Editorial on Advances in Biological Viruses
Introduction
Viruses is a very tiny and microscopic organism that exists almost everywhere on earth. It can infect all type of life forms from humans, animals, plants, fungi and even bacteria. It is not necessary that all viruses are harmful. The fact of virus is that they cannot replicate without a host cell and there is no cure for virus but vaccination can prevent them from spreading. Because of such huge list of virus they are classified in groups. Group I: dsDNA viruses; Group II: ssDNA viruses; Group III: dsRNA viruses; Group IV: (+) ssRNA viruses; Group V: (−) ssRNA viruses; Group VI: ssRNA-RT viruses; Group VII: dsDNA-RT viruses [1,2].
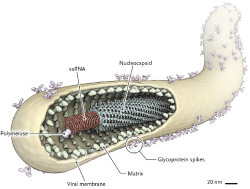
Below are few addressed viruses and it's aspects from abundant viruses that will help to study more about virus Figure 1.
Gene Edited Pigs Resistant to Billion Dollar Virus
Virus called Porcine Reproductive and Respiratory Syndrome (PRRS) which causes breathing problems and deaths in young animals. The virus infects pigs using a receptor on their cells surface called CD163. PRRS alone costs pig industry approximately $2.5 billion each year lost revenues in United States and Europe.
Researchers at University of Edinburgh's Roslin Institute have produced pigs that can resists one of the world's most costly animal disease. They invented a new method that use gene editing to create PRRS resistant pigs by removing the whole CD163 receptor. They also said that eliminating only a section CD163 allows the receptor to retain its ordinary function in the body and reduces side effects [3,4].
Giant Virus in the Waters of Oahu, Hawaii
Most of the phytoplankton that produces in the ocean everyday gets eaten, thereby supporting animals in the marine food web. However, it is common for viral infections to spread through inhabitants of phytoplankton. When this happens, the infected phytoplankton cells disintegrate and are decomposed by bacteria, diverting food source away from the animals.
Scientist at Daniel K Inouye Center for Microbial Oceanography at University of Hawaii at Manoa have characterized a novel, unusually large virus that infects common marine algae. "Most viruses are very tiny that requires an electron microscope to see them but these giants challenging bacteria size, and their genomes often code for functions that have never seen in viruses before". described by Schvarcz and Steward [5,6].
Virus Adapt Through Newly Revealed Path of Evolution
Normally viruses infect by attaching themselves to molecular receptors on surface of cells. These receptors are the "locks" that viruses open to enter cells. The "keys" to these locks are viral proteins called host recognition proteins. Scientist have focused on how mutations alter these protein keys and what kind of changes allow them to access new locks. Researchers have already know that virus can gain new keys with relatively few mutations but they have not solved the mysteries of how these mutations could appear.
Experts at University of California, San Diego have studied that how lambda virus that infects bacteria but not humans through lab experiments that provided evidence for a new path of evolution and with a deeper understanding that how quickly viruses can adapt to the environment [7,8].
Scientist Discover Marburg Virus Treatment
With a mortality rate of 88 percent, Marburg virus can rip through a community in days. In the year 2005, an outbreak of Marburg virus struck pediatric ward in the country of Angola. With no treatment available, virus killed three hundred and twenty-nine out of three hundred and seventy-four infected patients.
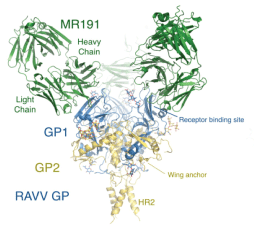
The Scripps Research Institute (TSRI) created a map of the virus structure and discovered first treatment for Marburg virus, a pathogen with same pandemic potential as Ebola virus. They revealed that through high-resolution imaging about MR191.
This antibody could finally give a way to successfully treat the disease. "This is the unique antibody that could treat Marburg virus" Figure 2 was confirmed by Dr. Erica Ollmann Saphire, PhD, a TSRI professor, experienced and senior author of the study [9,10].
Pandoravirus Invent Their Own Genes
In the year 2013, the discovery of two gigantic viruses unlike anything seen before confused the line between the viral and cellular world. Pandoraviruses are as big as bacteria and contain genomes that are more complex than those already found in some eukaryotic organisms; their strange amphora shape and massive, atypical genome led the researchers to wonder where they came from.
As per The National Center for Scientific Research (CNRS) three new members have been isolated and added to the Pandoravirus family by scientist at Structural and Genomic Information Laboratory. They offer an explanation to their perplexing massive genomes with many orphan genes; Pandoraviruses appears to be factories for new genes and therefore new tasks. From odds of nature to evolutionary innovators, giant viruses continue to shake branches on the tree of life [11,12].
To conclude, Annals of Microbiology and Research Journal will be committed to move forward with the international research community to succeed toughest possible scientific picture on coming up extent for exceptional quality for human mankind.
References
- https://www.medicalnewstoday.com/articles/158179.php.
- https://en.wikipedia.org/wiki/Virus.
- Christine Burkard, Tanja Opriessnig, Alan J Mileham, et al. (2018) Pigs lacking the scavenger receptor cysteine-rich domain 5 of CD163 are resistant to PRRSV-1 infection. Journal of Virology.
- www.sciencedaily.com/releases/2018/06/180620150139.htm.
- Christopher R Schvarcz, Grieg F Steward (2018) A giant virus infecting green algae encodes key fermentation genes. Virology 518: 423-433.
- www.sciencedaily.com/releases/2018/05/180503101643.htm.
- Katherine L Petrie, Nathan D Palmer, Daniel T Johnson et al. (2018) Destabilizing mutations encode nongenetic variation that drives evolutionary innovation. Science 359: 1542-1545.
- www.sciencedaily.com/releases/2018/03/180329141037.htm.
- Liam B King, Marnie L Fusco, Andrew I Flyak, et al. (2018). The Marburgvirus-Neutralizing Human Monoclonal Antibody MR191 Targets a Conserved Site to Block Virus Receptor Binding. Cell Host Microbe 23: 101-109.
- www.sciencedaily.com/releases/2018/01/180110123921.htm.
- Matthieu Legendre, Elisabeth Fabre, Olivier Poirot, et al. (2018) Diversity and evolution of the emerging Pandoraviridae family. Nat Commun 9: 2285.
- www.sciencedaily.com/releases/2018/06/180611133505.htm.
- https://www.nejm.org/doi/full/10.1056/NEJMp1405314.
- https://www.sciencedaily.com/releases/2018/01/180110123921.htm.
Corresponding Author
Manu Mitra, Member IEEE, University of Bridgeport, Connecticut, USA.
Copyright
© 2018 Mitra M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.