Bacterial Biofilm and its Clinical Implications
Abstract
Microbial biofilm created huge burden in treatment of both community and hospital infections. A biofilm is complex communities of bacteria attached to a surface or interface enclosed in an exopolysaccharide matrix and protected from unfavorable conditions such as presence of antibiotics, host defense or oxidative stresses. Biofilms are often considered hot spot for horizontal gene transfer among same or different bacterial species. Furthermore, bacteria with increased hydrophobicity facilitate biofilm formation by reducing repulsion between the extracellular matrix and the bacterium. There is a marked increase in the rate of persons nonresponsive to antibiotic therapy for infections of the Urinary Tract (UTIs), burns and upper respiratory tract due to biofilm formations. It is estimated that 90% of nosocomial infections are mediated by biofilm. The role of biofilm in infections has become so great that the treatment of such antibiotic resistance infections is proving difficult and costly to health care systems. The biofilm related infections varied from dental plaque, destruction of prosthetic valve to death of cystic fibrosis patients. This review aims to provide a summary of role of bacterial biofilm and its clinical implications for the patients.
Keywords
Biofilm, Bacterial infections, Antibiotic resistance, Plasmids
Introduction
Bacteria can be found in natural ecosystem in two forms, planktonic cells; better freedom of migration, more prone to mutation, sensitivity to environment, more active metabolically, sensitive to antibiotics and other antimicrobial agents and biofilm form; better protection, less subjects to mutation, are resistance to antibiotics and disinfectants and are less active metabolically. Biofilms are surface attached communities of bacteria, encased in an extracellular matrix of secreted proteins, carbohydrates, and/or eDNA, that creates phenotypes distinct from those of planktonic cells [1]. Microbes form a biofilm in response to various different factors which may include cellular recognition of specific or non-specific attachment sites on a surface, nutritional scarcity, or exposure of planktonic cells to subinhibitory concentrations of antibiotics or disinfectants [2]. When a cell switches to the biofilm mode of growth, it undergoes a phenotypic shift in behavior in which large number of genes are differentially regulated [3]. For example, biofilm formation in Escherichia coli was shown to involve the differential expression of 230 genes, including those encoding proteins associated with adhesion and auto-aggregation, outer membrane proteins (OmpC, OmpF, OmpT, and Slp), and proteins involved in lipid A biosynthesis [4]. Modarressi, et al. showed RND efflux pump and quorum sensing genes influenced by iron limitation in biofilm Acinetobacter baumannii [5].
Places in Which the Biofilms are Mostly Form
Nearly 80% of bacteria in natural environment can form biofilm, recent studies indicated biofilms can grow in the most extreme environments from the extremely hot, briny waters of hot springs ranging from very acidic to very alkaline, to frozen glaciers [6]. However, a detailed examination of biofilms would await the electron microscope, which allowed high-resolution scanning electron microscopy (SEM) at much higher magnifications than did the light microscope [7]. Most places that biofilm are formed in hospitals are medical devices on catheters, ventilators or other hospital devices [8]. In addition, biofilm can form on teeth, middle ear, GI, and respiratory tract (cystic fibrosis patients). Biofilms also form on soft tissue surfaces in living organisms or at liquid-air interfaces [9].
The biofilms on teeth can either be in an uncalcified state that can be removed by dental instruments, or a calcified state which is more difficult to remove. On the surface of teeth, they frequently subject to oxidative and acid stresses. Dietary carbohydrates can cause a dramatic decrease in pH in oral biofilms to values of 4 and below (acid stress). A pH of 4 at body temperature of 37 ℃ causes depurination of DNA, leaving apurinic (AP) sites in DNA especially loss of guanine [10].
Biofilms Impacts
Richard Chole, Washington University School of Medicine in St. Louis was the first who discovered that bacteria often form biofilms in the wet and warm folds of the tonsils, and that these may serve as reservoirs of infection [11]. Recurring tonsil infections and sore throats are a fact of life for many children. The pediatrician prescribes antibiotics, and they help for a while, but the infection returns. The pattern repeats itself until the doctor or the frustrated parents finally decide that the wrinkly olive-sized ovals of inflamed tonsil tissue must come out. Why, they wonder, can't the body eliminate these infections, even with the help of antibiotics? This question later resolved by discovery of biofilm and its clinical implications. Sanclement, et al. [12] reported that biofilms are prevailed on the removed tissue of 80% of patients had surgery for chronic sinusitis. Infections associated with the biofilm growth usually are difficult to eradicate and has several implications on our life, for example, disease prolongation, collapse of antibiotic therapy, prolong hospital stay and increase in severity of infections [13]. In ecosystems biofilm has positive role in acceleration of natural cycles of biogenic elements like C, N2, P, S, O2 [14].
Steps in Biofilm Formation
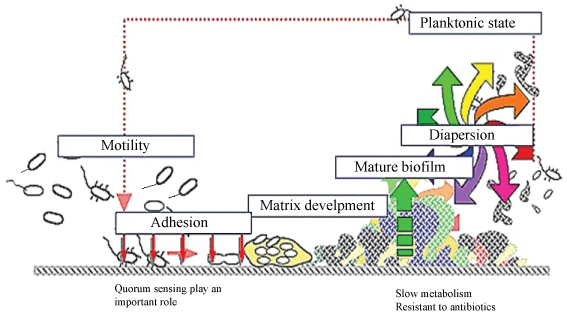
Biofilm formation is initiated by interaction of cells with a surface or with each other. It is thought that the planktonic bacteria adhere to the surface initially through weak, reversible adhesion via van der Waals forces and hydrophobic effects aggregate, then the cells form an extracellular matrix. This matrix encases the cells within it and facilitates communication among them through biochemical signals as well as genetic exchange. It contained the microbial cells, exopolysaccharide and water [15]. Other substances often found in the biofilm include extracellular DNA, RNA, and proteins reaching approximately 2% in total [16]. Dispersion is final state in biofilm, at the time of dispersal, microcolonies undergo cell death and lysis along with active dispersal of motile bacteria to leave behind hollow colonies. A biofilm is thought to maintain equilibrium via growth and dispersal. Dispersal is believed to occur either as single cells or as small microcolonies [17]. Dispersion help the biofilm producing bacteria to detach from biofilm body and form another biofilm microcolonies spread to the environment. It should be noted that structure of biofilms is dramatically different due to the specific organisms in the film and environmental conditions. Steps in biofilm formation are illustrated in Figure 1. The in-vitro observations available today suggest that it is more likely that biofilm formation proceeds through a series of temporal events that reflect adaptation to nutritional and environmental conditions [18].
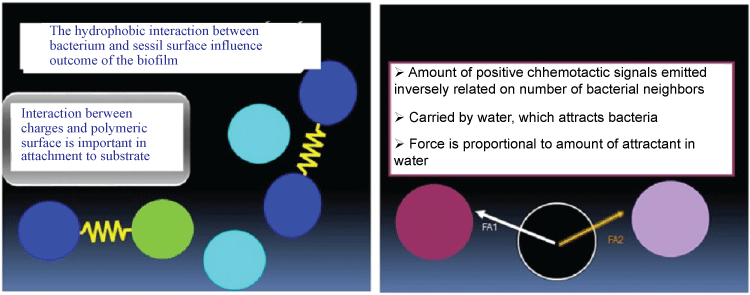
Hydrophobicity and electro kinetic potential of bacterial cells depend on their surface composition and structure, where lipopolysaccharide, in Gram-negative bacteria, may affect the ability of bacteria to form biofilms. Bacteria with increased hydrophobicity have reduced repulsion between the extracellular matrix and the bacterium [19]. Some bacteria species are not able to attach to a surface on their own due to their limited motility but are instead able to anchor themselves to the matrix or directly to other, earlier bacteria colonists. Non-motile bacteria cannot recognize surfaces or aggregate together as easily as motile bacteria [20]. Figure 2 shows the mechanism of interaction, hydrophobicity and charges play in biofilm matrix formation. Further studies revealed that iron play an important role in biofilm formation and expression of biofilm related genes in Acinetobacter baumannii [21]. Analysis of bap expression by rqRT-PCR revealed five isolates with four-fold bap overexpression in the presence of low iron concentration (20 µM) [21]. Similarly, the ability of A. baumannii to form biofilms is also largely dependent on pili, which mediate attachment and biofilm formation. In addition, csuE, is a member of the usher-chaperone assembly system. The genes are cluster together in form of the csu operon, the products of which form a pilus-like bundle structure in this bacterium. This gene has proved to be an important factor of A. baumannii biofilm formation [22].
Role of Plasmids in Bacterial Biofilms
It is well established that the biofilm is an important niche for horizontal gene transfer (HGT) by transformation in naturally competent bacteria and that biofilm development and competence are mediated and regulated by many of the same gene products [23,24]. Plasmid transfer has been shown to occur in many natural biofilm communities, such as soil, water, plant leaves, river rocks, biofilm reactors and mouse intestines [25]. In situ surveys of plasmid transfer in biofilms grown in flow cells have also shown that plasmid invasion in an established biofilm was detected only at the interfaces, where bacteria were most metabolically active and dividing [26]. The role of plasmids in biofilm formation described the effects of the well-studied conjugative F plasmid of E. coli biofilms. The experiments demonstrated that addition of the F plasmid to E. coli cells greatly increased their ability to form biofilms a conjugation-independent and plasmid-encoded pilus-dependent fashion [24]. Most published studies of plasmid biology and conjugation in biofilms have focused on Gram negative spp. such as Pseudomonas aeruginosa and E. coli. Many chromosomal genes have now been shown to be involved in different stages of biofilm development. By contrast, the contribution of the plasmid gene pool (representing as much as 10-20% of total bacterial DNA) to biofilm biology is poorly understood. As a consequence, with the exception of biotechnology application and antibiotic resistance spread, the role of plasmids in bacterial ecology has been largely overlooked. Conjugative plasmids of E. coli including pOLA52 and pMAS2027 have been shown to enhance biofilm formation through type 3 fimbriae [27]. The pOLA52 plasmid can also be transferred to a variety of organisms and retains its ability to induce biofilm formation in Salmonella typhimurium, Kluyvera spp. and Enterobacter aerogenes [28]. Co-culture studies with P. putida, E. coli, and Kluyvera spp. also demonstrated the impact of conjugative plasmids on biofilm development. In these experiments, the conjugative plasmid pKJK5, an IncP-1 plasmid [29].
Role of Biofilm in Microbial Infections
Increasing evidence suggests that the chronicity of persistent bacterial infections is due to bacterial biofilm formation, which contrasts with the planktonic bacteria found in acute infections [17]. It is more often that infectious biofilm form in a host that is in a compromised state due to immune deficiency, drug treatment, trauma, tissue damage (burns and surgery) or has an underlying physiological diseases such as cystic fibrosis or diabetes [30]. Cystic fibrosis is the most common lethal inherited disease in Caucasians. It is a monogenic, autosomal recessive multi-organ disease with a worldwide incidence of gene defects in the range of 1: 32000 to 1: 2000 live births. It is proved that biofilm play vital role in this syndrome and increases mortality of the patients considerably [18]. In this case, formation of immune complexes stimulates release of proteolytic enzymes that destroy adjacent tissues causing more damage than the invading P. aeruginosa [31]. Biofilm related infections are primarily caused by opportunistic pathogens, once, having access to a host, are able to evade the immune system by surrounding themselves in an exopolymer matrix or glycocalyx [32]. For example, Proteus mirabilis inhabits the environment and causes a number of infections including catheter related urinary tract through biofilm formation. Factors relevant to P. mirabilis biofilm formation include adhesion factors, proteins involved in LPS production, transporters, transcription factors, two component systems, and communication factors [33]. Scanning electron microscopy has revealed the rough, irregular nature of catheter surfaces, Latex-based catheters have particularly uneven surfaces facilitate attachment of P. mirabilis on catheters [32]. It is important to note that, the exopolysaccharides that cover biofilm bacteria have been found to be less immunogenic, thus hiding the proteins and lipopolysaccharides on bacteria surfaces.
In most of the studies conducted to date host-pathogen interactions concern bacteria in planktonic state, however, recent studies have begun to investigate immune response to biofilms. Their tolerance of host defences is dramatically increased [34]. This definition differs in one respect from most other biofilm definitions because it no longer requires that a biotic or abiotic surface is a hallmark. Antimicrobial factors such as lactoferrin and the human cationic host defense peptide LL-37 found at mucosal surfaces or in secondary granules of PMN, when used in vitro at very low concentrations, strongly inhibit formation of pseudomonas aeruginosa biofilm. However, bacteria are known to counter such host defense by secreting protease able to degrade both lactoferrin and LL-37 [35]. Recent research adds further aspects to this phenomenon because phagocytes do come in contact with the bacteria in biofilms and they can even penetrate biofilms. However, the bacteria in the biofilms are not killed [36].
Biofilm and Antibiotic Resistance
Tolerance to antimicrobial agents is common feature of microbial biofilm formation. Biofilm reduced susceptibility to antibiotics in quantified by a tolerance factor, TF, defined as: TF = (LRP × tB × CB/LRB × tP × CP).
Where CP and CB shows planktonic and biofilm dose concentration, respectively. tP and tB indicate planktonic and biofilm dose duration and LRp and LRB show the measured log reduction in planktonic and biofilm populations, respectively. TF compares the rate of killing in planktonic and biofilm states. TF = 10 means that biofilm killing is 10 times slower than in planktonic condition.
Biofilm are 1000-1500 times more resistant to antibiotics than planktonic state. Treatment of infections with biofilm forming bacteria is extremely difficult, requires higher doses or combination of antibiotics, and removal of foreign bodies when implicated in device related infections [37]. In biofilms, poor antibiotic penetration, nutrient limitation and slow growth, adaptive stress responses, and formation of persister cells are hypothesized to constitute a multi-layered defense. Recent report suggest that the biofilm matrix can act as a barrier to delay the diffusion of antibiotics into biofilms because antibiotics may either react chemically with biofilm matrix components or attach to anionic polysaccharides [2]. It must to be noted that biofilm antibiotic tolerance should not be confused with antibiotic resistance because, although bacteria within a biofilm tend to survive antibiotic treatment, they become susceptible to the treatment when the biofilm is disrupted [38]. In study conducted by my research group on 85 isolates of K. pneumoniae from four hospitals in Kerman, Iran. The antibiotic susceptibility under biofilm and planktonic growths was compared by microdilution method. A considerable increase in MIC to piperacillin/tazobactam, tetracycline and cefotaxime occurred for the cells taken from 24 h biofilm but all were sensitive to colistin and tigecycline [39]. Rodriguez-Baño, et al. [40] showed that biofilm-forming A. baumannii isolates were more susceptible to imipenem and ciprofloxacin than non-biofilm-forming counterparts, which suggests that the survival of these isolates in the hospital environment was less dependent on antibiotic resistance than on biofilm formation. In a study carried out on biofilm production and antibiotic resistance in Proteus spp. out of 88 patients infected by Proteus, 81 isolates were identified as P. mirabilis and 7 identified as P. vulgaris. 17% [n = 15] of the isolates exhibited strong biofilm and showed high resistance to ceftriaxone, chloramphenicol, ciprofloxacin, tetracycline and trimethoprim- sulfamethoxazole [41]. Furthermore, recent study by my research group [42] on effect of nano-silver, nano-copper, deconex and benzalkonium chloride on biofilm formation and expression of transcription regulatory quorum sensing gene (rh1R) in drug-resistance P. aeruginosa burn isolates showed that Ag NPs exerted highest antibiofilm activity, follow by deconex and benzalkonium chloride and Benzalkonium chloride, Ag NPs and deconex increased the expression of rhlR gene 64, 2 and 7 folds, respectively. Results suggested that, there is direct relationship between decrease in antibiofilm activity and increase in expression of the rhlR gene in the presence of benzalkonium chloride [42].
Conclusion
From above results it can be concluded that biofilms play an important role in survival of bacteria under natural harsh condition and protecting them from antimicrobial agents and toxic compounds. In medicine, biofilm forming bacteria are responsible for chronic and persistence infections. They are especially important in hospitals where most of the hospital acquired infecting bacteria are capable of producing biofilm. The biofilm can tolerate high concentrations of antibiotics because antibiotics may either react chemically with biofilm matrix components or attach to anionic polysaccharides. The antibiotic resistant biofilm producing bacteria are responsible for major death among patients with adverse conditions such as immune deficiency or cancer.
References
-
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr 3: 10.
-
Lebeaux D, Ghigo JM, Beloin C (2014) Biofilm-related infections: Bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev 78: 510-543.
-
Mataraci E, Dosler S (2012) In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob Agents Chemother 56: 6366-6371.
-
Schembri MA, Kjaergaard K, Klemm P (2003) Global gene expression in Escherichia coli biofilms. Mol Microbiol 48: 253-267.
-
Modarresi F, Azizi O, Shakibaie MR, et al. (2015) Effect of iron on expression of efflux pump (adeABC) and quorum sensing (luxI, luxR) genes in clinical isolates of Acinetobacter baumannii. APMIS 123: 959-968.
-
Rampelotto PH (2013) Extremophiles and extreme environments. Life 3: 482-485.
-
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol 2: 95-108.
-
Guggenbichler JP, Assadian O, Boeswald M, et al. (2011) Incidence and clinical implication of nosocomial infections associated with implantable biomaterials-catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip 6.
-
Gbejuade H, Lovering AM, Webb JC (2015) The role of microbial biofilms in prosthetic joint infections: A review. Acta Orthop 86: 147-158.
-
Marquis RE (1995) Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J Ind Microbiol 15: 198-207.
-
Chole RA, Faddis BT (2003) Anatomical evidence of microbial biofilms in tonsillar tissues: A possible mechanism to explain chronicity. Arch Otolaryngol Head Neck Surg 129: 634-636.
-
Sanclement J, Webster P, Thomas J, et al. (2005) Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope 115: 578-582.
-
Parsek MR, Singh PK (2003) Bacterial biofilms: An emerging link to disease pathogenesis. Annu Rev Microbiol 57: 677-701.
-
Dang H, Chen CT (2017) Ecological energetic perspectives on responses of nitrogen-transforming chemolithoautotrophic microbiota to changes in the marine environment. Front Microbiol 8: 1246.
-
Sutherland IW (2001) The biofilm matrix-an immobilized but dynamic microbial environment. Trends Microbiol 9: 222-227.
-
Southey-Pillig CJ, Davies DG, Sauer K (2005) Characterization of temporal protein production in pseudomonas aeruginosa biofilms. J Bacteriol 187: 8114-8126.
-
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: A common cause of persistent infections. Science 284: 1318-1322.
-
Bjarnsholt T (2013) The role of bacterial biofilms in chronic infections. APMIS Suppl 136: 1-51.
-
Gaddy JA, Actis LA (2009) Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol 4: 273-278.
-
López D, Vlamakis H, Kolter R (2010) Biofilms. Cold Spring Harb Perspect Biol 2.
-
Azizi O, Shahcheraghi F, Modarresi F, et al. (2016) Molecular analysis and expression of bap Gene in Biofilm-Forming Multi-Drug-Resistant Acinetobacter baumannii. Rep Biochem Mol Biol 5: 62-72.
-
Lee HW, Koh Y, Kim J, et al. (2008) Capacity of multidrug‐resistant clinical isolates of Acinetobacter baumannii to form biofilm and adhere to epithelial cell surfaces. Clin Microbiol Infect 14: 49-54.
-
Donlan RM (2002) Biofilms: Microbial life on surfaces. Emerg Infect Dis 8: 881-890.
-
Cook LC, Dunny GM (2014) The influence of biofilms in the biology of plasmids. Microbiol Spectr 2: 0012.
-
Van Elsas JD, Bailey MJ (2002) The ecology of transfer of mobile genetic elements. FEMS Microbiol Ecol 42: 187-197.
-
Król JE, Nguyen HD, Rogers LM, et al. (2011) Increased transfer of a multidrug resistance plasmid in Escherichia coli biofilms at the air-liquid interface. Appl Environ Microbiol 77: 5079-5088.
-
Burmølle M, Bahl MI, Jensen LB, et al. (2008) Type 3 fimbriae, encoded by the conjugative plasmid pOLA52, enhances biofilm formation and transfer frequencies in Enterobacteriaceae strains. Microbiology 154: 187-195.
-
Ong CL, Beatson SA, Mc Ewan AG, et al. (2009) Conjugative plasmid transfer and adhesion dynamics in an Escherichia coli biofilm. Appl Environ Microbiol 75: 6783-6791.
-
Bahl MI, Hansen LH, Goesmann A, et al. (2007) The multiple antibiotic resistance IncP-1 plasmid pKJK5 isolated from a soil environment is phylogenetically divergent from members of the previously established alpha, beta and delta sub-groups. Plasmid 58: 31-43.
-
Costerton JW, Lewandowski Z, Cadwell DE, et al. (1955) Microbial biofilm. Annu Rev Microbiol 49: 711-745.
-
Kronborg G, Hansen M, Sevenson M, et al. (1993) Cytokines in sputum and serum from patients with cystic fibrosis and chronic Pseudomonas aeruginosa infection as markers of destructive inflammation in the lungs. Pediatr Pulmonol 15: 292-297.
-
Stickler D, Young R, Jones G, et al. (2003) Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol Res 31: 306-311.
-
Jacobsen SM, Shirtliff ME (2011) Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence 2: 460-465
-
Hoiby N, Bjarnsholt T, Givskov M, et al. (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35: 322-332
-
Singh PK, Parsek MR, Greenberg EP, et al. (2002) A component of innate immunity prevents bacterial biofilm development. Nature 417: 552-555
-
Haagensen JA, Klausen M, Tolker-Nielsen T, et al. (2006) Differentiation and distribution of colistin/SDS tolerant cells in Pseudomonas aeruginosa flow-cell biofilms. J Bacteriol 189: 28-37.
-
Modarresi F, Azizi O, Shakibaie MR, et al. (2015) Iron limitation enhances acyl homoserine lactone (AHL) production and biofilm formation in clinical isolates of Acinetobacter baumannii. Virulence 6: 152-161.
-
Bayles KW (2007) The biological role of death and lysis in biofilm development. Nat Rev Microbiol 5: 721-726.
-
Monirzadeh F, Shakibaie MR, Gholamrezazadeh M, et al. (2018) Susceptibility of Klebsiella pneumoniae clinical strains to antibiotics and two quaternary ammonium compounds (benzalkonium chloride and deconex) under biofilm and planktonic conditions. Applied Biol and Biotechnol.
-
Rodríguez-Baño J, Martí S, Soto S, et al. (2008) Biofilm formation in Acinetobacter baumannii: Associated features and clinical implications. Clin Microbiol Infect 14: 276-278.
-
Shikh-Bardsiri H, Shakibaie MR (2013) Antibiotic resistance pattern among biofilm producing and non-producing Proteus strains isolated from hospitalized patients; matter of hospital hygiene and antimicrobial stewardship. Pak J Biol Sci 16: 1496-1502.
-
Gholamrezazadeh M, Shakibaie MR, Monirzadeh F, et al. (2018) Effect of nano-silver, nano-copper, deconex and benzalkonium chloride on biofilm formation and expression of transcription regulatory quorum sensing gene (rh1R) in drug-resistance Pseudomonas aeruginosa burn isolates. Burns 44: 700-708.
Corresponding Author
Mohammad Reza Shakibaie, Department of Microbiology and Virology, Research Center for Infectious Diseases and Tropical Medicine, Environmental Health Sciences and Engineering Research Center, Kerman University of Medical Sciences, Kerman, Iran, Tel: +98-340-322-1660, Fax: +98-340-322-1671.
Copyright
© 2018 Shakibaie MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.