Early Outcome of Mitral Valve Surgery comparing Minimally Invasive Versus Standard Median Sternotomy Approach
Abstract
Background: This study compares our experience of early outcome of mitral valve surgery (MVS) after minimally invasive (MI) versus standard median sternotomy (SMS) approach.
Objective: Minimal invasive mitral valve surgery (MIMVS) aims to avoid complications of SMS like, bleeding, postoperative pain, and sternal wound infection. It provides better cosmesis and early recovery. The aim of this study is to evaluate early clinical outcome of MIMVS.
Patient and Method: It is prospective comparative cohort study in adult patients with mitral valve disease who perform MVS using either MI or SMS. From January 2020 to December 2021, early outcome of MVS between [120 patients] MI group through right mini thoracotomy (RMT) with CPB peripheral cannulation and [120 patients] SMS group are compared.
Result: Females are more in MIMVS (80%). ACC and CPB time are longer in MIMVS than SMS (118 ± 15.5 vs. 74.4 ± 32.3; 155 ± 28.5 vs. 115 ± 48.8). Tricuspid repair and left atrial appendage (LAA) occlusion are performed only in SMS. Blood loss is lesser in MIMVS (250 ± 60.6 ml) than in SMS (550 ± 230 ml). Blood transfusion required (0.1 ± 0.53) in MIMVS; and (0.9 ± 0.7) in SMS. Re-exploration for bleeding is required in (4) cases of SMS. Mechanical ventilation time is shorter in MIMVS (6.4 ± 1.3) than in SMS (12.4 ± 6.8). ICU duration and hospital stay are shorter in MIMVS than SMS (2 ± 0.4 vs. 3.5 ± 1.3; 7.2 ± 1.3 vs. 12 ± 0.5). Wound infections present in (20) cases of SMS. Spirometric studies in MIMVS reveal better postoperative pulmonary functions than SMS group. Pain Visual Analog Score at discharge is better in MIMVS (1.4 ± 0.6) than in SMS (8.5 ± 1.5). There is no hospital mortality in both groups
Conclusion: Minimal invasive mitral valve surgery is a safe procedure and improves cosmesis and patient’s satisfaction.
Keywords
Minimal invasive cardiac surgery, Right mini-thoracotomy, Minimal invasive mitral valve surgery, Standard median sternotomy
Abbreviations
ACC: Aortic cross clamp; AF: Atrial fibrillation; BMI: Body mass index; BSA: Body surface area; DM: Diabetes mellitus; DSWI: Deep sternal wound infection; CPB: Cardiopulmonary bypass; CABG: Coronary artery bypass grafting; CTA: Computed tomography aortography; ICU: Intensive care unit; IVC: Inferior vena cava; IJV: Internal jugular vein; INB: Intercostal nerve block; IMR: Ischemic mitral regurge; LAA: Left atrial appendage; FEV1: Forced expiratory volume in one second; FVC: Forced volume capacity; MI: Minimal invasive; MICS: Minimal invasive cardiac surgery; MIMVS: Minimal invasive mitral valve surgery; MVS: Mitral valve surgery; LOS: Length of stay; PCI: Percutaneous coronary intervention; PFT: Pulmonary function test; POD: Postoperative day; PVD: Peripheral vascular disease; RMT: Right minithoracotomy; SMS: Standard median sternotomy; SSWI: Superficial sternal wound infection; SVC: Superior vena cava; TEE: Transesophageal echo; VAS: Visual analog scale
Introduction
Inspite of availability of minimally invasive cardiac surgery (MICS) technique for long time ago, most of cardiac surgeons remained reluctant to perform MICS [1]. Many obstacles included a learning curve and special instruments required. Also, it had disadvantages of longer cardiopulmonary bypass (CPB) time, difficult visualization, and poor exposure of operative field [2]. Moreover, it carried a high risk of stroke because of inadequate deairing [3]. Poor exposure of the surgical field in MICS was present in patients with average body weight; obese patients were added more high surgical risk [4,5].
MICS was introduced to overcome morbidity associated with standard median sternotomy (SMS). Its access achieved through right minithoracotomy (RMT). The advances in instruments and cannula systems had allowed surgeons to perform MICS easily. Moreover, there was a need to overcome limitations of increased CPB time, difficult deairing and added more complex surgery [4].
The advantages of smaller surgical incisions were including early recovery, less postoperative pain, better cosmesis and carried low risk of wound infection [5,6]. With MICS approach, high-risk patients might benefit more from limiting surgical trauma with this approach [4]. There are efforts to decrease incision length, enhanced recovery, and better patient’s satisfaction, without drawback on surgical techniques [7]. Improved cosmesis should not be only indication for MICS; however, it is a welcomed added benefit [4].
Other benefits of MICS included reduced blood loss, blood transfusion, and atrial fibrillation (AF), shorter intensive care unit (ICU) and hospital stays [2]. Minimal invasive mitral valve surgery (MIMVS) had been associated with mortality rates of 1.2% - 5.8% [6].
Patient and Method
After approval by the local ethical committee of our center 240 consecutive patients with mitral valve disease were prospectively included in the study from January 2020 to December 2021. All patients gave written informed consent after the study protocol and the potential risks associated with the procedure had been outlined in details. Patients who had undergone MIMVS compared with patients who had undergone SMS. The mitral valve was either [8] repaired or (112) replaced in (120) patients by means of MIMVS approach through RMT. MIMIVS was performed according to patients` acceptance and desire if there were no contraindications. Demographics and preoperative patient data were summarized in (Table 1). Inclusion criteria were elective mitral valve surgery. Exclusion criteria were mitral surgery with other concomitant cardiac surgery, emergency and redo surgery, endocarditis, ischemic mitral regurgitation (IMR), low EF (less than 30%), chest wall deformity, previous thoracotomy or thoracic radiation, chronic obstructive pulmonary disease (FEV1 < 1 L), and liver or kidney failure.
Patients for MIMVR should include careful history for relevant comorbidities, clinical examination, echocardiography, pulmonary function testing (PFT) and coronary angiography when indicated. Preoperative computed tomography aortography (CTA) provides valuable information regarding aortic aneurysm, tortuosity, atherosclerosis, and femoral artery suitability for cannulation.
Operative data were including CPB time, and aortic cross clamp (ACC) time, mortality and morbidity (stroke, prolonged ventilation, bleeding, renal failure, and wound infection) were recorded.
Sternal wound infection (SWI) are either superficial (SSWI) including skin and subcutaneous, or deep (DSWI) including sternal bone exposure with/out stability, necrotic bone, and heart exposure, with/out septicemia.
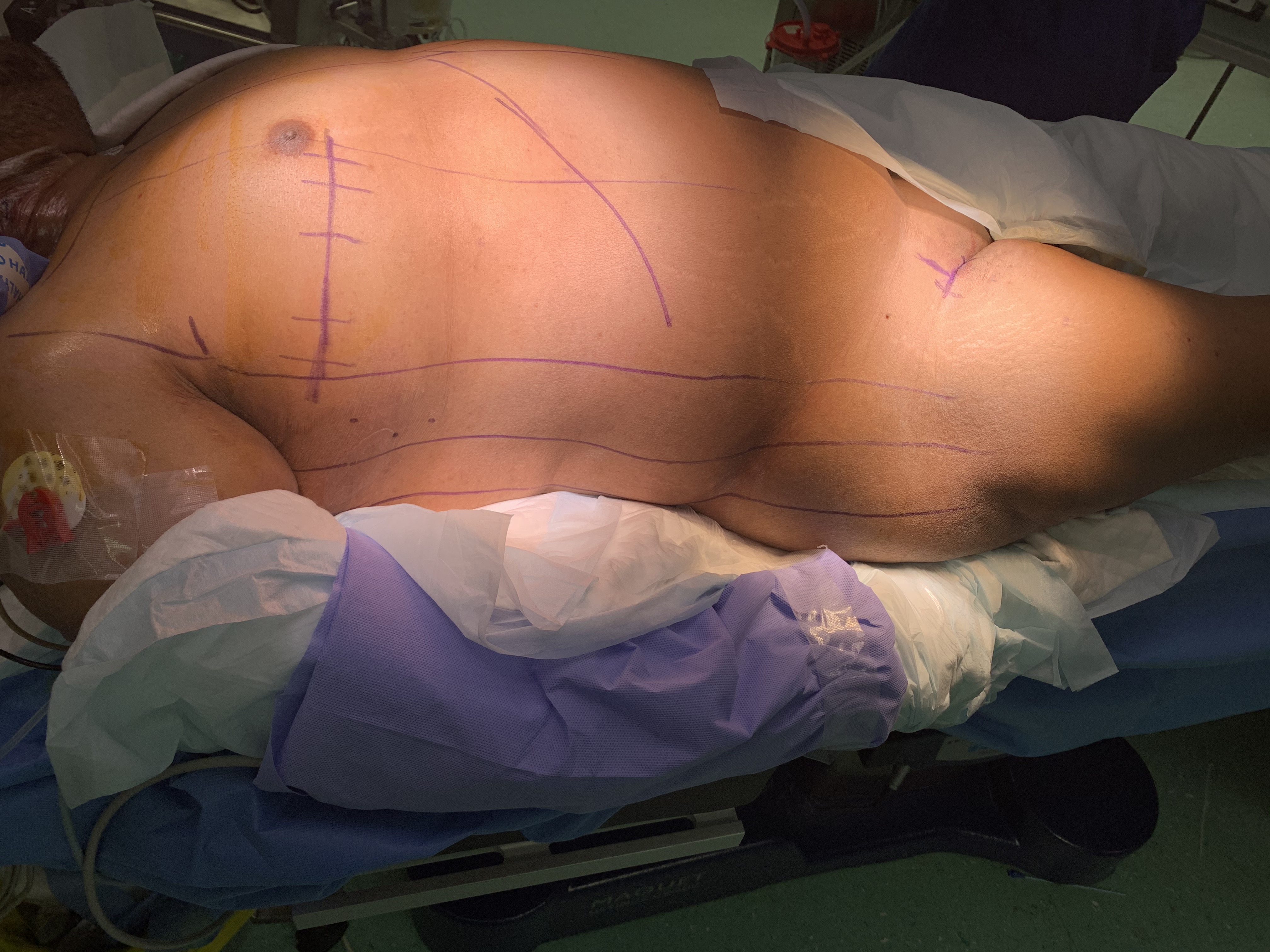
Patients were positioned in a supine position with the right side of the chest slightly elevated (Photo 1). External defibrillator pads were placed on the chest. Intraoperative trans-esophageal echo (TEE) was obtained for all the patients. CPB was performed through peripheral cannulation technique. Femoral artery was cannulated with a 16F - 18F arterial cannula, and femoral vein was cannulated with a 25F venous cannula through open technique. Two venous drainage cannulas were routinely used at our center; the anesthesiologist placed a wire in right internal jugular vein (IJV) before draping. The cannula for superior vena cava (SVC) was placed percutaneously from the neck. Cannulation for Bicaval cannulation was performed; the tip of the femoral cannula was positioned just below the junction of inferior vena cava (IVC) and right atrium.
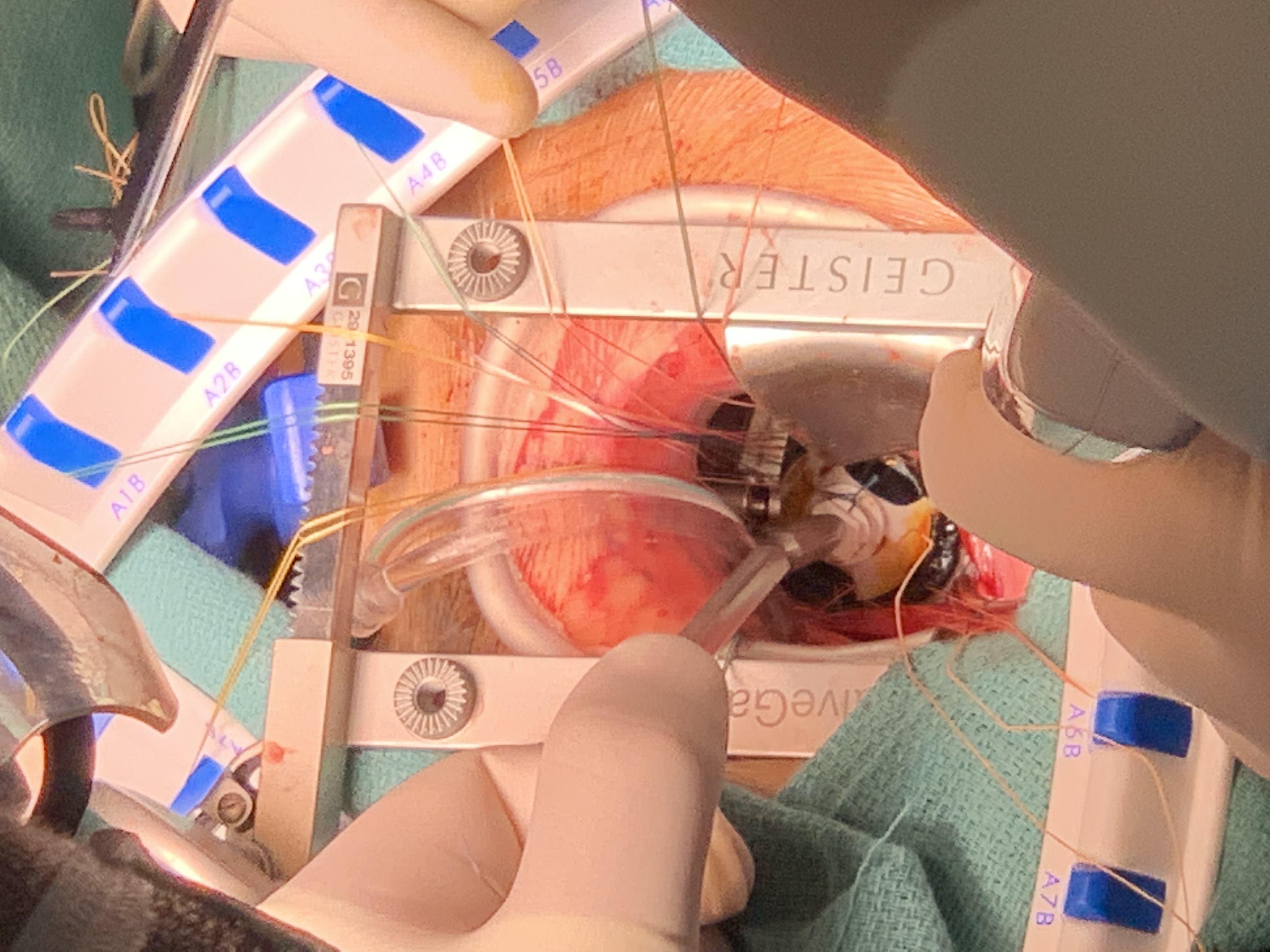
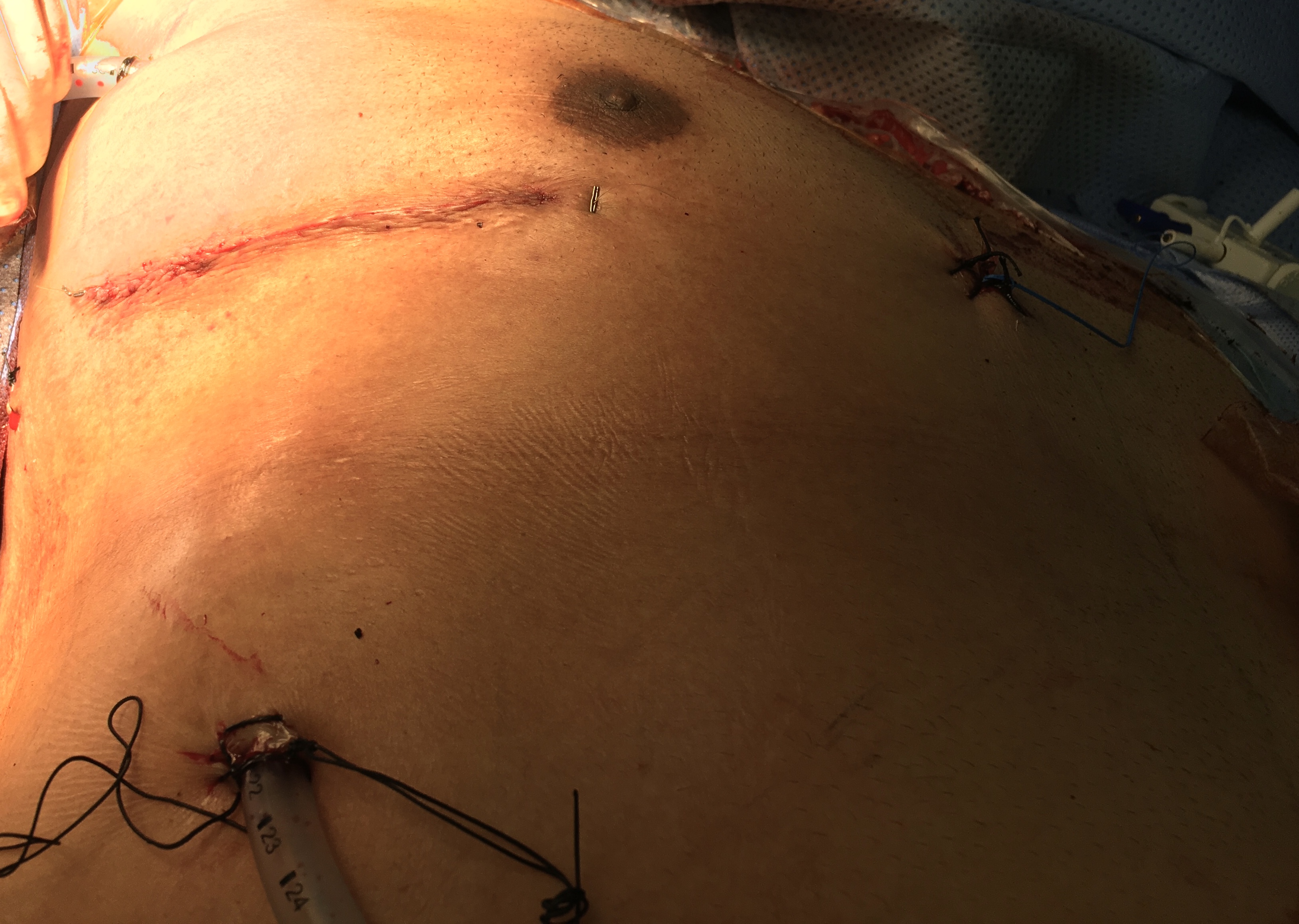
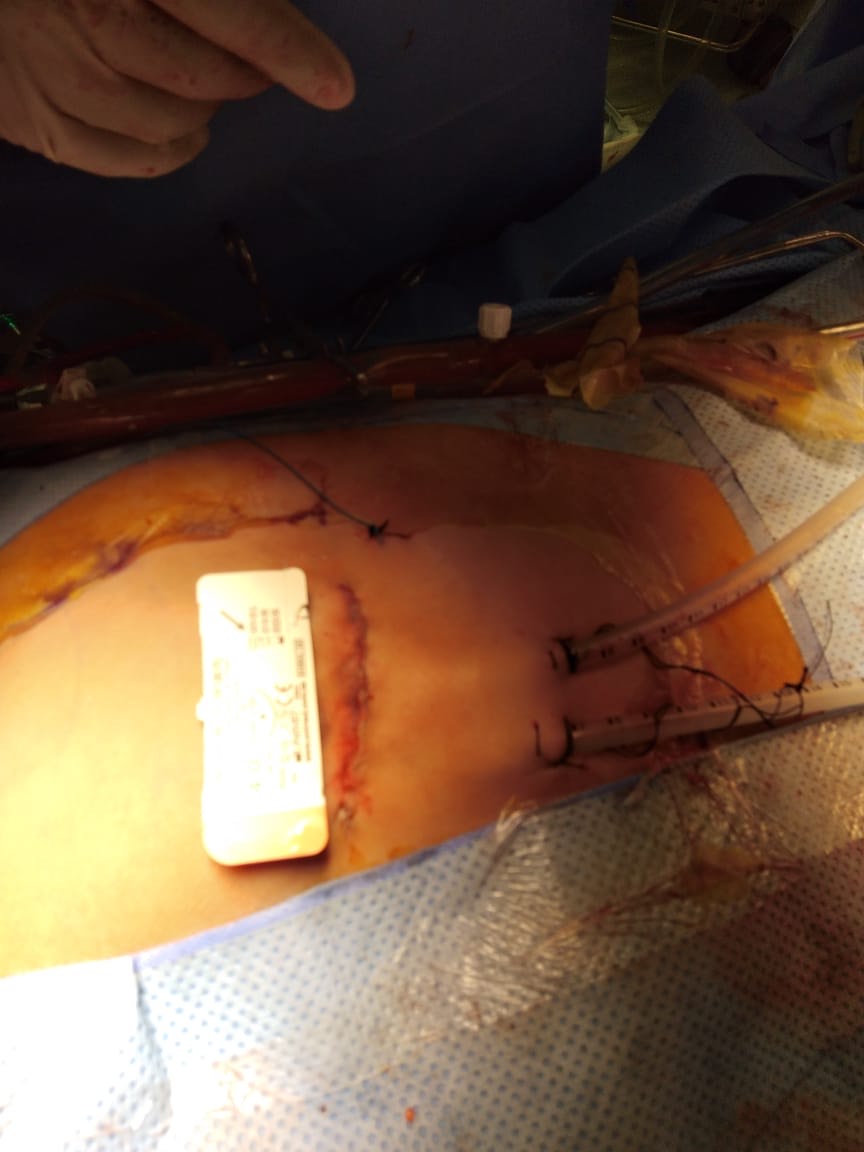
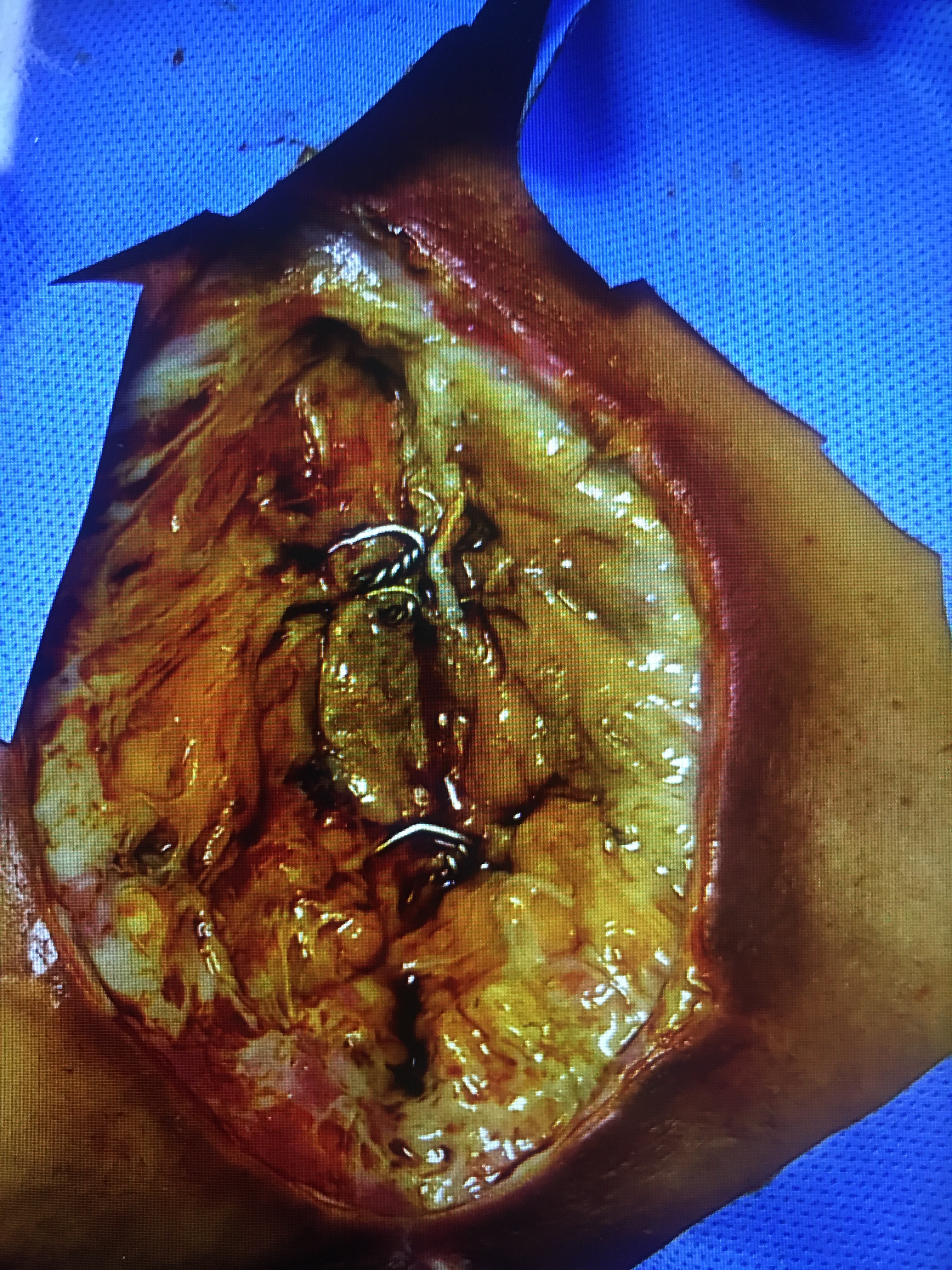
The mitral valve is approached through RMT, and infra-mammary incision (5 cm- 6 cm) is made. This incision is made (1 cm-2 cm) inferior to the nipple in men and about (1 cm) above the breast crease in women, with subsequent soft tissue dissection directed toward the chest wall to allow entry into the thoracic cavity through 4th intercostal space. The soft tissue retractor and metallic multiuse retractors allow optimal exposure (Photo 2). The pericardium was opened above and parallel to the right phrenic nerve to expose the roof of the left atrium extending from aorta to diaphragm. Exposure was enhanced by placing three stay sutures in the pericardium; small hooks (crochet) pulled the stay sutures outside chest. Poor exposure of operative field, due to high diaphragmatic dome, overcame by silk stay suture placed on the central tendon of the diaphragm and pulled out of chest. This stitch should not be released but be tied tightly at the end of the operation, to avoid any oozing from diaphragm. A combined Y-shape cardioplegia/aortic vent catheter is placed into the ascending aorta. CPB was initiated with moderate hypothermia (32℃). Venous drainage is achieved with vacuum assistance of approximately - 40 mmHg. Chitwood clamp was then inserted through a separate stab. Carbon dioxide flow used to reduce retained intracardiac gas. A special atrial retractor used to obtain MV exposure. The surgeon should size the atrial retractor blade to properly elevate the intra-atrial septum anteriorly and slightly to the left. A stab incision is made over the right sternal margin, and the retractor handle is bluntly passed though skin, and then screwed to the blade which is manually introduced into the chest (Photo 3). When the heart was empty before releasing aortic cross-clamp, a ventricular pacing wire was placed. During deairing patient was in a deep Trendelenburg position, aggressive filling the heart, positive pressure ventilation, and confirmed by TEE. After discontinuing CPB and administering protamine, decannulation was performed. The purse string sutures were tied, and femoral artery was reinforced using 5/0 Prolene suture. The thoracotomy incision was closed in the routine manner (Photo 4). Careful hemostasis is crucial and should be checked before chest closure by dental mirror to avoid bleeding from chest wall. When the hemostasis secured, two 28Fr chest drains placed into the pericardium and right pleural space. The pericardium is closed with 2-3 single sutures.
The statistical analysis was performed using the SPSS software package (version 20.0; SPSS Inc., Chicago, IL, USA). The analyzed data were expressed as number (N), percentage (%), mean (M) and standard deviation (SD) or as proportions. P-value < 0.05 was considered statistically significant.
Results
Demographics and preoperative patient data of both groups were similar except, MIMVS was more common in female gender (80%) (Table 1). Also, intra-operative data in both groups were similar except, tricuspid repair in SMS group has significant p-value < 0.05 (Table 2).
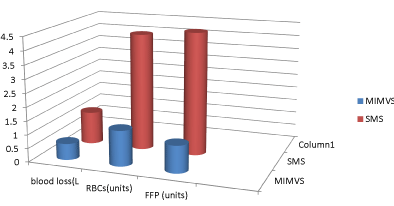
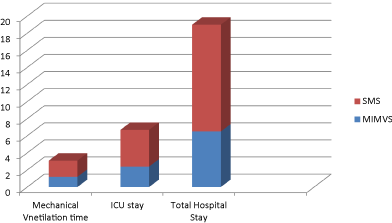
In our study, postoperative data in both groups were showing more blood loss and more blood transfusion required (Figure 1), high rate of wound infection (Photo 5), prolonged hospital stay, and more pain according to pain visual analog scale (VAS) at time of discharge in group SMS (Table 3). Duration of mechanical ventilation, ICU, and hospital stay time were more in SMS group (Figure 2).
Blood loss in MIMVS was (250 ± 60.6) vs. (550 ± 230) in SMS group, which showed significant statistically difference. Wound infection was (9) cases of SMS. In SMS group, there were (15) cases superficial sternal wound infection (SSWI), and (5) cases deep sternal wound infection (DSWI) needed vacuum [VAC]. DSWI had sternal bone exposed but still stable with positive culture (Staph. Aureus) (Photo 6).
In our study, preoperative PFT was done to all patients prior to surgery, during the morning in sitting position. The preoperative spirometric study showed no significant statistically difference between the two groups (Table 4). Postoperative Spirometric study was performed to all patients on the 7th postoperative day (POD). In group MIMVS, spirometric study revealed that, PFT had no significant reduction after surgery denoting better postoperative PFT than SMS group (Table 5).
Mitral valve replacement (prosthetic or bioprosthetic valve), and repair were similar in both groups. However, tricuspid repair (ring or mini band) and left atrial occlusion were recorded only in SMS group. There were postoperative significant p-value of FVC and FEV1, however insignificant p-value of FEV1/FVC between both groups.
There were no hospital mortality, and all patients were discharged with normal valve function according to postoperative echo. At most recent follow-up, all patients were in functional class I, with resumption of normal activity earlier than SMS group.
Discussion
Some surgeons remained concerned about the risk of groin wound infection, and risk of vascular injury due to peripheral cannulation in MICS [8]. In our study, there was not any wound infection at cannulation site. There were no recorded wound infection, vascular or neurological complications because of good preoperative preparation of cases, proper learning curve, clinical history, and CT aortography.
Peripheral cannulation and retrograde arterial perfusion carried a risk of retrograde dissection, embolization, and ipsilateral limb ischemia and was associated with an increased risk of stroke in patients with severe peripheral vascular disease (PVD) [9,10]. In our study, full clinical history, CT aortography, and with TEE guidance all those complications were avoided.
MIMVS through RMT was safe procedure as SMS approach in elderly [11]. In our study, patients aged (42.6 ± 12.8 years) in MIMVS group, however it is promising in all age groups when contraindications are absent.
MV repair through RMT provides a durable and safe alternative to SMS with benefits of improved cosmesis, reduced postoperative pain, less blood loss with fewer blood transfusions, low incidence of infection, shorter hospital stay, and faster return to activity [12]. It can be performed with the same incidence of mortality, and morbidity to SMS [13]. In our MIMVS group, 12 patients underwent mitral repair [9 with ring and 3 with mini-band] with good results with competent repair within 3-month follow up.
Some surgeons believed that RMT might be as painful as SMS. There were methods to reduce postoperative pain like, minimization of rib-spreading, and intercostal nerve block (INB) by bupivacaine injection [14]. In our study, Pain VAS at the time of discharge was better in MIMVS than in SMS with significant p-value (< 0.01) because of use of soft tissue retractor, use of INB, reduce rib spreader, and shorter incision length, avoid rib fracture, and avoid cases with chest wall deformity.
Patient selection was the first important step to prevent complications [14]. With time, despite a steep learning curve, surgeons expanded this approach to perform more complex procedures, and included patients with more comorbidity, and high body mass index (BMI) [16]. Although, obese patients had more comorbidity, they did not have an increase in mortality during cardiac surgery. MIMVS in obese patients had a lower morbidity and mortality when compared with SMS [4]. In our study, there were no significant statistically difference in preoperative comorbidity conditions; DM (3% MIMVS, 2% SMS), BSA (1.7 ± 0.1 MIMVS, 1.67 ± 0.1 SMS), NYHA class (3.50 ± 0.5 MIMVS, 3.2 ± 0.6 SMS), AF (15 MIMVA, 18 SMS), EF (53.8 ± 16.2 MIMVS, 56.8 ± 9.8 SMS), and FEV1 (2.15 ± 0.5 MIMVS, 2.7 ± 0.6 SMS)
Hybrid PCI and MIMVS in patients with prior cardiac surgery is more advantageous than CABG and mitral valve surgery by SMS [16]. However, there were neither redo nor ischemic MR cases in our study.
Many surgeons and their patients are beginning to believe that smaller incisions are always better because of less pain, faster recovery, and more satisfactory cosmetic result. However, CPB, and total operative times are significantly longer 40% or more. Smaller incisions are more “patient friendly” for the surgeons [17]. In our study, length of incision was (6.2 ± 1.3) in MIMVS, and (16.3 ± 5.8) in SMS with significant p-value.
RMT approach was associated with less new-onset AF, pneumonia, respiratory failure, and acute renal failure, lower drain output, and fewer blood transfusions [3]. Chest drains output was (250 ± 60.6ml) in MIMVS, and (550 ± 230ml) in SMS, with significant p-value < 0.01. Blood transfusion was (0.1 ± 0.53) in MIMVS, and (0.9 ± 0.7) in SMS with significant p-value < 0.05.
MIMVS is a safe procedure associated with low operative mortality. In addition to improved cosmetics, it provides shorter ventilation time; shorter ICU and hospital stay, and earlier return to full activities [18]. Preoperative predictors of mortality included advanced age, DM, smoking, dialysis, lung disease, congestive heart failure, and PVD [3]. In our study, there was not any perioperative mortality. There was no significant p-value between both groups in mechanical ventilation time (6.4 ± 1.3 MIMVS, 12.4 ± 6.8 SMS), and ICU stay (2 ± 0.4 MIMVS, 3.5 ± 1.3 SMS). However, significant p-value (< 0.001) between both groups in total hospital stay (7.2 ± 1.3 MIMVS, 12 ± 0.5 SMS)
MIMVS in elderly patients yields a lower morbidity and mortality when compared with SMS and should be considered when such individuals require valve surgery [19]. In our study, both groups included adult age. Also, we excluded ischemic or redo cases which were common in elder.
MIMVS has been associated with mortality rates of 1.2% - 5.8%. Moreover, it is often associated with enhanced recovery and better patient satisfaction [6]. Today, MIMVS became preferred at many centers owing to less postoperative bleeding and AF, reduced incidence of wound infection, and shorter hospital stays, quicker recovery, and improved cosmesis [20]. In our center, patients asked to perform MICS for cosmesis, less painful, short hospital stay especially with era of covid-19.
Cardiac surgery has shown a progressive and increasing interest towards the development of MICS. It represents a significant change in the way cardiac surgeons treat their patients today. It brings new challenges to the surgeon as well as the anesthesiologist. However, it significantly demands learning curves for the team and technical challenges for the operating surgeons are commonly required. MIMVS is rapidly growing with excellent results comparable with SMS approach: it has set equivalent perioperative mortality and less pain, less wound infection, less blood loss, less transfusions and re-explorations for bleeding, shorter hospital stay, and faster recovery.
Conclusion
Minimally invasive mitral valve surgery has been proven a feasible alternative to standard median sternotomy approach with low early morbidity and short-term mortality.
Limitations
Each group has small number of patients because of short time of the study. One center experience with some bias of selection of cases was presented. Inspite of that was prospective study, it was not randomized.
Ethics approval and consent to participate
Patient confirms that have read and understood the information about the research as provided in the participant information sheet inside his file. The study has got the formal approval and permission from Heart Team Meeting Ethical Committee at MCC approval before to start the study. The study conformed to the principles of “Declaration of Helsinki” and the investigator followed the appropriate safeguards regarding the rights and welfare of the human participants that have been included in the performed study.
Consent for publication
It was obtained written consent from patients.
Availability of data and material
It is available from recording (paper and in computer system) files and data at Madinah cardiac center adult cardiac surgery department.
Competing interest
None to declare.
Funding
None.
References
- Nissen AP, Nguyen S, Abreu J, et al. (2019) The first 5 years: Building a minimally invasive valve Program. J Thorac Cardiovasc Surg 157: 1958-1965.
- Hawkins RB, Mehaffey JH, Kessel SM, et al. (2018) Minimally invasive mitral valve surgery is associated with excellent resource utilization, cost, and outcomes. J Thorac Cardiovasc Surg 156: 611-616.
- Tang P, Onaitis M, Gaca JG, et al. (2015) Right minithoracotomy versus median sternotomy for mitral valve surgery: A propensity matched study. Ann Thorac Surg 100: 575-581.
- Santana O, Reyna J, Grana R, et al. (2011) Outcomes of minimally invasive valve surgery versus standard sternotomy in obese patients undergoing isolated valve surgery. Ann Thorac Surg 91: 406-410.
- Grossi EA, LaPietra A, Ribakove GH, et al. (2001) Minimal invasive versus sternotomy approaches for mitral reconstruction: Comparison of intermediate term results. J Thorac Cardiovasc Surg 121: 708-713.
- Saunders PC, Grossi EA, Sharony R, et al. (2004) Minimally invasive technology for mitral valve surgery via left thoracotomy: Experience with 40 cases. J Thorac Cardiovasc Surg 127: 1026-1032.
- McClure RC, Cohn LH, Wiegerinck E, et al. (2009) Early and late outcomes in minimally invasive mitral valve repair: An eleven-year experience in 707 patients. J Thorac Cardiovasc Surg 137: 70-75.
- Barbero C, Marchetto G, Ricci D, et al. (2016) Right minithoracotomy for mitral valve surgery: Impact of tailored strategies on early outcome. Ann Thorac Surg 102: 1989-1995.
- Grossi EA, Loulmet DF, Schwartz CF, et al. (2012) Evolution of operative techniques and perfusion strategies for minimally invasive mitral valve repair. J Thorac Cardiovasc Surg 143: S68-S70.
- Kandakure PR, Batra M, Garre S, et al. (2020) Direct cannulation in minimally invasive cardiac surgery with limited resources .Ann Thorac Surg 109: 512-516.
- Holzhey DM, Shi W, Borger MA, et al. (2011) Minimally invasive versus sternotomy approach for mitral valve surgery in patients greater than 70 years old: A propensity-matched comparison. Ann Thorac Surg 91: 40-415.
- Ward AF, Grossi EA, Galloway AC (2013) Minimally invasive mitral surgery through right mini-thoracotomy under direct vision. J Thorac Dis 5: 673-679.
- Vassileva CM (2016) Minimally invasive mitral repair: The cost is the same, but what is the price? J Thorac Cardiovasc Surg 151: 389-90.
- Czesla M, Mogilansky C, Balan R, et al. (2016) Evolution of a minimally invasive mitral valve program . J Vis Surg 2: 169-204.
- Santana O, Xydas S, Williams RF, et al. (2017) Minimally invasive valve surgery in high-risk patients. J Thorac Dis 9: 614-623.
- Santana O, Xydas S, Williams RF, et al. (2017) Percutaneous coronary intervention followed by minimally invasive valve surgery compared with median sternotomy coronary artery bypass graft and valve surgery in patients with prior cardiac surgery. J Thorac Dis 9: 575-581.
- Cooley DA (1998) Minimally invasive valve surgery versus the conventional approach. Ann Thorac Surg 66: 1101-1105.
- Lange R, Voss B, Kehl V, et al. (2017) Right minithoracotomy versus full sternotomy for mitral valve repair: A propensity matched comparison. Ann Thorac Surg 103: 573-579.
- Lamelas J, Sarria A, Orlando O, et al. (2011) Outcomes of minimally invasive valve surgery versus median sternotomy in patients age 75 years or greater. Ann Thorac Surg 91:79-84.
- Ailawadi G, Agnihotri AK, Mehall JR, et al. (2016) Minimally invasive mitral valve surgery i: Patient selection, evaluation, and planning. Innovations 11: 243-250.
Corresponding Author
Yasser Mubarak, MD, Department of Cardiothoracic Surgery, Faculty of Medicine, Minia University, Egypt, Tel: +201002554078, 966560708223, ORCID: 0000-0002-3068-1607.
Copyright
© 2022 Mubarak Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.