Effects of Pasteurization and High Pressure Treatments on Two Bacteriophages (Φ Apr-1 and Φ Apr-2) Active against Lactococcus Lactis from Buffalo Milk
Summary
Water buffalo mozzarella is a worldwide highly valued Italian stretched-curd cheese, produced with "wild" starter cultures with bacteriophage contaminations of raw milk still being a major cause of fermentation failure during cheese making.
The aim of this study was to evaluate the effect of pasteurization and high pressure treatments (200, 400, 600 and 800 MPa) against two lytic bacteriophages (Φ Apr-1 and Φ Apr-2) active against Lactococcus lactis. Phage sensitivity was tested in M17 and PLGYG [1] broths and in raw buffalo milk and whey collected from 25 dairy factories in Southern Italy.
The results showed a high sensitivity of Φ Apr-1 and Φ Apr-2 to those treatments especially in case of experimentally infected whey. In particular, the highest phage reduction and the less impairment of lactic acid bacteria was observed after treatment of whey with 200 MPa pressures and these are the first results about the thermal and pressure sensitivity of bacteriophages isolated from buffalo milk.
Keywords
Bacteriophage, Buffalo, High pressure, Lactic Acid Bacteria (LAB), Pasteurization, Raw milk, Starter cultures, Whey
Introduction
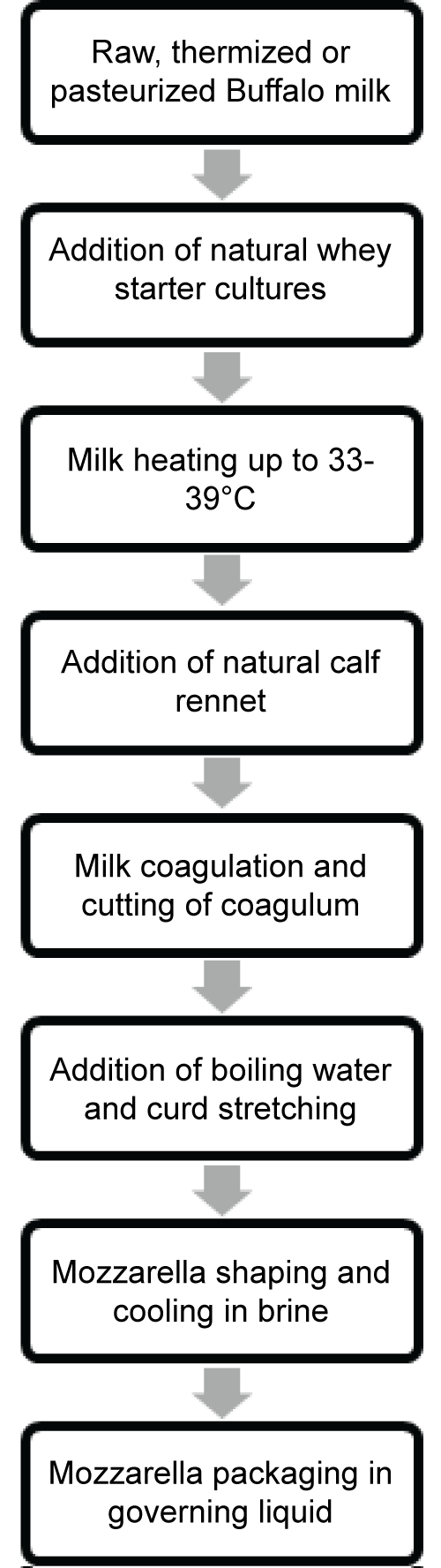
Water Buffalo Mozzarella (WBM) is a typical Italian stretched-curd cheese, traditionally produced in central-southern Italy. The Protected Designation of Origin (PDO) mark was obtained in 1996, according to Reg. (CE) 510/2006 and it represents a guarantee in relation to the geographical production area, milk quality, production phases and labelling. With its porcelain-white colour, thin rind and delicate taste and with a market value of about 500 million of euros per year, WBM represents one of the most important Italian food trademark and it is exported worldwide. The traditional production (Figure 1) is characterized by the use of raw milk. In particular, starter cultures are "wild" and derive from the whey of a previous batch of cheese production after being stored at room temperature for 24 hours to ensure the Lactic Acid Bacteria (LAB) growth. In fact, LAB from buffalo milk are essential not only for the success of mozzarella production but also for conferring the typical flavours. Only few authors have studied and characterized this particular LAB flora [2,3].
A seasonal acidification problem leading to complete fermentation failure during cheese making was experienced in few WBM dairy-plants in Italy. Bacteriophage occurrence in raw milk and natural whey has been previously investigated and two bacteriophages, Φ Apr-1 and Φ Apr-2 active against Lactococcus lactis (L. lactis) were isolated and characterized from buffalo whey starter cultures. This reflects what is reported in literature about phage-infection that is still considered a major problem in the field of industrial fermentations [4], especially in the dairy industry [5,6] and in buffalo milk productions [7,8].
The aim of this study was to investigate the sensitivities of Φ Apr-1 and Φ Apr-2 to pasteurization and to a range of high pressures in order to assess some potential solutions against phage contaminations compromising WBM productions. The interest in high pressure treatment is based on its little impact on milk qualities [9-12] when compared with the effects of heat on milk properties [13]. This research also focused on phage sensitivity to pasteurization because this is a mandatory treatment to export dairy products in some Countries, e.g. USA [14].
Even though few authors reported dairy-phage inactivation by heat [15,16] and high pressures [17-19] this is the first assessment on LAB phages isolated from buffalo milk and the first results about their sensitivity to those treatments in natural buffalo milk and whey.
Finally, since acid development during cheese making and traditional Mozzarella flavours are granted from natural starter cultures, we also tested Total Viable Count (TVC) and LAB resistance to pasteurization and high pressures in order to evaluate the grade of potential impairment that the treatments could provoke to these useful bacteria.
Materials and Methods
Phages, host and media
Phages Apr-1 and Apr-2 were previously isolated from buffalo whey starter cultures, identified as the major cause of curdling failures during WBM production and their genomes were partially characterized [7,8]. In fact, buffalo milk samples from this research were previously investigated and found contaminated with different phages infecting the LAB flora [20]. In particular, Φ Apr-1 and Φ Apr-2 belonged to Caudovirales order of the Siphoviridae family and showed a genome size of 31.4 Kb and 31 Kb, respectively [20]. Their host, a Lactococcus lactis strain, was isolated from buffalo whey and it was identified by API 50 CHL computer-assisted identification scheme (API Laboratory Products Ltd., Basinstoke, England) and confirmed by 16S rRNA gene sequence analysis. The host was harvested in M17 broth at 30 ℃ for 14-16 hours before being used for phage replication.
Bacteriophages were propagated in liquid media. Briefly, 10 ml of PLGYG broth was inoculated with 0.1 ml of overnight host culture, 0.5 ml of 0.1 M CaCl2 and 0.1 ml of each bacteriophage. The suspension was incubated for 6 hours at 30 ℃ until complete bacterial lysis occurred. Than lysates were filtered (0.45 micron filters) to remove cells and cell debris and phage titres were assayed according with the double-layer method [21]; briefly, M17 soft agar (2.5 ml; 0.7% w/v) constantly heated at the temperate of 46 ℃ by heating block was inoculated with the host (100 µl), CaCl2 0.185 M (100 µl) and with one ml of each dilution of phage lysate. The media was then poured onto M17 agar plates and incubated at 37 ℃ for 24-48 h. Plaques were counted after incubation.
Fresh phage dilutions were used to inoculate raw buffalo milk and whey for pasteurization and high pressure treatments. Raw buffalo milk and whey were collected from a pool of 25 dairy factories located in Southern Italy and were stored at -20 ℃. Immediately before performing the assays, milk and whey were thawed at 37 ℃ in water bath and kept in ice before bacteriophage inoculation. Before the inoculation with Φ Apr-1 and Φ Apr-2, milk and whey samples were screened for absence of natural phage contaminations [2,22].
Thermal treatment
Bacteriophage sensitivity to pasteurization was tested in PLGYG broth, buffalo milk and whey. Fresh Φ Apr-1 and Φ Apr-2 suspensions were used for contamination with a final titre of 106 pfu/ml of each media. Thus, contaminated media were dispensed into sterile plastic tubes (1.5 ml and no head space left), in triplicate. Samples were placed in a preheated heating block at the temperature of 63 ℃. Once each sample reached 63 ℃ (less than 1 min), control tubes were removed to evaluate the phage titre at the starting time. The other samples were heated at 63 ℃ for 30 min as the equivalent treatment for pasteurization commonly applied to milk during cheese-making (71.7 ℃ for 15 sec). The temperature was constantly monitored with an electronic thermometer. After the treatment, all samples were rapidly placed in ice in order to stop the heat effect and immediately assessed for phage-titre count.
Some samples of raw buffalo milk and whey (without phage contamination) were assayed in order to test TVC and LAB titre before and after the thermal treatment.
The experiment was carried on three different times and the results derived from the arithmetic mean value.
High pressure treatment
High pressures were applied to Φ Apr-1 and Φ Apr-2 in three different media: PLGYG broth, raw buffalo milk and whey. The contaminated suspensions were dispensed into sterile stomacher bags with a final phage titre of 106 pfu/ml. Samples were triple-packaged and heat sealed. The two phages were assayed separately and in triplicate for each media. The bags were placed in a Stansted Fluid Power Iso-Lab 900 High Pressure Food Processor (Stansted Fluid Power Ltd., Stansted, Essex, UK) and subjected to pressures of 200, 400 and 600 MPa, with holding times of 10 min. Values of 800 MPa were applied only to phage-contaminated milk, since a stronger phage-protection was expected for this media. All treatments were performed at 20 ℃ and adiabatic heating on compression resulted in an increase in the temperature of the processing liquids by up to 15 ℃ at 800 MPa. After depressurisation, samples were immediately assessed for phage titres.
Also, not contaminated samples of raw buffalo milk and whey were assayed in order to assess TVC and LAB titres before and after the high pressure treatments.
The experiment was carried on three different times and the results derived from the arithmetic mean value.
Determination of phage titre
The titres of of Φ Apr-1 and Φ Apr-2 before and after pasteurization and high pressure treatments were determined according with the double-layer method [21].
Determination of TVC and LAB titres
Before and after pasteurization and pressure treatments, milk and whey samples were diluted in Ringer solution containing (in mM) 125 NaCl, 5 KCl, 1 MgSO4, 32 N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), 5 glucose, 1 CaCl2 (pH 7.4) and dilutions were pour-plated in PCA and MRS agar [23]. The plates were incubated at 30 ℃ for 3 days before colony counting and MRS plates were incubated in microaerophilic conditions (5% O2; 10% CO2, 85% N2).
Results
Phage inactivation by pasteurization
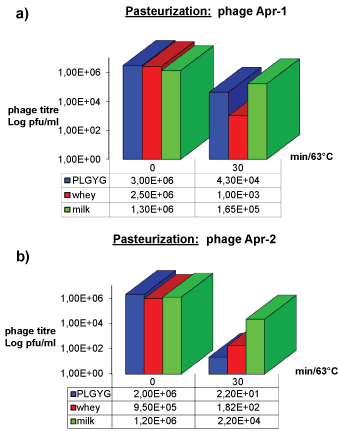
The effects of pasteurization on Φ Apr-1 and Φ Apr-2 are shown in Figure 2a and Figure 2b respectively. Phage Apr-1 was reduced by 2, 3 and 1 Log when it was tested respectively in PLGYG broth, whey and raw buffalo milk. Instead, Φ Apr-2 was reduced respectively by 5, 4 and 2 Log when tested in the same media.
TVC and LAB viability after pasteurization
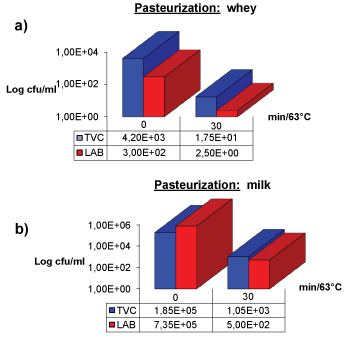
The results concerning TVC and LAB reductions in whey and milk after pasteurization are shown in Figure 3a and Figure 3b. In particular, 2 Log reduction occurred in TVC and LAB titre in whey, while 2 and 3 Log reductions were shown respectively for TVC and LAB in milk after thermal treatment.
Phage inactivation by high pressures
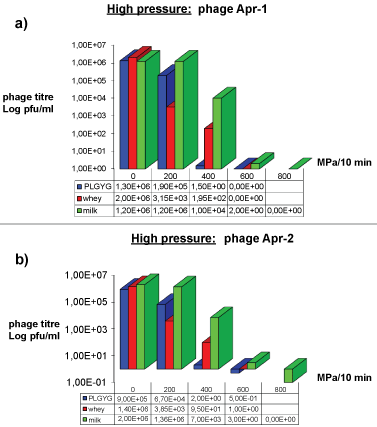
The effects of high pressure treatments against Φ Apr-1 and Φ Apr-2 are shown in Figure 4a and Figure 4b respectively. As regards Φ Apr-1, reductions of 1 and 6 Log were showed at 200 and 400 MPa respectively in PLGYG broth. In whey reductions of 3 and 4 Log at 200 and 400 MPa respectively were observed. No reduction was noticed at 200 MPa in milk but 2 and 6 Log reductions occurred respectively at 400 and 600 MPa pressures. Phage Apr-1 was completely inactivated after treatment at 600 MPa in PLGYG broth and whey and at 800 MPa in milk.
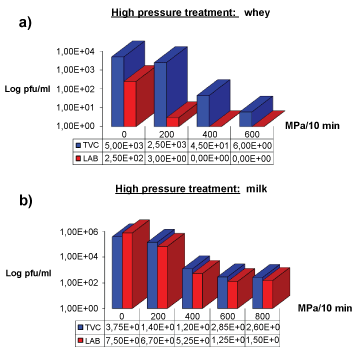
TVC and LAB sensitivity to high pressures
The results of TVC and LAB reductions in whey and milk after high pressure treatments are shown in Figure 5a and Figure 5b. In whey, TVC resulted in 2 and 3 Log reductions at 400 and 600 MPa pressure treatments respectively while the titre only slightly decreased at 200 MPa. LAB flora in whey was subjected to 2 log reduction at 200 MPa application.
In milk, TVC resulted in 2 and 3 Log reductions at 400 and 600 MPa pressure treatment respectively while only a little decrease was observed after 200 MPa, respect to the starting titre. Also, the difference in titre after 600 and 800 MPa treatments was minimal.
As regards LAB count in milk, 1 and 3 Log reductions occurred at 200 and 400 MPa pressures respectively with no relevant difference observed after application of 400, 600 and 800 MPa pressures.
Discussion
The higher reduction grade (3 Log) observed after pasteurization for Φ Apr-1 contaminating the whey could be explained by the less protein content of whey acting as protective agent when comparing the other tested media (milk and PLGYG broth) [24]. Moreover, Φ Apr-2 resulted more heat-sensitive than Φ Apr-1.
Phage Apr-2 showed a very similar resistance pattern in the same media and with the same high pressure values when compared to Φ Apr-1. However, Φ Apr-2 resulted slightly more pressure stabile than Φ Apr-1.
Also for the pressure assays, as for pasteurization, the highest phage-sensitivity at 200 MPa was observed for phage-contaminated whey when compared to PLGYG broth. Moreover, both phages were much more protected in milk and the effect of their inactivation in this medium was low up to 400 MPa (2 Log). A very interesting topic to investigate is the reason why phages are inactivated. A possibility might be represented by the phage heads being damaged when the pressure is released. This could be illuminated by examining pressure-inactivated phages by Transmission Electon Microscope and it will be exploited in the next future.
In relation to TVC and LAB sensitivity to high pressures, the results suggest that 200 MPa treatments could guarantee the less impairment of TVC and LAB in whey and milk when comparing them with those deriving from the application of higher pressure values.
Up to now, very little research has been carried out to evaluate LAB phage sensitivity to thermal and pressure treatments for industrial purposes. In particular Chen, et al. [25], differently from us, described a strong protection of milk toward Lactobacillus virulent phage P1 at 63 ℃ for 10 min. Fifty-three bacteriophages of three lactic acid bacteria species (Lactococcus lactis, Leuconostoc pseudomesenteroides and Streptococcus thermophilus) isolated from whey powders and whey powder formulations, were tested for their thermal stability [26]. Also in this case, phages were particularly resistant to pasteurisation conditions, such as 9 Leuconostoc mesenteroides phages, isolated during blue cheese manufacture, from Puiato, et al. [27]. The same authors also reported a partial inactivation of Leuconostoc phages after treatment with high pressures at 100 MPa, values that have not been assayed in our research.
The effect of high hydrostatic pressure on the inactivation of Streptococcus thermophilus bacteriophages Abc2, ALQ13.2 and DT1 was also investigated [28]. The authors showed similar results to ours for pressure values of 400 and 600 MPa (holding time 0-30 min). In particular, the treatment at 600 MPa for less than 5 min was sufficient to completely inactivate phages Abc2 and DT1.
Conclusions
The principal aim of this research was to investigate the effects of pasteurization and high pressure treatments on the activity of two phages, Φ Apr-1 and Φ Apr-2, active against L. lactis and responsible of failures during Water Buffalo Mozzarella cheese making with consequential important economic loss.
We estimated that a treatment can be considered efficient if it is able to reduce the phage titre of at least 3 Log. This speculation derives from the fact that the authors experienced an average of natural buffalo whey contamination from LAB phages of about 103 pfu/ml and it was always associated with slow acid production. Neverthless, the treatments mentioned in the study should be optimized as possible for total removal of the virus particles and yet not interfere with the raw material in order not to impair the lactic fermentation and thus not affecting the final product. Based on our results, a potential action to adopt in case of natural bacteriophage-contamination in dairy industries could be based on pasteurization of whey starter cultures or their treatment with high pressures of 200 MPa. Tools based on high pressure techniques would be preferred to high temperatures not only for flavour preservation in the final product but also for the high phage inactivation grade associated with a small reduction in TVC and LAB count. However, TVC and LAB titres in whey and milk in this research are underestimated because of the partial inactivation effect determined by storage-temperature (-20 ℃). Since LAB count in fresh buffalo starter cultures is estimated to reach about 8 Log UFC/mL [3], a low risk of drastic impairment of the starter culture-activity caused by thermal/pressure treatments should be considered. In fact, the small reduction of LAB count observed after the treatments would be recovered during the 24 hr period of whey fermentation during the traditional production, with potential no negative implication in cheese making.
This technical guide lines are in compliance with the WBM product specifications that would allow the use of these techniques also for PDO productions.
References
- Douglas J, Qanber-Agha A, Phillips V (1974) Medium for the propagation and assay of lactic and other phages. Lab Pract 23: 3-4.
- Aprea G, Mullan WMA, Mullan A, et al. (2005) Isolation of polyphosphate-accumulating lactic acid bacteria from natural whey starters. Milchwissenschalft 60: 256-258.
- Coppola S, Parente E, Dumontet S, et al. (1988) The microflora of natural whey cultures utilized as starters in the manufacture of Mozzarella cheese from water-buffalo milk. Le Lait 68: 295-310.
- Emond E, Moineau S (2007) Bacteriophages and food fermentations. Bacteriophage: Genetics and Molecular Biology, Caister Academic Press, UK, 93-124.
- Garneau JE, Moineau S (2011) Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb Cell Fact 10: S1-S20.
- Kleppen HP, Bang T, Nes IF, et al. (2011) Bacteriophages in milk fermentations: diversity fluctuations of normal and failed fermentations. International Dairy Journal 21: 592-600.
- Aprea G (2006) Evaluation of the causes implicated in fermentation delay and complete fermentation failure during the manufacture of "Mozzarella di Bufala". Università degli studi di Napoli "Federico II", Italy.
- Aprea G, Fusco G, Buonanno M, et al. (2011) Identification of a new lytic bacteriophage against Lactococcus lactis from natural whey starter cultures used in the production of the Italian buffalo Mozzarella cheese. IV International Conference on Environmental, Industrial and Applied Microbiology (BioMicroWorld 2011), Torremolinos, Spain.
- Bravo FI, Molina E, Lopez-Fandino R (2012) Effect of the high-pressure-release phase on the protein composition of the soluble milk fraction. J Dairy Sci 95: 6293-6299.
- Delgado FJ, Contador R, Alvarez-Barrientos A, et al. (2013) Effect of high pressure thermal processing on some essential nutrients and immunological components present in breast milk. Innovative Food Science & Emerging Technologies 19: 50-56.
- Mazri C, Sanchez L, Ramos SJ, et al. (2012) Effect of high-pressure treatment on denaturation of bovine lactoferrin and lactoperoxidase. J Dairy Sci 95: 549-557.
- Ozturk M, Govindasamy-Lucey S, Jaeggi JJ, et al. (2013) Effect of various high-pressure treatments on the properties of reduced-fat Cheddar cheese. J Dairy Sci 96: 6792-6806.
- Chang JC, Chen CH, Fang LJ, et al. (2013) Influence of prolonged storage process, pasteurization, and heat treatment on biologically-active human milk proteins. Pediatr Neonatol 54: 360-366.
- (2012) 21 CFR 1240.61 - Mandatory pasteurization for all milk and milk products in final package form intended for direct human consumption. United States Government Printing Office.
- Atamer Z, Hinrichs J (2010) Thermal inactivation of the heat-resistant Lactococcus lactis bacteriophage P680 in modern cheese processing. International Dairy Journal 20: 163-168.
- Muller-Merbach M, Rauscher T, Hinrichs J (2005) Inactivation of bacteriophages by thermal and high-pressure treatment. International Dairy Journal 15: 777-784.
- Avsaroglu MD, Buzrul S, Alpas H, et al. (2006) Use of the Weibull model for lactococcal bacteriophage inactivation by high hydrostatic pressure. Int J Food Microbiol 108: 78-83.
- Capra ML, Patrignani F, Quiberoni ADL, et al. (2009) Effect of high pressure homogenization on lactic acid bacteria phages and probiotic bacteria phages. International Dairy Journal 19: 336-341.
- Mercanti DJ, Guglielmotti DM, Patrignani F, et al. (2012) Resistance of two temperate Lactobacillus paracasei bacteriophages to high pressure homogenization, thermal treatments and chemical biocides of industrial application. Food Microbiol 29: 99-104.
- Aprea G, Mullan WM, Murru N, et al. (2017) Multiplex PCR to detect bacteriophages from natural whey cultures of buffalo milk and characterisation of two phages active against Lactococcus lactis, φApr-1 and φApr-2. DOI 10.12834/VetIt 315.1238
- Terzaghi BE, Sandine WE (1975) Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol 29: 807-813.
- Del Rio B, Binetti AG, Martin MC, et al. (2007) Multiplex PCR for the detection and identification of dairy bacteriophages in milk. Food Microbiol 24: 75-81.
- De Man JC, Rogosa D, Sharpe ME (1960) A medium for the cultivation of lactobacilli. Journal of Applied Microbiology 23: 130-135.
- Atamer Z, Dietrich J, Neve H, et al. (2010) Influence of the suspension media on the thermal treatment of mesophilic lactococcal bacteriophages. International Dairy Journal 20: 408-414.
- Chen X, Liu Y, Fan M, et al. (2017) Thermal and chemical inactivation of Lactobacillus virulent bacteriophage. J Dairy Sci 100: 7041-7050.
- Wagner N, Samtlebe M, Franz C, et al. (2017) Dairy bacteriophages isolated from whey powder: Thermal inactivation and kinetic characterisation. International Dairy Journal 68: 95-104.
- Pujato SA, Guglielmotti DM, Ackermann HW, et al. (2014) Leuconostoc bacteriophages from blue cheese manufacture: long-term survival, resistance to thermal treatments, high pressure homogenization and chemical biocides of industrial application. Int J Food Microbiol 177: 81-88.
- Zhang L, Qu M, Yao J, et al. (2015) Effect of high hydrostatic pressure on the viability of Streptococcus thermophilus bacteriophages isolated from cheese. Innovative Food Science & Emerging Technologies 29: 113-118.
Corresponding Author
Giuseppe Aprea, Department of Hygiene in Food Technology and Animal Feeds, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise "G. Caporale" Teramo, Italy.
Copyright
© 2017 Aprea G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.