaging (MRI) findings in the early stage of AVN have been reported [10-14].
Based on the impact of Extracorporeal Shock Wave Therapy (ESWT) on vascularization and osteogenesis, a number of researchers employed it as a treatment for early stage AVN and reported improved clinical prognosis and decreases in osteonecrosis [15].
Further, there are a number of studies that compared clinical outcomes of ESWT to conservative drug treatments (e.g., the prostacyclin-analogue iloprost [16] and alendronate [17]) and surgical treatments (e.g., coredecompression, bone graft, and total hip arthroplasty), yet scant information is available regarding changes in clinical aspects and the effects across varying radiographic stages.
However, head preserving surgical procedures which aim at decompression and revascularization of the femoral head have given encouraging results in precollapse stages [18-20].
The commonly done revascularization procedures are core decompression by a large, single or small multiple holes drilled into the avascular area [20-24], core decompression with non-vascularized fibular strut grafting [25,26], muscle pedicle bone grafting [27], core decompression with vascularized fibular grafting [28], and osteotomies [29,30], etc.
Less invasive alternatives have been suggested to prevent the progression of AVN. Among these, core decompression alone may often lead to a transient benefit with discouraging failure [31]. It is therefore conceivable that bone regeneration may be promoted by a supply of autologous osteogenic cells at the site of AVN [32-34].
The actual efficacy of cell therapy for AVN, however, is still controversial because of the harsh conditions at the osteonecrotic site, where ischemia and hypoxia cause apoptosis of bone cells [35]. Another recent introduction therapy but still under development is that of quantum medicine, working on the patient's energy-electromagnetic appearance and consequently on the physiological and pathological aspect [36].
Already in 1985, physicist Abraham R, and his associates demonstrated that coupling a static magnetic field (Terrestrial) to a variable electromagnetic field at lower intensity and at room temperature, By specific frequencies (called cyclotronics), some ions (Ca2+, Na+, K+, Li+, Mg+) can overcome the cell membrane barrier. According to the fact that many pathologies are caused by the loss of substances and ions, redirecting these substances back into the cell constitutes a truly interesting therapeutic possibility [37,38].
This physical therapy, which causes vasodilation, appears to be applicable in the presence of flogic and degenerative processes [39].
Case Report
AB patient of 175 cm, 73 kg (BMI: 23.84), who are diagnosed lumbar back pain at another hospital. To clinical examination the lumbar pain appears to be caused by erroneous walking. According to this fact the left hip was suffering to clinical exam. Absence of leg length discrepancies.
In terms of range of motion showed flexion of 90°, complete extension, abduction of 30°, adduction of 20°. He walked with crutches for intensive groin and leg pain, with inability to run, to climb stairs and carrying out the normal play activities. Symptomatology had idiopathic origin. He had performed hip and spine radiographs without showing significant pathologies.
In first step we have given him antalgic drug therapy and required to perform pelvis and spine Magnetic Resonance Imaging (MRI).
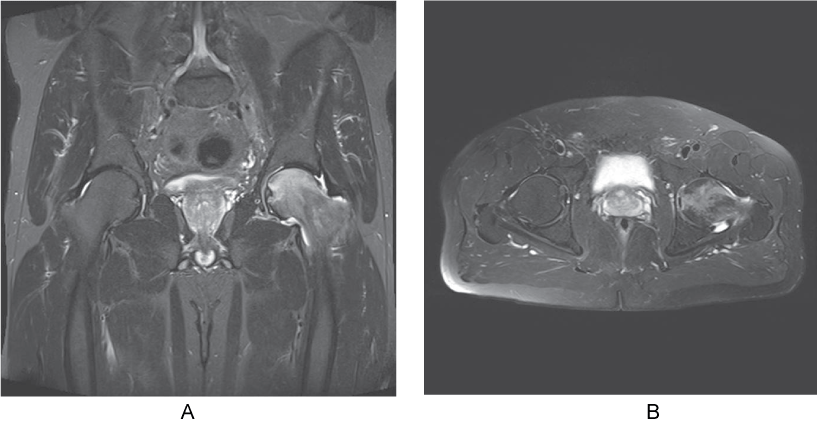
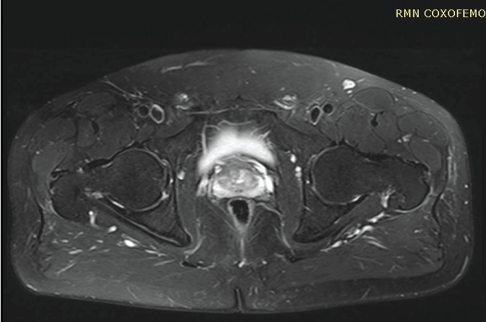
Ten days later he comes back with slight improvement in symptoms, but still with lameness. Instrumental imaging (Figure 1) showed AVN of 1st degree according to Ficat classification [9].
At this time we have prescribed Seqex-magnetotherapy (2 sessions per week) and confirm the same antalgic therapy.
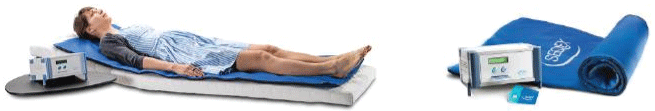
Seqex HC is a Pulsed Magnetic Fields (PEMFs) of new generation. Unlike the others PEMFs it has solenoid mat, the size of an ambulatory bed (Figure 2), voltage pulses from 1 to 80 Hz, magnetic field intensity of 1 Gauss, is sufficient a therapeutic sessions for a maximum of 20 minutes [39]. Compared to other PEMFs, Seqex HC has a wider frequency that allows it to be applied in multiple body 'areas and a less powerful field to allow it to be used even in presence of metal bodies.
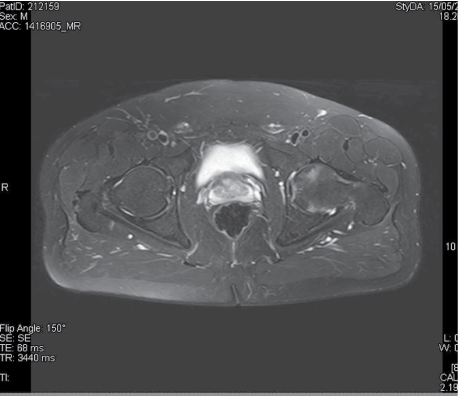
Another magnetic resonance step is performed after 2 months and showed improvement of the problem, but not total healing (Figure 3). At this time patient did not need further antalgic therapy.
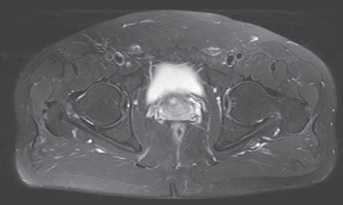
After 4 months from the beginning of the Seqex-magnetotherapy another MRI (Figure 4) was checked and showed total resolution of the problem. The left hip was completely pain free and with normal range of motion. He walked and running without problems.
After 1 year the patient did another pelvis MRI (Figure 5) for another problem and it was possible to see the absence of any AVN recurrence.
Clinical data were collected at the beginning of the problem, at 4 months and after 1 year with the Harris Hip Score (HHS) scale, VAS score and with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Table 1) [40,41].
Discussion
AVN is a relatively common disease, often affecting young and active individuals, that almost inevitably progresses toward osteoarthritis and severe disability of the hip joint. The etiopathology is not clearly understood. However, it is known that interruption of the blood supply to the femoral head leads to cellular death, subchondral fracture, and segmental collapse and at a later stage, secondary degenerative arthrosis [21-25].
To prevent invasive procedures, such as total joint replacement, maximum effort should be made to halt the progression of osteoarticular damage and enhance the inherent regenerative potential of bone tissue.
Core decompression is the most popular revascularization procedure which reduces the intra-osseous pressure caused by interstitial edema, improves vascularity, enhances bone healing and therefore relieves pain. It can be achieved by drilling a large single track into the femoral head or by drilling multiple drill holes [42].
Kim, et al. [43] reported better results with multiple drilling compared with single-core decompression.
To prevent the collapse of the subchondral area after removal of the necrotic bone, some authors advocate bone grafting [25,26]. This structural support can be achieved by fibular bone grafting. Core decompression with bone grafting has shown survival rate from 36 to 87% [25,24,45]. Vascularized fibular grafting and muscle pedicle bone grafting further improve the blood supply [11]. Meyers muscle pedicle bone graft was found to be effective in stage I and stage II. However, in stage III, only 4 out of 11 hips had good outcome [46]. In a long-term follow-up in 152 patients, Bakshi, et al. [27] reported excellent to good results in 86% hips following multiple drilling and muscle pedicle bone grafting with tensor fascia lata.
There are several non-invasive therapies for the treatment of AVN; Lee JY, et al. [47] show that all patients with early and mid osteonecrosis (i.e., Ficat I and II stages) who underwent ESWT had significant improvements in pain reduction and HHS.
ESWT is a type of sound wave with high pressure and velocity that can pass through liquid and soft tissues; when it goes toward bone, reflection and precipitation of the shock wave are made on the boundary surface between soft tissue and bone [48]. It has been suggested that these precipitations might positively impact necrosis and angiogenesis and some studies demonstrated its therapeutic potential on AVN [49].
In another study, Levin, et al. [50] demonstrated that hyperbaric oxygen reduces the ranges of femoral head necrosis in rats.
Regional ischemia causes joint structure hypoxia, which is a common feature of AVN. By providing 100% oxygen at elevated atmospheric pressure, Hyperbaric Oxygenation (HBO) increases the partial pressure of oxygen in plasma and in tissues.
As an adjunctive therapy, HBOT has been reported to improve the outcomes in patients suffering from bone diseases [51] and femoral head necrosis [52-54].
Recent works reveal that hyperbaric oxygenation may also accelerate osteoblast differentiation and suppress osteoclasts genesis-activation, shifting the balance between bone formation and bone resorption in a direction that promotes regeneration [55-57].
Recently, cell therapy was introduced with advances in stem cell research and regenerative medicine [34,58-60]. Cell therapy for AVN is typically used at the early stage [61]. For this reason, accurate diagnosis is increasingly important.
Studies have demonstrated the efficacy of Mesenchymal Stem Cells (MSCs) implanted into the femoral head for the treatment of AVN [62,63]. MSCs can be isolated from bone marrow and adipose tissues in adult stages and from Umbilical Cord (UC) blood, and connective tissue of human UC [64-67]. MSCs can differentiate into specialized cells to repair injured tissues, under certain conditions based on their potential capacity of multidirectional differentiations [68,69] and studies demonstrated the safety and efficacy of osteoblastic differentiation of MSCs [70].
Pulsed magnetic fields therapy has been used in orthopedics disease for several years [71-75].
Few are studies that document satisfactory results in the use of pulsed electromagnetic fields in the AVN [76,77], but in 1983 the first attempts were made [78].
The recent Seqex therapy has allowed a therapeutic improvement, since it is possible to select between 30 different waveforms that provide the best organism response based on the problem to be taken [39]. The computer makes an initial test on the patient and recording data on a personal smart card that is reused at each therapeutic session.
Also with this kind of PEMFs the lesions in early stage respond well to treatment, but with Seqex there is a significant reduction in the number of therapeutic sessions and their duration, with greater patient satisfaction for cost/benefit ratio.
The USA Food and Drug Administration (FDA) has approved in 1987 the use of this method for the care of "Bone disorders" and shortly after the "spine bifida".
More than a million people have been treated in the US and never are harmful effects from its use have been observed. It should also be noted that magnetic fields that can be generated by these devices are based on the use of extremely low frequencies and "intensity", which does not therefore generate no heat or "ionization" [79].
In case of patients with metal prosthesis, the treatment does not presents contraindication according to the fact that the devices do not generate any thermal effect [37,80].
Conclusion
Hips with AVN in the precollapse stage can be salvaged by core decompression with or without fibular grafting. Core decompression by multiple small diameters drilling carries less morbidity and hence preferred in stage I and stage II. However, in stage III disease, core decompression with fibular strut grafting gives better results [43].
ESWT improved the VAS pain score and HHS at 24 months follow-up for AVN patients [49].
Autologous BM-MSCs is based on regenerative approach might increase the chance to obtain an efficient bone tissue regeneration because of the ability of autologous mesenchymal cells to face the hypoxic microenvironment and to undergo the mineralization process [68-70].
Uzun G, et al. [81] make a reviewer in which assess that in some studies HBOT was combined with other treatment modalities. Hip survivorship wherein HBOT was used alone was 95.5% in Steinberg Stage I lesions, 89% in Steinberg Stage II lesions and 100% in Ficat Stage II lesions [81]. Seqex seems to be a method that, in view of the choice of the most suitable electromagnetic waves, allows fewer sessions than normal pulsed electromagnetic fields, and being a non-thermogenic therapy makes it usable in everybody district. His target is exclusively the membrane cell.
Probably the effect of pulsed electromagnetic field stimulation may be to protect the articular cartilage from the catabolic effect of inflammation and subchondral bone-marrow edema and promote osteogenic activity at the necrotic area and prevent trabecular fracture and subchondral bone collapse.
References
- Aldridge JM, Urbaniak JR (2004) Avascular necrosis of the femoral head: Etiology, pathophysiology, classification, and current treatment guidelines. Am J Orthop 33: 327-332.
- Koo KH, Kim R, Kim YS, et al. (2002) Risk period for developing osteonecrosis of the femoral head in patients on steroid treatment. Clin Rheumatol 21: 299-303.
- Wang GJ, Cui Q, Balian G (2000) The nicolas andry award. The pathogenesis and prevention of steroid-induced osteonecrosis. Clin Orthop Relat Res 295-310.
- Wang CJ, Wang FS, Yang KD, et al. (2008) Treatment of osteonecrosis of the hip: Comparison of extracorporeal shockwave with shockwave and alendronate. Arch Orthop Trauma Surg 128: 901-908.
- Wang CJ, Wang FS, Huang CC, et al. (2005) Treatment for osteonecrosis of the femoral head: Comparison of extracorporeal shock waves with core decompression and bone-grafting. J Bone Joint Surg Am 87: 2380-2387.
- Mont MA, Jones LC, Hungerford DS (2006) Nontraumatic osteonecrosis of the femoral head: Ten years later. J Bone Joint Surg Am 88: 1117-1132.
- Wang CJ, Wang FS, Ko JY, et al. (2008) Extracorporeal shockwave therapy shows regeneration in hip necrosis. Rheumatology 47: 542-546.
- Steinberg ME, Hayken GD, Steinberg DR (1995) A quantitative system for staging avascular necrosis. J Bone Joint Surg Br 77: 34-41.
- Ficat RP (1985) Idiopathic bone necrosis of the femoral head. Early diagnosis and treatment. J Bone Joint Surg Br 67: 3-9.
- Mitchell DG, Kressel HY, Arger PH, et al. (1986) Avascular necrosis of the femoral head: Morphologic assessment by MR imaging, with CT correlation. Radiology 161: 739-742.
- Koo KH, Ahn IO, Kim R, et al. (1999) Bone marrow edema and associated pain in early stage osteonecrosis of the femoral head: Prospective study with serial MR images. Radiology 213: 715-722.
- Sugano N, Ohzono K, Masuhara K, et al. (1994) Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res 190-199.
- Sugano N, Masuhara K, Nakamura N, et al. (1996) MRI of early osteonecrosis of the femoral head after transcervical fracture. J Bone Joint Surg Br 78: 253-257.
- Kubo T, Yamazoe S, Sugano N, et al. (1997) Initial MRI findings of non-traumatic osteonecrosis of the femoral head in renal allograft recipients. Magn Reson Imaging 15: 1017-1023.
- Ludwig J, Lauber S, Lauber HJ, et al. (2001) High-energy shock wave treatment of femoral head necrosis in adults. Clin Orthop Relat Res 119-126.
- Disch AC, Matziolis G, Perka C (2005) The management of necrosis-associated and idiopathic bone-marrow oedema of the proximal femur by intravenous iloprost. J Bone Joint Surg Br 87: 560-564.
- Lai KA, Shen WJ, Yang CY, et al. (2005) The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am 87: 2155-2159.
- Mont MA, Carbone JJ, Fairbank AC (1996) Core decompression versus nonoperative management for osteonecrosis of the hip. Clin Orthop Relat Res 169-178.
- Warner JJ, Philip JH, Brodsky GL, et al. (1987) Studies of non-traumatic osteonecrosis: The role of core decompression in the treatment of no traumatic osteonecrosis of the femoral head. Clin Orthop Relat Res 225: 104-127.
- Mont MA, Ragland PS, Etienne G (2004) Core decompression of the femoral head for osteonecrosis using percutaneous multiple small diameter drilling. Clin Orthop Relat Res 131-138.
- Steinberg ME (1995) Core decompression of the femoral head for avascular necrosis: Indications and results. Can J Surg 38: S18-S24.
- Bellot F, Havet E, Gabrion A, et al. (2005) Core decompression of the femoral head for avascular necrosis. Rev Chir Orthop Reparatrice Appar Mot 91: 114-123.
- Israelite C, Nelson CL, Ziarani CF, et al. (2005) Bilateral core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res 441: 285-290.
- Song WS, Yoo JJ, Kim YM, et al. (2007) Results of multiple drilling compared with those of conventional methods of core decompression. Clin Orthop Relat Res 454: 139-146.
- Keizer SB, Kock NB, Dijkstra PD, et al. (2006) Treatment of avascular necrosis of the hip by a non-vascularised cortical graft. J Bone Joint Surg Br 88: 460-466.
- Al-Edanny M Sh (2012) Non vascularized bone graft versus core decompression in treatment of early stages of non traumatic hip osteonecrosis. Karbala J Med 5: 1295-1305.
- Baksi DP, Pal AK, Baksi DD (2009) Long-term results of decompression and muscle-pedicle bone grafting for osteonecrosis of the femoral head. Int Orthop 33: 41-47.
- Eward WC, Rineer CA, Urbaniak JR, et al. (2012) The vascularized fibular graft in precollapse osteonecrosis: Is long term hip preservation possible? Clin Orthop Relat Res 470: 2819-2826.
- Zhao G, Yamamoto T, Ikemura S, et al. (2010) Radiological outcome analysis of transtrochanteric curved varus osteotomy for osteonecrosis of the femoral head at a mean follow-up of 12.4 years. J Bone Joint Surg Br 92: 781-786.
- Sakano S, Hasegawa Y, Torii Y, et al. (2004) Curved intertrochanteric varus osteotomy for osteonecrosis of the femoral head. J Bone Joint Surg Br 86: 359-365.
- Moya-Angeler J, Gianakos AL, Villa JC, et al. (2015) Current concepts on osteonecrosis of the femoral head. World J Orthop 6: 590-601.
- Hernigou P, Flouzat-Lachaniette CH, Delambre J, et al. (2015) Osteonecrosis repair with bone marrow cell therapies: State of the clinical art. Bone 70: 102-109.
- Banerjee S, Issa K, Pivec R, et al. (2013) Osteonecrosis of the hip: Treatment options and outcomes. Orthop Clin North Am 44: 463-476.
- Mac Queen L, Sun Y, Simmons CA (2013) Mesenchymal stem cell mechanobiology and emerging experimental platforms. J R Soc Interface 10: 20130179.
- Seamon J, Keller T, Saleh J, et al. (2012) The pathogenesis of nontraumatic osteonecrosis. Arthritis 2012: 601763.
- Tribbia C (2005) Da "Atti del convegno Nazionale della Società Italiana di Biofisica". 21-22.
- Vallesi G, Raggi F, Rufini S, et al. (2007) Effects of cyclotronic ion resonance on human metabolic processes: A clinical trial and one case report. Electromagn Biol Med 26: 283-288.
- Ciafaloni A (2007) Cyclotronic ion resonance therapy and arthralgia. Electromagn Biol Med 26: 299-303.
- Rivasanseverino E, Castellacci P (2005) Da "Atti del convegno Nazionale della Società Italiana di Biofisica". 16-20.
- Whitehouse SL, Lingard EA, Katz JN, et al. (2003) Development and testing of a reduced WOMAC function scale. J Bone Joint Surg Br 85: 706-711.
- Harris WH (1969) Traumatic arthritis of the hip after dislocation and acetabular fractures: Treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 51: 737-755.
- Camp JF, Colwell CW Jr (1986) Core decompression of the femoral head for osteonecrosis. J Bone Joint Surg Am 68: 1313-1319.
- Kim SY, Kim DH, Park IH, et al. (2004) Multiple drilling compared with core decompression for the treatment of osteonecrosis of the femoral head. Orthopaedic Proceedings.
- Rosenwasser MP, Garino JP, Kiernan HA, et al. (1994) Long term follow-up of thorough debridement and cancellous bone grafting of the femoral head for avascular necrosis. Clin Orthop Relat Res 17-27.
- Plakseychuk AY, Kim SY, Park BC, et al. (2003) Vascularized compared with nonvascularized fibular grafting for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am 85: 589-596.
- Meyers MH (1978) The treatment of osteonecrosis of the hip with fresh osteochondral allografts and with the muscle pedicle graft technique. Clin Orthop Relat Res 130: 202-209.
- Lee JY, Kwon JW, Park JS, et al. (2015) Osteonecrosis of Femoral Head Treated with Extracorporeal Shock Wave Therapy: Analysis of Short-term Clinical Outcomes of Treatment with Radiologic Staging. Hip Pelvis 27: 250-257.
- Ohzono K, Takaoka K, Saito S, et al. (1992) Intraosseous arterial architecture in nontraumatic avascular necrosis of the femoral head. Microangiographic and histologic study. Clin Orthop Relat Res 79-88.
- Zhou Q, Li Q, Yang L, et al. (2000) Changes of blood vessels in glucocorticoid-induced avascular necrosis of femoral head in rabbits. Zhonghua Wai Ke Za Zhi 38: 212-215.
- Levin D, Norman D, Zinman C, et al. (1999) Treatment of experimental avascular necrosis of the femoral head with hyperbaric oxygen in rats: Histological evaluation of the femoral heads during the early phase of the reparative process. Exp Mol Pathol 67: 99-108.
- Sen RK (2009) Management of avascular necrosis of femoral head at pre-collapse stage. Indian J Orthop 43: 6-16.
- Reis ND, Schwartz O, Militianu D, et al. (2003) Hyperbaric oxygen therapy as a treatment for stage-I avascular necrosis of the femoral head. J Bone Joint Surg Br 85: 371-375.
- Camporesi EM, Vezzani G, Bosco G, et al. (2010) Hyperbaric oxygen therapy in femoral head necrosis. J Arthroplasty 25: 118-123.
- Bennett M (2011) Hyperbaric oxygen therapy improved both pain scores and range of motion in patients with early idiopathic femoral head necrosis (Ficat stage II). Diving Hyperb Med 41: 105.
- Al Hadi H, Smerdon GR, Fox SW (2015) Hyperbaric oxygen therapy accelerates osteoblast differentiation and promotes bone formation. J Dent 43: 382-388.
- Al Hadi H, Smerdon GR, Fox SW (2013) Hyperbaric oxygen therapy suppresses osteoclast formation and bone resorption. J Orthop Res 31: 1839-1844.
- Vezzani G, Quartesan S, Cancellara P, et al. (2017) Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necrosis patients. J Enzyme Inhib Med Chem 32: 707-711.
- Hernigou P, Beaujean F (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res 14-23.
- Yamasaki T, Yasunaga Y, Ishikawa M, et al. (2010) Bone-marrow-derived mononuclear cells with a porous hydroxyapatite scaffold for the treatment of osteonecrosis of the femoral head: A preliminary study. J Bone Joint Surg Br 92: 337-341.
- Mao Q, Wang W, Xu T, et al. (2015) Combination treatment of biomechanical support and targeted intra-arterial infusion of peripheral blood stem cells mobilized by granulocyte-colony stimulating factor for the osteonecrosis of the femoral head: A randomized controlled clinical trial. J Bone Miner Res 30: 647-656.
- Massari L, Osti R, Lorusso V, et al. (2015) Biophysical stimulation and the periprosthetic bone: Is there a rationale in the use of Pulsed Electromagnetic Fields after a hip or knee implant? J Biol Regul Homeost Agents 29: 1013-1015.
- Zhao D, Liu B, Wang B, et al. (2015) Autologous bone marrow mesenchymal stem cells associated with tantalum rod implantation and vascularized iliac grafting for the treatment of end stage osteonecrosis of the femoral head. Biomed Res Int 2015: 240506.
- Jin H, Xu T, Chen Q, et al. (2016) The fate and distribution of autologous bone marrow mesenchymal stem cells with intra arterial infusion in osteonecrosis of the femoral head in dogs. Stem Cells International 2016: 8616143.
- Park HW, Chang JW, Yang YS, et al. (2015) The effect of donor dependent administration of human umbilical cord blood derived mesenchymal stem cells following focal cerebral ischemia in rats. Exp Neurobiol 24: 358 365.
- Qu Z, Guo S, Fang G, et al. (2014) AKT pathway affects bone regeneration in nonunion treated with umbilical cord derived mesenchymal stem cells. Cell Biochem Biophys 71: 1543-1551.
- Qu Z, Guo L, Fang G, et al. (2012) Biological characteristics and effect of human umbilical cord mesenchymal stem cells (hUC MSCs) grafting with blood plasma on bone regeneration in rats. Cell Biochem Biophys 63: 171 181.
- Qu Z, Fang G, Cui Z, et al. (2015) Cell therapy for bone nonunion: A retrospective study. Minerva Med 106: 315 321.
- Klimczak A, Kozlowska U (2016) Mesenchymal stromal cells and tissue specific progenitor cells: Their role in tissue homeostasis. Stem Cells International 2016: 4285215.
- Mattar P, Bieback K (2015) Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth associated tissue mesenchymal stromal cells. Front Immunol 6: 560.
- Chen C, Qu Z, Yin X, et al. (2016) Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: A three-year follow-up study. Mol Med Rep 14: 4209-4215.
- Aragona SE, Mereghetti G, Lotti J, et al. (2017) Electromagnetic field in control tissue regeneration, pelvic pain, neuro-inflammation and modulation of non-neuronal cells. J Biol Regul Homeost Agents 31: 219-225.
- Li B, Bi J, Li W, et al. (2016) Effects of pulsed electromagnetic fields on histomorphometry and osteocalcin in disuse osteoporosis rats. Technol Health Care 25: 13-20.
- Yang X, He H, Zhou Y, et al. (2017) Pulsed electromagnetic field at different stages of knee osteoarthritis in rats induced by low-dose monosodium iodoacetate: Effect on subchondral trabecular bone microarchitecture and cartilage degradation. Bio Electro Magnetics 38: 227-238.
- Randelli P, Menon A, Ragone V, et al. (2016) Effects of the pulsed electromagnetic field PST® on human tendon stem cells: A controlled laboratory study. BMC Complement Altern Med 16: 293.
- Bagnato GL, Miceli G, Marino N, et al. (2016) Pulsed electromagnetic fields in knee osteoarthritis: A double blind, placebo-controlled, randomized clinical trial. Rheumatology 55: 755-762.
- Li JP, Chen S, Peng H, et al. (2014) Pulsed electromagnetic fields protect the balance between adipogenesis and osteogenesis on steroid-induced osteonecrosis of femoral head at the pre-collapse stage in rats. Bioelectromagnetics 35: 170-180.
- Massari L, Fini M, Cadossi R, et al. (2006) Biophysical stimulation with pulsed electromagnetic fields in osteonecrosis of the femoral head. J Bone Joint Surg Am 88: 56-60.
- Eftekhar NS, Schink-Ascani MM, Mitchell SN, et al. (1983) Osteonecrosis of the femoral head treated by pulsed electromagnetic fields (PEMFs): A preliminary report. Hip 306-330.
- Mancuso M, Ghezzi V, Di Fede G (2007) Utilization of extremely low frequency (ELF) magnetic fields in chronic disease; five years experience: Three case reports. Electromagn Biol Med 26: 311-313.
- Raggi F, Vallesi G, Rufini S, et al. (2008) ELF magnetic therapy and oxidative balance. Electromagn Biol Med 27: 325-339.
- Uzun G, Mutluoglu M, Ersen O, et al. (2016) Hyperbaric oxygen therapy in the treatment of osteonecrosis of the femoral head: A review of the current literature. Undersea Hyperb Med 43: 189-199.
Corresponding Author
Manuel Bondi, MD, ASST-Mantova, Carlo Poma, Struttura Complessa di Ortopedia e Traumatologia, Strada Lago Paiolo 10, 46100 Mantova, Italy.
Copyright
© 2017 Bondi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.