Modelling the Amounts of Carbon Dioxide in the Headspace to Assess the Coliforms of Mozzarella Cheese
Abstract
This article proposes an approach based on the evaluation of CO2 in the headspace produced by coliforms to assess their viable count in mozzarella cheese, packaged in diluted salt solution with and without lysozyme and Na2-EDTA. The data were modelled by a re-parameterized Gompertz equation in order to calculate the Minimum Detection Time (MDT), defined as the time to attain 3% of CO2.
The proposed method showed a good correlation with the standard plate count and could be an alternative and simple approach to study the shelf-life of mozzarella-cheese.
Keywords
Coliforms, Lysozyme, Minimum detection time, Mozzarella cheese.
Introduction
Mozzarella is a stretched curd cheese, originally produced in Italy and nowadays-worldwide spread [1]. It is produced by traditional procedures or by using commercial starter cultures of lactic acid bacteria, and/or direct acidification by addition of citric acid [2,3]. It is an ideal medium for the growth of a wide range of microorganisms [4]. The natural microbiota includes thermophilic or mesophilic lactic acid bacteria (Streptococcus thermophilus, Lactobacillus delbrueckii, L. helveticus, Lactococcus lactis, Leuconostoc, Enterococcus), psychrophilic bacteria, and some yeasts, such as Debaryomyces hansenii and Kluyveromyces marxianus [5-11].
Coliforms live in the intestine of mammals and the recovery of some of them in dairy products (Enterobacter, Escherichia, Citrobacter, and Serratia) could be the result of poor sanitation conditions and/or the use of unpasteurized milk [12]. Coliforms are responsible of gas defects, small holes, and off-odours of mozzarella [13].
The standard plate count on a selective medium (Violet Red Bile Agar) is the method usually employed to assess the viable count of coliforms; however, bacterial cells could experience the state on VBNC (viable but not cultivable cells) for different factors (e.g. the presence of antimicrobial compounds etc…).
Some alternative and indirect methods have been proposed. A promising technique is the gas-chromatographic approach, because of its simplicity, linearity, high sensitivity and recovery capacity [14,15].
Therefore, the main goal of this paper is a preliminary approach to design and optimize an alternative method to assess the coliforms in mozzarella cheese, with and without antimicrobial compounds, and exploit the possible benefits and limits of the protocol.
Materials and Methods
Samples preparation
Mozzarella cheese was purchased from a local market in Foggia (Italy) and packaged with diluted salt (50 g of mozzarella cheese in 200 ml of solution), containing phosphate buffer (50 mM K2HPO4/KH2PO4; J.T. Baker, Milan, Italy), lysozyme (0.25 mg/ml; Sigma-Aldrich, Milan), and Na2-EDTA (50 mM or 20 mM; J.T. Baker). Samples of mozzarella cheese packaged in salt solution without lysozyme and Na2-EDTA were used as control. The samples were stored at 4 ℃ and analyzed immediately after packaging and 1, 3, 6 and 8 days.
Microbiological analyses
Twenty grams of mozzarella cheese were diluted with 180 ml of a sterile saline solution (0.9% w/v NaCl) in a Stomacher bag and blended for 1 min with a Stomacher Lab Blender 400 (PBI International, Milan, Italy). Decimal dilutions of cheese homogenates were performed and microbiological counts of total coliforms were carried out on VRBA (Violet Red Bile Agar, Oxoid, Milan), incubated at 37 ℃ for 18-24 h. The experiments were performed over two independent batches and for each batch the analyses were made in duplicate; the data were submitted to One-Way Analysis of Variance (ANOVA) and Tukey’s test, through the software Statistica for Windows (Statsoft, Tulsa, USA).
Metabolism of coliforms
The metabolism of coliforms was studied in a model system as follows: 1 ml of cheese homogenate from each time of sampling (0, 1, 3, 6, and 8 days of storage) and each sample (with and without antimicrobials) was added to 20 ml vials, containing 10 ml of a synthetic medium (yeast extract, 3.0 g/l; bacteriological peptone, 7.0 g/l; NaCl, 5.0 g/l and biliar salts n. 3,1.5 g/l; Oxoid). The composition of the medium is the same of VRBA, the solid medium traditionally used to assess coliforms through the standard plate count; the only modification is the lack of agar. After the inoculation, the vials were hermetically sealed, incubated at 37 ℃ and analyzed for 72 h to assess the amount of CO2 (v/v) in the headspace through a Gas Analyzer Checkmate 9900 O2/CO2 (PBI Dansensor, Ringsted, Denmark). The experiments were performed over two independent batches and for each batch the analyses were made in duplicate; the results were modelled through the Gompertz equation, reparameterized by Corbo, et al. [16] and cast in the following form, as reported by Bevilacqua, et al. [15]:
Where: (CO2)max is the maximum increase in CO2 attained in the headspace (% v/v); µmax, the maximal rate of CO2 production (CO2/h); λ, the time (h) before the beginning of the production of CO2; Lc is the critical level of CO2, (3%) and MDT, the Minimum Detection Time, defined the time necessary to attain a level of CO2 in the headspace of 3% [14].
Confirmation of the prevalence of coliforms in the sealed vials
The prevalence of coliforms in the sealed vials was assessed by the isolation of at least 10-15 colonies from each vial after 24, 48, and 72 h of incubation. The isolates were preliminary characterized through microscopic examination, Gram staining, catalase test, and oxido-fermentative metabolism. Some isolates were also recovered from VRBA to confirm the results of the standard plate count.
Results and Discussion
Many food spoilers consume oxygen and produce carbon dioxide in the headspace of packed foods; thus, the evaluation of the content of CO2 is expected to increase because of the increase of the level of spoiling microorganisms [17,18]. Therefore, the evaluation of CO2 in the headspace could be successfully used to directly assess the beginning of a spoiling event and determine the level of some microorganism, as reported for sausages [19], ready-to-eat salads [20], milk [15], and mushrooms [21].
Gardini, et al. [14] proposed the use of the content of CO2 in the headspace of a sealed system to indirectly determine the level of Salmonella sp. and Saccharomyces cerevisiae, and proposed the Minimum Detection Time (defined as the time to attain 3% of CO2 in the headspace) as a reliable instrumental parameter related to microbial growth. Recently, Bevilacqua, et al. [15] proposed a similar approach to determine the level of Pseudomonas spp. in milk. This paper represents an up-grade of the research of Bevilacqua, et al. [15], as it focuses on the head-space method to evaluate the coliforms of mozzarella cheese with a special emphasis on the preliminary steps (laboratory and mathematical models) required to develop and optimize a new protocol.
The optimization of the protocol was done in two steps:
a) Assessment of the level of coliforms through the standard plate count and study of their metabolic activity (CO2 produced in the vials);
b) Building a simple regression model to correlate the standard plate count and an indirect parameter (Minimum Detection Time, MDT) related to the production of CO2.
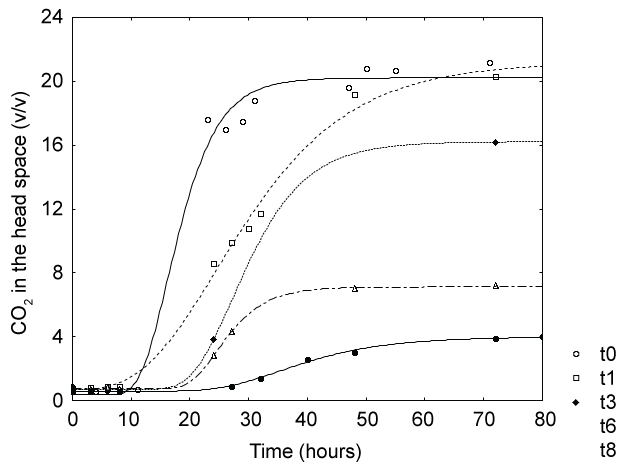
Table 1 reports the results from the standard plate count (last column), as well as the results from the production of CO2 in the headspace. These results were shown in the form of fitting parameters of a modified Gompertz equation, as the evolution of CO2 followed a typical sigmoid function (see for example Figure 1). The fitting parameters of the Gompertz equation were analyzed through one-way ANOVA to assess the effect of the storage time and antimicrobials.
In the control samples, no differences were found in the maximum amount of the CO2 in the headspace. On the other hand, the parameters λ and MDT were significantly lower in the vials inoculated with cheese homogenate after 8 days of storage; this result was probably related to a higher count of coliforms (5.87 log CFU/g).
The presence of the antimicrobials exerted a significant effect. In the samples containing the highest amount of Na2-EDTA, the amount of CO2 was similar to control for cheese homogenate immediately after the storage and after 1 day (ca. 20%); then it decreased (15.52% for cheese homogenate after 3 day-storage, 6.37% after 6 days, and 3.40% after 8 days). The antimicrobial compounds also determined a decrease of the rate (from 1.55% CO2/h immediately after the packaging to 0.12% CO2/h after 8 days), as well as an increase of the Minimum Detection Time up to 45.87 h.
This effect was related to a strong antibacterial activity, as one could infer from the decrease of the viable count of coliforms from 3.46 log CFU/g immediately after the packaging to the undetectable level after 8 days.
The significance of the antimicrobials on the metabolism of coliforms was found also at the lowest amount of Na2-EDTA (20 mM), although the effect on the maximum amount of CO2 and lag/MDT was less strong: in fact, after 8 days (CO2)max was 10.97% and 28.19 h the MDT. As previously stated, this effect was probably related to the contemporary reduction of the viable count of coliforms throughout storage.
This approach is a promising way to focus on the metabolism of coliforms of mozzarella cheese in presence of some antimicrobials and suggests a limit of the traditional approach. After 8 days, the inoculation of cheese homogenate in the sealed vials determined an increase of CO2, thus suggesting a residual level of coliforms, although the result of the plate count was below the detection limit.
Mozzarella cheese could support the growth of microorganisms other than coliforms (lactic acid bacteria, yeasts, other Gram negative bacteria) and some of them could experience different level of resistance to bile salts; therefore, the results of the standard plate count, as well as the prevalence of coliforms in the sealed vials were confirmed. All isolates recovered from VRBA plates were preliminary identified as coliforms; concerning the broth in the vials, the isolates were all coliforms after 24 h. Some other bacteria (mainly Gram positive) were recovered after 48 and 72 h; however, their prevalence was low (5-7%), thus suggesting that CO2 could be mainly attributed to coliforms.
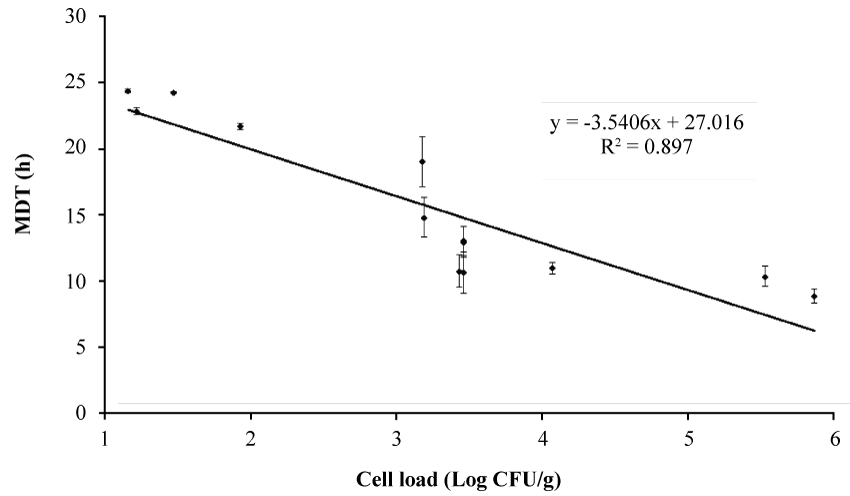
The second step of the research was a regression analysis “MDT/viable count” to assess how the evaluation of CO2 is a reliable tool to study coliforms of mozzarella cheese (Figure 2). The determination coefficient (0.897) highlights the adequacy of the approach and pinpoints the existence of a correlation of the kinetic of the production of CO2 in the vials with the initial count of coliforms in mozzarella cheese.
Coliforms were for a long period the target group to label the acceptability of mozzarella cheese, as Italian law set up a critical level to 5 log CFU/g. The traditional plate count could offer the results after 24 h; on the other hand, the correlation MDT vs. plate count could give a feedback in a lower time. Figure 2, in fact, suggests that for a viable count of 5 log CFU/g or higher the MDT should be 10 h or lower, thus a MDT < 10 h could highlight a high level of coliforms, and a poor microbiological quality.
The traditional pour plate on VRBA is a time-consuming method; although some other approaches can be used to determine coliforms (e.g. MPN, Most Probable Number), the standard plate count is still the traditional protocol used in Italian factories and the official method of Italian law. Therefore, this paper explores the possibility of using an alternative approach, based on the evaluation of the kinetic of the production of CO2 in the headspace and on MDT (Minimum Detection Time).
The results showed some interesting evidences:
1. The linear relationship of MDT vs cell load of coliforms and the possibility to find out a level of 5 log CFU/g or higher in 10 h;
2. The possibility to highlight a residual level of coliforms in presence of antimicrobials, even if the feedback of the traditional plate count gives as feedback “below the detection limit”.
Further efforts are required to effectively design and propose this method for a validation at industrial level. This paper gives a first insight and highlights a possibility.
References
- Mijan MA, Haque MA, Habib MA, et al. (2010) Evaluation of quality of mozzarella cheese. The Bangladesh Veterinarian 27: 36-42.
- Coppola S, Blaiotta G, Ercolini D, et al. (2001) Molecular evaluation of microbial diversity occurring in different types of Mozzarella cheese. J Appl Microbiol 90: 414-420.
- De Angelis M, Gobbetti M (2011) Pasta-filata cheeses: traditional Pasta-filata cheese. In: Fuquay JW, Encyclopedia of Dairy Sciences. (2nd edn), Elsevier, New York, USA 1: 745-752.
- Ruegg PL (2003) Practical food safety interventions for dairy production. Journal of Dairy Science 86: E1-E9.
- De Candia S, De Angelis M, Dunlea E, et al. (2007) Molecular identification and typing of natural whey starter cultures and microbiological and compositional properties of related traditional mozzarella cheeses. Int J Food Microbiol 119: 182-191.
- De Filippis F, La Storia A, Stellato G, et al. (2014) A selected core microbiome drives the early stages of three popular italian cheese manufactures. PLoS One 9: e89680.
- Ercolini D, Mauriello G, Blaiotta G, et al. (2004) PCR-DGGE fingerprints of microbial succession during a manufacture of traditional water buffalo mozzarella cheese. J Appl Microbiol 96: 263-270.
- Paola Bellia, Anna FA Cantaforaa, Simone Stella, et al. (2013) Microbiological survey of milk and dairy products from a small scale dairy processing unit in Maroua (Cameroon). Food Control 32: 366-370.
- Leonardo Caputoa, Laura Quintieria, Daniela Manila Bianchi, et al. (2015) Pepsin-digested bovine lactoferrin prevents Mozzarella cheese blue discoloration caused by Pseudomonas fluorescens. Food Microbiology 46: 15-24.
- Maria Angélica Costa Diasa, Anderson S Sant Anab, Adriano G Cruzc, et al. (2012) On the implementation of good manufacturing practices in a small processing unity of mozzarella cheese in Brazil. Food Control 24: 199-205.
- Faccia M, Trani A, Loizzo P, et al. (2014) Detection of αs1-I casein in mozzarella Fiordilatte: a possible tool to reveal the use of stored curd in cheesemaking. Food Control 42: 101-108.
- Milena Sinigaglia, Antonio Bevilacqua, Maria Rosaria Corbo, et al. (2008) Use of active compounds for prolonging the shelf life of mozzarella cheese. International Dairy Journal 18: 624-630.
- Tamime AY (2000) Cheese. In: Robinson RK, Batt CA, Patel PD, Encyclopedia of Food Microbiology. Academic Press, London, 372-403.
- Gardini F, Lanciotti R, Sinigaglia M, et al. (1997) A headspace gas chromatographic approach for the monitoring of the microbial cell activity and the cell viability evaluation. Journal of Microbiological Methods 29: 103-114.
- Antonio Bevilacqua, Maria Rosaria Corbo, Giuseppe Martino, et al. (2013) Evaluation of Pseudomonas spp. through O2 and CO2 head-space analysis. International Journal of Food Science and Technology 48: 1618-1625.
- Maria Rosaria Corbo, Matteo Alessandro Del Nobile, Milena Sinigaglia (2006) A novel approach for calculating shelf-life of minimally processed vegetables. International Journal of Food Microbiology 106: 69-73.
- Fiach C O'Mahony, Tomas C O'Riordan, Natalia Papkovskaia, et al. (2004) Assessment of oxygen levels in convenience-style muscle-based sous vide products through optical means and impact on shelf-life stability. Packaging Technology and Science 17: 225-234.
- Aylin Ozturk, Neriman Yilmaz, Gurbuz Gunes (2010) Effect of different modified atmosphere packaging on microbial quality, oxidation and colour of a seasoned ground beef product (meatball). Packaging Technology and Science 23: 19-25.
- Jacob Gotterupa, Karsten Olsena, Susanne Knochel, et al. (2008) Colour formation in fermented sausages by meat-associated staphylococci with different nitrite- and nitrate-reductase activities. Meat Science 78: 492-501.
- Nicolas Borcherta, Andreas Hempela, Helena Walsha, et al. (2012) High throughput quality and safety assessment of packaged green produce using two optical oxygen sensor based systems. Food Control 28: 87-93.
- Nicolas B Borcherta, Malco C Cruz-Romeroa, Pramod V Mahajan, et al. (2014) Application of gas sensing technologies for non-destructive monitoring of headspace gases (O2 and CO2) during chilled storage of packaged mushrooms (Agaricus bisporus) and their correlation with product quality parameters. Food Packaging and Shelf Life 2: 17-29.
Corresponding Author
Antonio Bevilacqua, Department of the Science of Agriculture, Food and Environment, University of Foggia, Foggia, Italy.
Copyright
© 2017 Speranza B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.