Early Pfizer Covid-19 Vaccine Elicited Spike Antibody (total, IgG, IgM) Responses Compared to Chemiluminescent Neutralizing Antibodies
Abstract
Introduction: We compared the total, IgG and IgM spike antibodies (S-Ab) to neutralizing antibody (N-Ab) responses in seronegative subjects after the first and second doses of the BNT162b2 mRNA COVID-19 vaccine.
Methods: sixty-five volunteers were tested pre-vaccination, 10 days after the first dose of vaccine, and 20 days after dose two (17 males, 48 females, mean age 41.5 ± 14.1-years). Total/IgG/IgM were assessed with Roche and Abbott immunoassays, with N-Ab using the Snibe Maglumi SARS-CoV-2 immunoassay.
Results: Ten days after first vaccination, 64.6% (n = 42) and 66.2% (n = 43) were S-Ab (total and IgG) positive; however, only 13.8% (n = 9) and 10.8% (n = 7) were IgM and N-Ab positive. Younger patients (< 50-years-old) had significantly higher S-Ab responses after the first vaccine dose (difference between age groups: total 1.12 BAU/mL, 95% CI 0-3.69, p = 0.02; IgG 11.4 BAU/mL, 95% CI 2.72-37.1, p = 0.01; IgM COI 0.24, 95% CI 0.05-0.52, p = 0.01). 20 days after second vaccination, ALL participants had high antibodies - IgG (265-7764 BAU/mL), total (274-6127 BAU/mL) and N-Ab (0.51-15.7 ug/mL). In total, females displayed greater IgG (52.5 BAU/mL, 95% CI 2.47-912, p = 0.02) and N-Ab (0.1 ug/mL, 95% CI 0.01-1.26, p = 0.03) responses than males. Total and IgG displayed better correlation with N-Ab after vaccination than IgM (total and IgG Pearson correlation coefficient both r = 0.96, IgM r = 0.78).
Conclusion: In seronegative subjects, S-Ab or N-Ab may not be reactive 10 days after a single dose of vaccine, especially males ≥ 50-years-old. However, all subjects had high total, IgG and N-Ab positive 20 days after 2 vaccinations.
Keywords
SARS-CoV-2, mRNA vaccine, COVID-19, Vaccination
Abbreviations
SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease 2019; S-Ab: Spike Antibody; N-Ab: Neutralizing Antibody; Nuc-Ab: Nucleocapsid Antibody; CAP: College of American Pathologists; ECLIA: Electrochemiluminescent Immunoassay; BAU/Ml: Binding Antibody Unit Per Ml; CLIA: Chemiluminescent Immunoassay; COI: Cut-Off Index
Introduction
With the rapid implementation of large-scale vaccination programs around the world, many people are being vaccinated against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). However, due to logistical issues, several regions are forced to extend the interval between vaccine doses [1] and some people forego the second dose entirely. According to the latest data from the Centers for Disease Control and Prevention, 53.4% of the US population has received at least one dose of SARS-CoV-2 vaccine, while only 45.2% have been fully vaccinated [2]. While these emergency measures may reduce the total number of hospitalizations and deaths in the short term, it is unclear whether the early response to a single vaccine dose is sufficiently robust. This is especially true for native seronegative patients without previous Coronavirus Disease 2019 (COVID-19). Some studies [3] have demonstrated that 9 to 12 days after vaccination, seronegative patients tend to have lower SARS-CoV-2 IgG responses than patients with previous infection. This is supported in another study [4], where 3 weeks after a single dose of the BNT162b2 SARS-CoV-2 mRNA vaccine, blocking antibodies were higher among patients with prior COVID-19 compared to initially seronegative patients (49.6 to 66.2% versus 96.0 to 97.0%). While a single dose of vaccine elicits a neutralizing antibody response in subjects with no known prior COVID-19 infection, a second dose is required to produce a significantly higher antibody titer [5], presumably offering a greater degree of protection (119 ELISA units at 56 days after a single dose of ChAdOx1 vaccine, versus 639 ELISA units at day 56 in subjects with a booster dose).
In Singapore, the national COVID-19 vaccination program stipulates a compulsory follow-up booster 21 days after the first dose for the Pfizer vaccine and 28 days for the Moderna vaccine. We undertook this study to compare the early spike antibody (S-Ab) (total, IgG and IgM) and neutralizing antibody (N-Ab) responses in seronegative subjects after the first dose of BNT162b2 mRNA Covid-19 vaccine against the subsequent responses after a second booster dose using four different antibody assays. All subjects were tested for total and IgG nucleocapsid antibodies (Nuc-Ab) at all time points to ascertain that they are truly COVID-19 naïve.
Several studies have also evaluated the difference in antibody responses to SARS-CoV-2 vaccines between different groups. While antibody responses may differ based on the presence of comorbidities such as cardiovascular disease and diabetes [6]. Several studies [7-9] have demonstrated that older individuals display a less robust antibody response than younger subjects. However, other studies [10] show a similar neutralising antibody response across different age groups of 18-55-years, 56-69-years, and 70-years or older. Some studies have also demonstrated a difference in antibody responses between genders [7], with females having a higher antibody response than males (LIAISON IgG S-Ab 339 AU/mL vs. 213 AU/mL, p = 0.001) after two doses of BNT162b2 vaccine. Thus, we also evaluated the influence of gender and age on antibody responses in our study.
Methods
Study participants
Between January to June 2021, sixty-five hospital staff (26.2% (17/65) males and 73.8% (48/65) female) from our institution (Changi General Hospital, Singapore) were tested for antibody levels pre-vaccination and 10 days after the first dose of BNT162b2 mRNA COVID-19 vaccine and 20 days after their second inoculation. All participants had no prior history of COVID-19. Participants’ ages ranged from 24-90 years-old (23.1% (15/46) ≥ 50-years-old, 70.8% (46/65) < 50-years-old, 6.2% (4/65) age unknown, mean age 41.5 ± 14.1 years). Because of different vaccination schedules, 59 out of 65 subjects were tested 20 days after the second dose of vaccine. Our hospital is a tertiary care, JCI-accredited, 1000-bed facility and our hospital laboratory is accredited by the College of American Pathologists (CAP).
Materials and instrumentation
Serum samples from peripheral venous blood were collected using the BD Vacutainer collection system in SST tubes. Serum was obtained post-centrifugation and stored at -70 degrees Celsius prior to analysis. Total S-Ab was assessed using the quantitative Roche Elecsys Anti-SARS-CoV-2 S double-antigen sandwich electro-chemiluminescent immunoassay (ECLIA) run on the Roche Elecsys e801 auto-analyser, and IgG S-Ab was assessed using the Abbott quantitative IgG S-Ab assay on the Abbott Architect. The Roche total S-Ab assay typically reports antibody titers in U/mL, with a result of ≥ 0.80 U/mL considered reactive. The Abbott IgG S-Ab reports titers in units of AU/mL, with ≥ 50 AU/mL considered positive. However, recently both the Roche and Abbott quantitative total/IgG S-Ab assays are now traceable to the 1st WHO International Standard units (Binding antibody unit per mL [BAU/mL]); Abbott Architect BAU/mL = 0.142 × AU/mL (Abbott user circular), and Roche total S-Ab BAU/mL = 0.97 × U/mL (Roche user circular), allowing comparability of results. As such, we used BAU/mL for all results for total/IgG S-Ab in our study. The Roche total S-Ab assay thus has a positive threshold of ≥ 0.78 BAU/mL, upper limit of 243 BAU/mL (dilution range up to 1:100), limit of detection 0.34 of BAU/mL, and reported precision of 2.9%/1.4% at 0.47/178 BAU/mL; with a reported assay sensitivity of 98.8% and specificity of 99.98%. The Abbott IgG S-Ab assay has a measuring range of 3.0-5680 BAU/mL, positive cut-off of ≥ 7.1 BAU/mL, reported precision 4.9%/5.1% at 6.8/5115 BAU/mL, limit of detection of 1.0 BAU/mL, and reported sensitivity 66-99% and specificity 99.6%. We subscribe to the CAP external quality assessment for COVID-19 serology assays.
Qualitative IgM S-Ab testing was performed on the Abbott Architect SARS-CoV-2 IgM S-Ab assay (positive cut-off index (COI) ≥ 1.0), whose performance has been previously reported [11]. N-Ab was assessed on the Snibe automated competitive quantitative SARS-CoV-2 chemiluminescent immunoassay (CLIA) (Snibe Maglumi). The Snibe N-Ab assay is a competitive chemiluminescence immunoassay, where the SARS-CoV-2 N-Ab in the sample competes with magnetic micro beads (coated with ACE2 antigen) for binding with ABEI labelled with recombinant SARS-CoV-2 S-receptor-binding domain antigens. After precipitation and washing, starter reagents are added to initiate a chemiluminescent reaction where the generated light signal is inversely proportional to the sample N-Ab. The assay has a measuring range of 0.05-30 ug/mL, with ≥ 0.3 ug/mL regarded as positive (reported inter-assay precision is 1.27% and 1.01% at 0.079 and 21.192 ug/mL, limit of detection 0.045 ug/mL, sensitivity of 100% and specificity 100%).
To exclude previous COVID-19 infection or asymptomatic COVID-19 infections during our study, all subjects were tested on two previously evaluated CLIA/ECLIA Nuc-Ab assays (Abbott IgG, positive COI ≥ 1.4; Roche total antibody, positive COI ≥ 1.0) [12,13] at all time points (baseline, 10 days after first dose, and 20 days after second dose).
Statistical analysis
Data were presented in either mean ± standard deviation or median [inter-quartile range], as appropriate. No indeterminate or missing results were used. Antibody titers at different time points were compared using Mann-Whitney U testing (MedCalc Statistical Software version 20, MedCalc Software Ltd, Ostend, Belgium), with p < 0.05 considered statistically significant. Regression analysis was also performed for results between Roche/Abbott S-Ab and N-Abs. Our IRB deemed this work exempt as this was part of routine laboratory evaluation of new assays as well as a seroprevalence survey using de-identified leftover sera. However, we obtained informed consent from all volunteers as they needed to provide blood samples on multiple occasions. Compliance with STARD and STROBE guidelines are enclosed (see Supplementary Table A and Supplementary Table B).
Results
Nucleocapsid antibodies and proof of COVID-19 naivety
As pre-vaccinated/vaccinated subjects may contract or have previously contracted asymptomatic/subclinical COVID-19 pre- or post-vaccination and confound the antibody response, Nuc-Abs were assessed at each blood draw (baseline, 10 days after the first vaccination, and 20 days after the second vaccination) on both the Roche and Abbott Nuc-Ab automated chemiluminescent assays. All subjects were Nuc-Ab negative on both Roche total Nuc-Ab and Abbott IgG Nuc-Ab assays at all time points, thus confirming their COVID-19 naïve status (see Supplementary Table C).
Post-vaccination responses
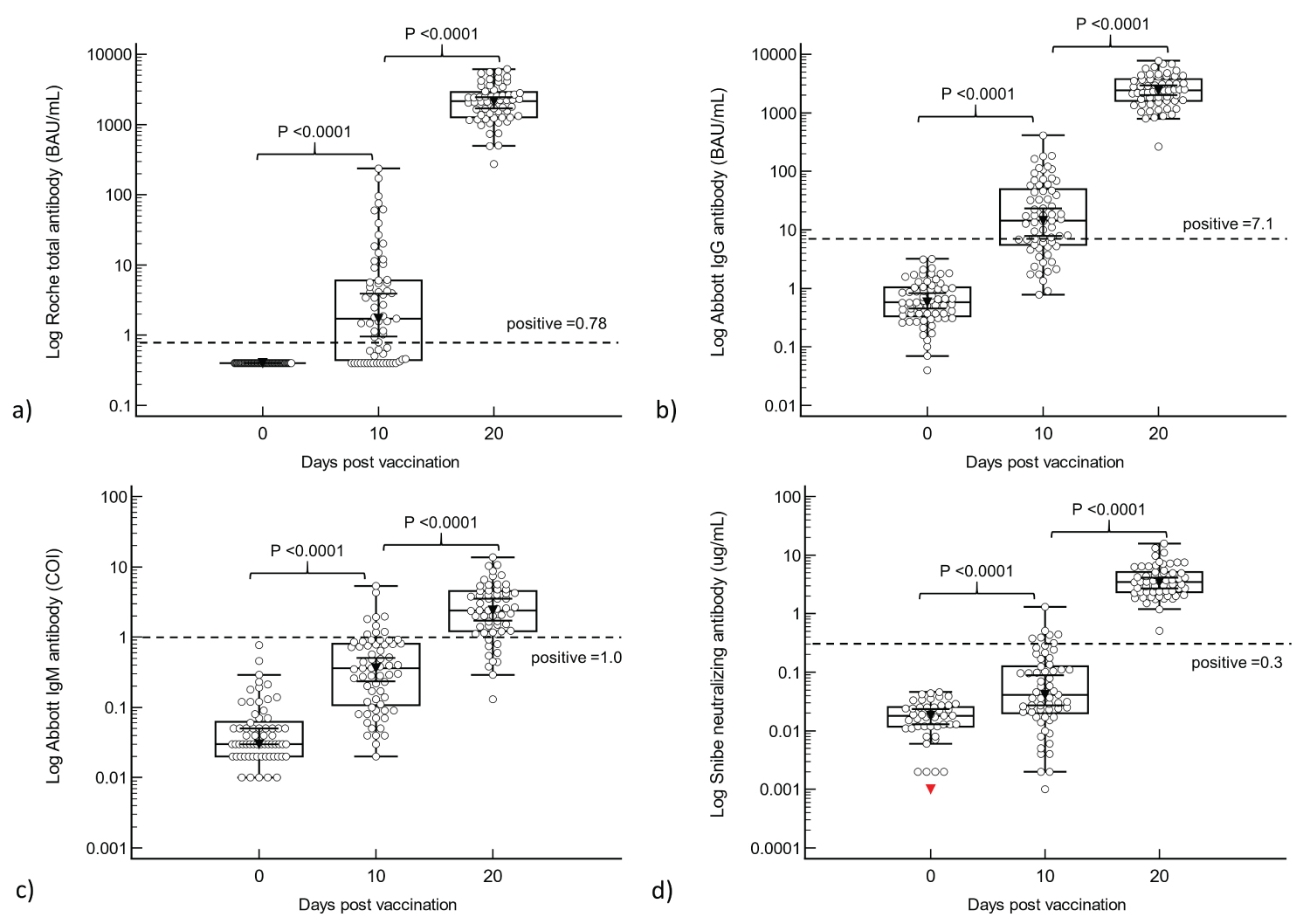
Both S-Ab (Total, IgG and IgM) and N-Ab titers rose in response to the first and second doses of vaccine (see Figure 1 and Supplementary Table D). At 10 days after the first vaccination, 64.6% (n = 42) and 66.2% (n = 43) were positive on the Roche total S-Ab and Abbott IgG S-Ab respectively. However, only 13.8% (n = 9) and 10.8% (n = 7) were positive on the IgM and N-Ab assays. In contrast, at 20 days after the second vaccination, ALL participants had high antibody titers: Abbott IgG range 265-7764 BAU/mL, median 2412 BAU/mL; Roche total range 274-6127 BAU/mL, median 2146 BAU/mL) and N-Ab (range 0.51-15.7 ug/mL, median 3.48 ug/mL). However, only 83% (48/58) were IgM S-Ab positive. With the upper limit of the on-board measuring range of the Abbott IgG S-Ab at 5680 BAU/mL, only 4 samples required dilution, whereas the Roche total S-Ab required dilution for all samples (on-board measuring range upper limit of 243 BAU/mL). Although Mann-Whitney U analysis showed that there was a significant increase in antibodies between each time point (see Figure 1), the increases between pre-vaccination and the first vaccination (total S-Ab: 1.32, 95% CI 0.73 to 3.02; IgG S-Ab 13.7, 95% CI 7.78 to 18.4; IgM S-Ab 0.28, 95% CI 0.19 to 0.42; N-Ab 0.03, 95% CI 0.02 to 0.05) were generally overshadowed by the marked increase between first vaccination and second vaccination (total S-Ab 2141, 95% CI 1754 to 2419; IgG S-Ab 2387, 95% CI 2113 to 2809; IgM S-Ab 1.92, 95% CI 1.36 to 2.64; N-Ab 3.33, 95% CI 2.71 to 3.77).
Age and gender analysis
Ten days after the first vaccination, females had a significantly higher IgM response, and at 20 days after the second vaccination, females had a significantly higher IgG response (see Table 1). When all results at 10 and 20 days were analysed, females displayed greater IgG (52.5 BAU/mL, 95% CI 2.47-912, p = 0.02) and N-Ab (0.1 ug/mL, 95% CI 0.01-1.26, p = 0.03) responses than males. When comparing antibody titers between groups ≥ 50 and < 50-years-old, the only significant difference was in the total/IgG/IgM S-Ab titers 10 days after the first vaccination, where younger subjects had a brisker antibody response than older subjects (see Table 2). However, by 20 days post-vaccination, there was no significant difference between the age groups (Table 1 and Table 2).
Agreement between antibodies
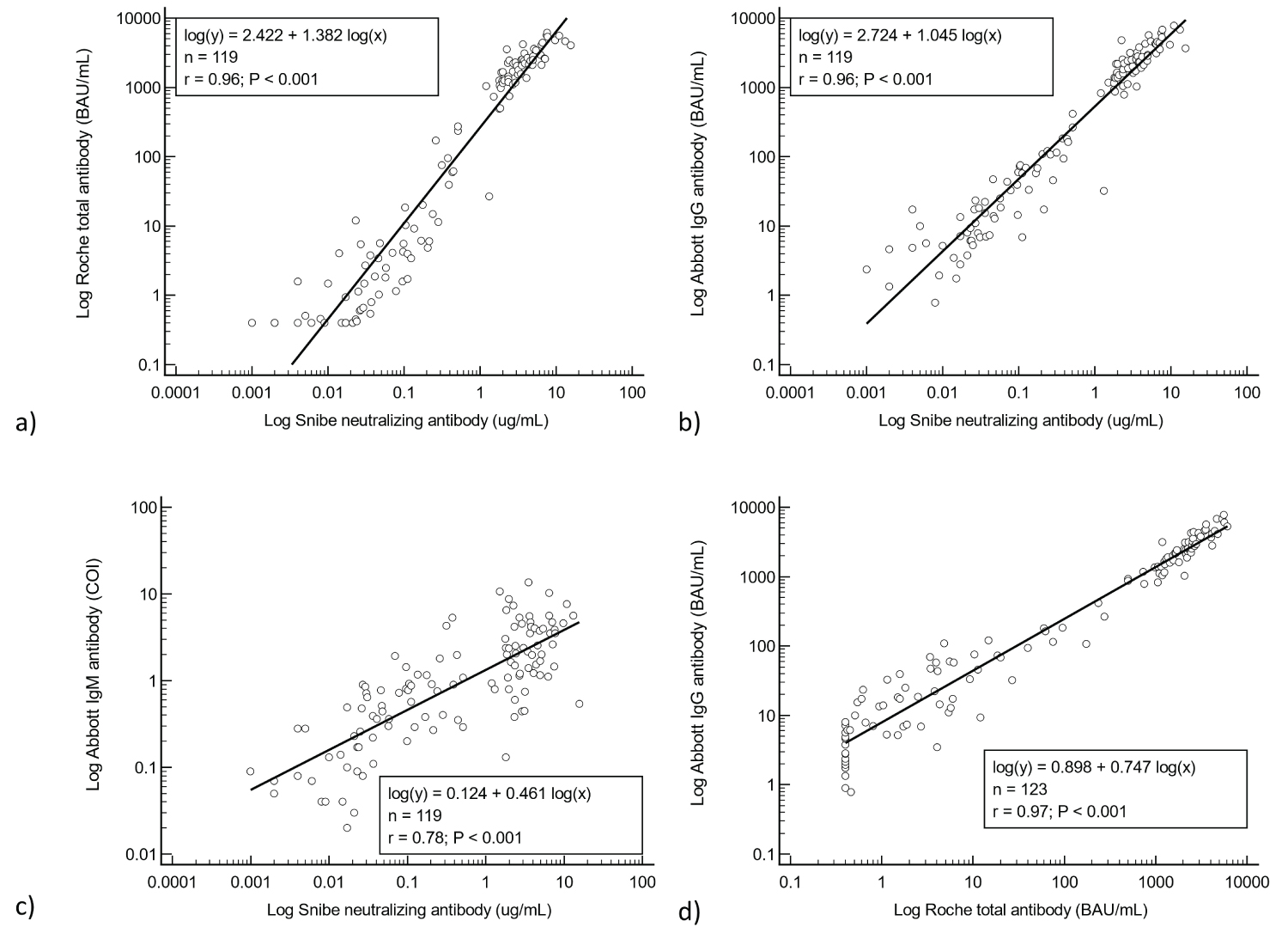
Regression analyses of all results after the first and second vaccination doses showed good agreement between the S-Abs and the N-Ab (Pearson correlation coefficients: total S-Ab R2 = 0.92, IgG S-Ab R2 = 0.93) (see Figure 2). However, the agreement between IgM S-Ab and N-Ab was less close (R2 = 0.61). The agreement between the total and IgG antibodies, both expressed in BAU/mL (see Figure 2d) was also very good (R2 = 0.95).
Discussion
Although it is well demonstrated that a single dose of vaccine elicits an antibody response in many studies [3,4,6], the practical efficacy of a single dose of vaccine has been called into question [14]. When the incidence of COVID-19 among vaccinated HCWs was compared between those who received a second dose and those who did not, there was a greater incidence of COVID-19 among the HCWs with only one dose (51.3/1000 workers vs. 11.5/1000 workers in week 4). In another study of 3052 HCWs who received at least 1 dose of Pfizer vaccine [15], 51 vaccinees tested positive for SARS-CoV-2 during follow-up, with a greater proportion of these positives after the first dose of vaccine (41 before and 10 after the second dose, with an incidence rate ratio 0.42-0.59 before second dose, 0.04-0.20 after the second dose).
This may be explained in part by our study, where only a small proportion (10.8%) of individuals were positive for neutralizing antibodies 10 days after a single vaccine dose, while the majority were positive for total and IgG spike antibodies at this point (64.6% and 66.2%); it was only after the second vaccination where 100% of subjects had positive total, IgG and neutralizing antibodies. This has implications for the use of single vaccine dosing in the general population, especially for those who are initially seronegative, as a single vaccine dose may not mount a sufficiently strong neutralizing antibody response. However, a second dose produced a much more robust surge in antibody levels, with all subjects having total S-Ab titers several times higher than the upper limit of the assay. Indeed, the increase in antibody levels between 10 days after the first vaccine dose and 20 days after the second vaccination was 1622x greater for total S-Ab, 174x greater for IgG S-Ab, 6.9x greater for IgM S-Ab, and 111x for N-Ab respectively. This lends support to the argument that adequate vaccination in initially seronegative subjects probably requires two vaccine doses. Notably, all samples at 20 days post-vaccination exceeded the measuring range of the total S-Ab assay, and modifications may be required on the total S-Ab assay to accommodate the assessment of post-vaccination samples.
We confirm that females displayed a greater IgG and IgM responses than males. We also demonstrated that older individuals develop a significantly weaker response to a single dose of vaccine. This has implications in single-dose vaccination programs, where older, male individuals aged 50-years and above may probably require an additional second dose of vaccine without delayed intervals between doses to ensure adequate protection. This is supported in a recent study [16] examining the performance of a single dose of BNT162b2 vaccine in 10,400 long-term care facility residents 80-91 years of age which found that the vaccine was only 65% effective 35-48 days after vaccination. Another study [17] showed that the same vaccine had an effectiveness of 69.4% in preventing hospital admissions with respiratory infection in patients > 80-years-old at least 14 days after receipt of the first dose. Further randomised control trials would be desirable before adoption of the one-dose vaccination schedule for the BNT162b2 vaccine, especially in the elderly.
Expectedly, nucleocapsid antibodies did not rise in response to either the first vaccine dose or the second inoculation in all our subjects. This finding has also been reported in other studies [4,18,19] underscoring the specificity of the mRNA vaccine. Nucleocapsid antibody assays may still have a role in the detection of recurrent COVID-19 in post-vaccinated subjects. The Nuc-Ab negative status of our subjects throughout the study also confirms their COVID-19 naivety even at pre-vaccination. Documenting COVID-19 sero-negativity is essential, as up to 56.9% of post-vaccination infection may be asymptomatic [15] and confound the antibody response.
Although all S-Ab titers have generally good agreement with N-Ab, we noted that N-Ab agreed less with IgM S-Ab. This is expected, as we also noted that IgM S-Ab was less sensitive than total/IgG responses in a previous evaluation in COVID-19 infections [11]. A recent study has also reported a muted IgM S-Ab response following vaccination [19]. In our study, a minority of individuals (13.8%) were IgM S-Ab positive 10 days after the first vaccination. This may be because IgM levels may have already peaked earlier or yet to peak 10 days after the first vaccination or may have declined 20 days after the second vaccination. In other studies [20], the post-vaccination IgM peak only occurred 14 days later. This underscores the importance of selecting a suitable time point for antibody testing, especially in seronegative individuals who mount a less prominent response than subjects with previous COVID-19 [21]. We also found that paired Abbott IgG and Roche total antibody results (with different cut-offs for positivity, due to the analysis of different antibodies), when expressed in BAU/mL, were much closer with good correlation (r = 0.97). This is in contrast to that reported by Perkmann, et al. [22] using the same assays and manufacturer recommended units (r = 0.88).
We report the following unique, novel findings in our study:
• Only a minority of initially seronegative subjects (10.8%) developed reactive titers of N-Ab 10 days after the first dose of vaccine, requiring a second booster dose before becoming N-Ab reactive. However, all subjects had very high S-Ab (total and IgG) and N-Ab titers after a second inoculation. This lends support to the notion that initially seronegative subjects may require two doses of vaccine before they mount a sufficiently robust antibody response.
• 20 days after the second vaccine dose, total S-Ab titers exceeded the assay upper limit on the Roche platform but not the Abbott assay; the Roche assay may require modification to accommodate post-vaccination samples with high antibody titers.
• Samples in this study were not contaminated by asymptomatic/subclinical disease as Nuc-Ab remained negative throughout, which proves that they had no new COVID-19 throughout the study and had no reported history of COVID-19 prior to and during the study. Thus, Nuc-Ab will still be useful as a marker for the assessment of COVID-19 infection in individuals who have been vaccinated with the mRNA vaccine.
• There is a significant difference in the S-Ab responses between gender and age groups after a single dose of vaccine, with older male subjects having a less robust response.
• Spike antibodies (Total/IgG/IgM) correlated closely with N-Ab; the relationship with IgM was less strong.
• There is very good agreement between total and IgG reported in international WHO units.
A limitation of our study is that we only managed to capture a single time point after the first vaccination. A second time point just prior to the second booster dose would have been useful to clarify our findings. We were only able to study the antibody responses to a single type of vaccine. We were unable to recruit cases with previous COVID-19, to study their response to vaccination. We were also limited by a small study population at a single center. Our study population was also predominantly female (73.8%) and < 50-years-old (70.8%), and future studies with larger numbers of male subjects ≥ 50-years-old would be desirable.
Conclusion
Whilst there is a brisk S-Ab and N-Ab response to vaccination, not all initially seronegative subjects may mount a sufficiently reactive N-Ab response from a single dose of vaccine, especially if male and ≥ 50-years-old. Such seronegative vaccines may require a second booster dose before developing a reactive N-Ab. This may have implications in vaccine allocation in population vaccination programs.
Disclosures
All co-authors have contributed to the study and manuscript.
Declarations of Interest
None.
References
- Plotkin SA, Halsey N (2021) Accelerate Coronavirus Disease 2019 (COVID-19) Vaccine Rollout by Delaying the Second Dose of mRNA Vaccines. Clin Infect Dis 73: 1320-1321.
- https://covid.cdc.gov/covid-data-tracker/#vaccinations.
- Krammer F, Srivastava K, Alshammary H, et al. (2021) Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 384: 1372-1374.
- Bradley T, Grundberg E, Selvarangan R, et al. (2021) Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med 384: 1959-1961.
- Folegatti PM, Ewer KJ, Aley PK, et al. (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396: 467-478.
- Shrotri M, Fragaszy E, Geismar C, et al. (2021) Spike-antibody responses to ChAdOx1 and BNT162b2 vaccines by demographic and clinical factors (Virus Watch study). medRxiv. doi: https://doi.org/10.1101/2021.05.12.21257102.
- Pellini R, Venuti A, Pimpinelli F, et al. (2021) Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 100928.
- Muller L, Andree M, Moskorz W, et al. (2021) Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis 381.
- Prendecki M, Clarke C, Brown J, et al. (2021) Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 397: 1178-1181.
- Ramasamy MN, Minassian AM, Ewer KJ, et al. (2021) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 396: 1979-1993.
- Lau CS, Hoo SP, Liang YL, et al. (2021) Performance of an automated chemiluminescent immunoassay for SARS-COV-2 IgM and head-to-head comparison of Abbott and Roche COVID-19 antibody assays. Pract Lab Med 25: e00230.
- Lau CS, Oh HML, Hoo SP, et al. (2020) Performance of an automated chemiluminescence SARS-CoV-2 IG-G assay. Clin Chim Acta 510: 760-766.
- Lau CS, Hoo SP, Yew SF, et al. (2020) Evaluation of an electrochemiluminescent SARS-CoV-2 antibody assay. J Appl Lab Med 5: 1313-1323.
- Benenson S, Oster Y, Cohen MJ, et al. (2021) BNT162b2 mRNA Covid-19 Vaccine Effectiveness among Health Care Workers. N Engl J Med 384: 1775-1777.
- Tang L, Hijano DR, Gaur AH, et al. (2021) Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA 325: 2500-2502.
- Shrotri M, Krutikov M, Palmer T, et al. (2021) Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis S1473-3099: 00289-00289.
- Hyams C, Marlow R, Maseko Z, et al. (2021) Effectiveness of BNT162b2 and ChAdOx1 nCoV-19 COVID-19 vaccination at preventing hospitalisations in people aged at least 80 years: A test-negative, case-control study. Lancet Infect Dis 3099: 330-333.
- Mueller T (2021) Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: A method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta 518: 9-16.
- Gobbi F, Buonfrate D, Moro L, et al. (2021) Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 Infection. Viruses 13: 422.
- Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. (2021) T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med 27: 270-278.
- Anichini G, Terrosi C, Gandolfo C, et al. (2021) SARS-CoV-2 Antibody response in persons with Past natural infection. N Engl J Med 385: 90-92.
- Perkmann T, Perkmann-Nagele N, Koller T, et al. (2021) Anti-Spike protein assays to determine post-vaccination antibody levels: A head-to-head comparison of five quantitative assays. Microbiol Spectr e0024721.
Corresponding Author
Tar-Choon AW, MBBS, Department of Laboratory Medicine, Changi General Hospital, 2 Simei Street 3, 529889, Singapore, Tel: +65-68504927, Fax: +65-64269507.
Copyright
© 2021 LAU CS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.