Strontium Barium Titanates Nanowires Sensors: Preparation and Application in Prediction of Cancers by Exhaled Biomarkers
Abstract
Sr0.6Ba0.4TiO3, SrTiO3, BaTiO3 nanowires were synthesized by hydrothermal method with all-inorganic raw materials and without any pH adjustment, organic precursors or templates, high temperature. The width of nanowires is 25-150 nm, the length is ten to hundred of micrometers. The array of sensors based on Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 nanowires was designed without any organic modifier. The array of sensors can distinguish the simulated cancers breath from healthy breath without the need for pre-concentration and dehumidification, accounts for > 98% variance. Nine volatile organic compounds that represent lung, liver, kidney, breast, diabetes cancers were used to train and optimize the sensors. Our results show that the array of sensors with a detection limit of ppt level is a low-cost, simple, rapid, sensitivity and non-invasive sensing technology, much simpler and lower detection limit than the gold nanoparticles sensors and colorimetric sensor.
Introduction
Volatile organic compounds (VOCs) generated by the human body have been much studied, especially in breath analysis. A number of VOCs in breath, e.g., ammonia [1,2], ketones [3], ethanol [2], propanol [4], pentane and hexane etc. of C4-C11 straight or monomethylated alkanes [5,6], benzene derivatives [7], have been identified as biomarkers of different systemic diseases, such as lung, liver, kidney, diabetes and breast cancers. The breath analysis has been proposed as a fast, convenient, non-invasive and painlessness procedure, complementary to blood and urine sampling [8-10]. Gas chromatography/mass spectrometry (GC-MS) [11,12], selected ion flow tube mass spectroscopy [3], laser adsorption spectrometry [13], infrared spectrometry [14], surface acoustic wave sensors [15,16] and coated quartz crystal microbalance sensors [17] have been used for this purpose. However, these techniques are expensive, slow, require complex instrument and, further more, require pre-concentration of the biomarkers.
Here, we report Sr0.6Ba0.4TiO3, SrTiO3, BaTiO3 nanowires were synthesized by hydrothermal methods. The synthesis involves low-cost, all-inorganic raw materials, unlike solid-state, sol-gel and other hydrothermal synthesis employing high temperature, organic precursors, pH adjustment [18-20]. The array of sensors based on Sr0.6Ba0.4TiO3 nanowires was designed and can distinguish the breath of cancers from the breath of healthy individuals without the need for pre-concentration and dehumidification.
Experimetal Methods
Hydrothermal synthesis of Sr0.6Ba0.4TiO3, SrTiO3, BaTiO3 nanowires from Na2Ti3O7 nanowires
The Na2Ti3O7 nanowires have been prepared by the hydrothermal method, 0.20g of TiO2 powder (Degussa P25) was mixed with a NaOH (Alfa Aesar) aqueous solution (concentration of 10 mol/L) and placed in a 150 mL poly(tetrafluoroethylene) lined autoclave, ultrasonic for 20 min. The synthesis was performed at 240 ℃ for 3 days. The products were isolated by centrifugation and washed several times with distilled water until pH of 7 and then dried at 60 ℃ overnight.
Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 nanowires were synthesized by the reaction of Na2Ti3O7 with Sr(OH)2 (Alfa Aesar) or Ba(OH)2 (Alfa Aesar) under a hydrothermal condition. A typical preparation procedure of Sr0.6Ba0.4TiO3 is shown as follows: 0.04 g of Na2Ti3O7 nanowires, 0.02g Sr(OH)2, 0.10g Ba(OH)2 and 10 mL DDW (deionized distilled water) were mixed and put into the 50 mL Teflon-lined autoclave. The autoclave was placed into an oven at 150 ℃ for 3 days. After reaction, the autoclave was cooled down to room temperature and the Sr0.6Ba0.4TiO3 nanowires were separated by centrifugation and repeatedly washed with 0.1 M aqueous nitric acid, centrifugalized and thoroughly washed with distilled water several times, and finally dried at 60 ℃ overnight.
Material characterization
The crystal structure and phase composition of the synthesized Sr0.6Ba0.4TiO3, SrTiO3, BaTiO3 and Na2Ti3O7 nanowires were characterized by X-ray diffraction (XRD, MiniFlex Rigaku) using CuKa radiation. Scanning electron microscope (SEM), Energy-dispersive X-ray analyses (EDX) and transmission electron microscopy (TEM) were carried out on Philips ESEM XL30 microscope and Titan STEM80-300, respectively.
Fabrication of the sensor
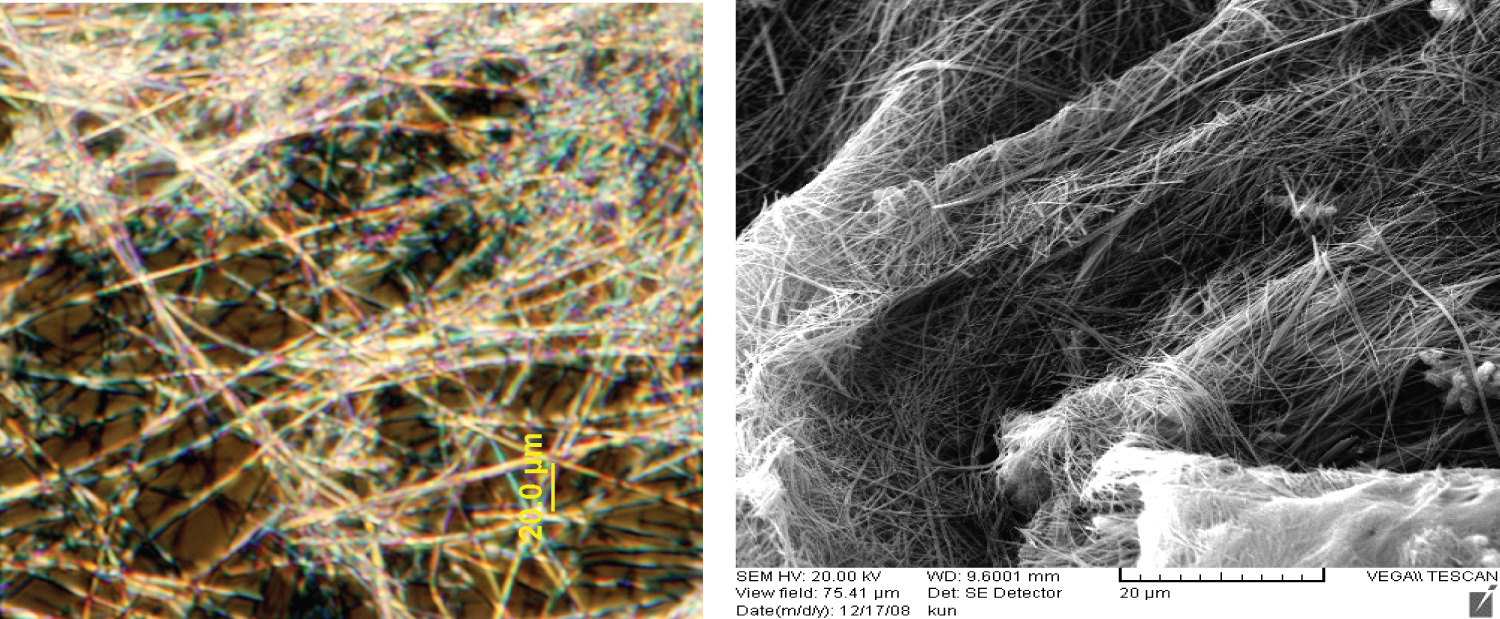
The nanowires were mixed with DDW in a weight ratio of 10:1 to form sensing film. The sensing film was drop-cast onto a silicon substrate (4 mm × 4 mm) with gold interdigital electrode and dried in oven at 60 ℃ overnight. Each electrode has 40 fingers, fingers width and gap distance is 25 μm, the thickness of the gold is about 2000 angstroms. The structure of sensor is shown in Figure 1 and Figure 2.
Simulation and test of breathe samples
Nine VOCs [1-7] were attained by dissolving ammonium hydroxide, acetic acid, ethanol, toluene, methylhydrazine, pentane, hexane, isopropanol and acetone solution into distilled water, respectively. Each compound's concentration is 5 ppt, 5 ppb and 5 ppm. The healthy breath and cancers breath were simulated by mixing the nine compounds into solution with each compound's concentration of 10 ppb and 100 ppb, respectively. The resistance values (R) of samples were measured using a SR810 DSP Lock-In Amplifier at room temperature (about 23 ℃) and 100 Hz.
Results and Discussion
Material characterization
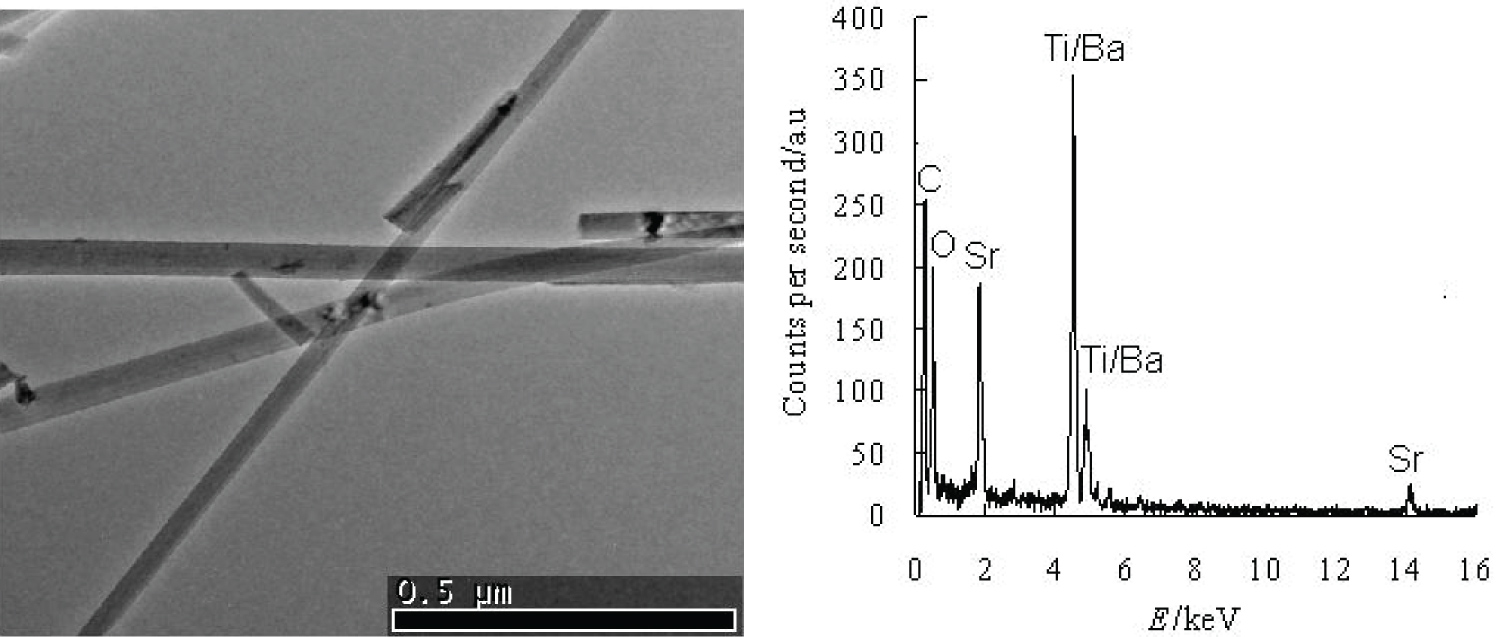
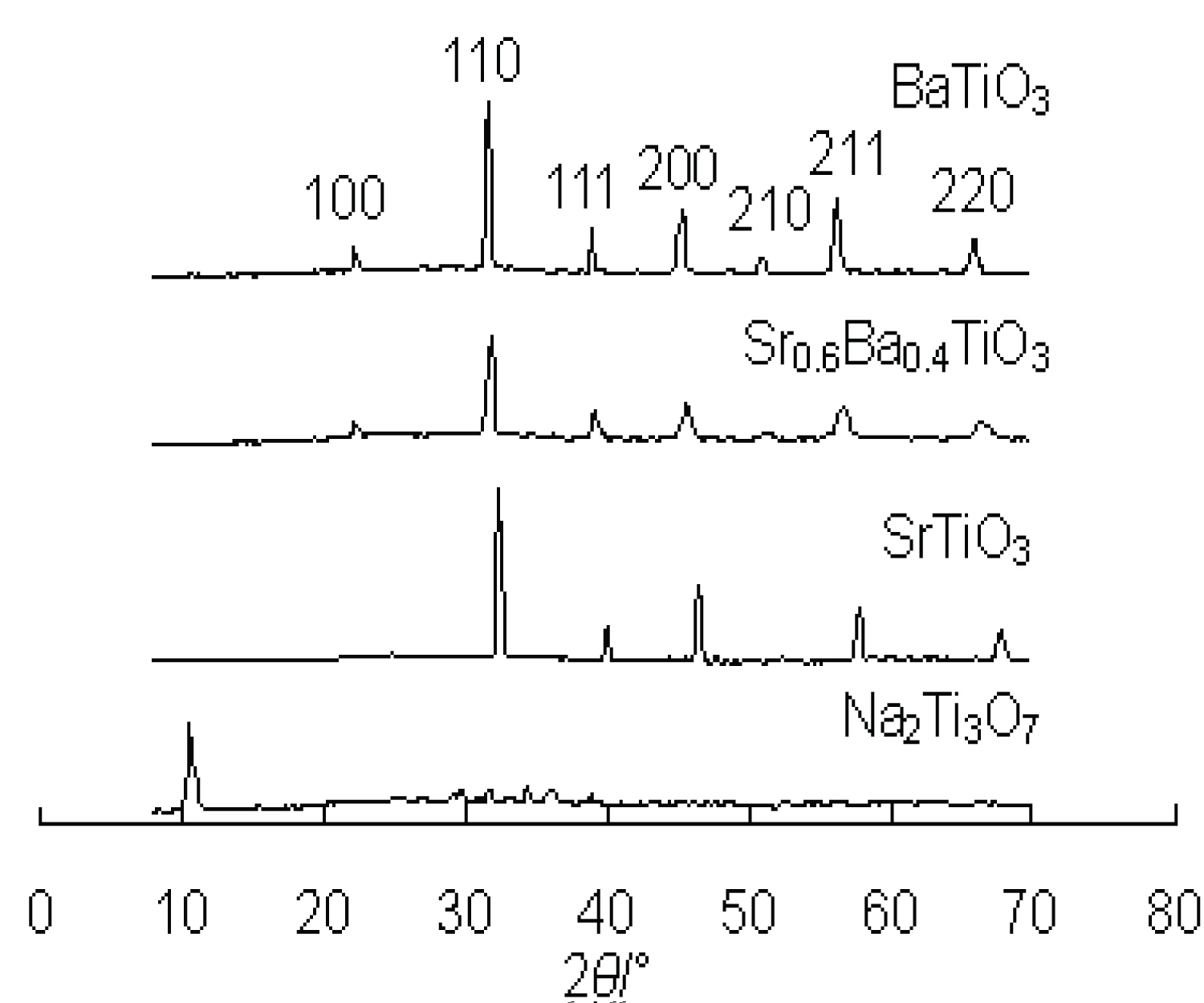
The XRD patterns of Sr0.6Ba0.4TiO3, SrTiO3, BaTiO3 and Na2Ti3O7 are shown in Figure 3. It is observed that all the peaks fit well to the peak positions of layered sodium titanate [21,22] (JCPDS No.31-1329), cubic phase SrTiO3 [18-20] (JCPDS No.35-734) and BaTiO3 [20,23] (JCPDS No.31-174), respectively. The peak positions of Sr0.6Ba0.4TiO3 (d110, 2θ = 31.8) locate between BaTiO3 (d110, 2θ = 31.6) and SrTiO3 (d110, 2θ = 32.4). The energy dispersive X-ray spectroscope (EDS) analysis in Figure 2 from different positions along one single nanowire shows that the nanowires are essentially composed of Sr, Ba, Ti and O. The element molar ratio of Sr and Ba is 6:4, which indicates that the molecular formula is Sr0.6Ba0.4TiO3. From the patterns of Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3, no peaks of Na2Ti3O7 can be found, it indicates that the layered structure of Na2Ti3O7 has been lost and relatively complete reaction between the Na2Ti3O7 and Sr (OH)2 or Ba(OH)2 takes place. The results demonstrate that pure Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 nanowires can be attained by hydrothermal method from Na2Ti3O7 nanowires. The processes involve all-inorganic raw materials, without pH adjustment, organic precursors or templates, high temperature, not like recent report [18-24].
Electric Microscope, Representative scanning electron microscope (SEM) and transmission electron microscope (TEM) images are presented in Figure 1 and Figure 2. The images show that straight Sr0.6Ba0.4TiO3 nanowires have a uniform cylindrical structure with diameters of 25-150 nm and the lengths from ten to one hundred micrometers.
Detection of breath biomarkers
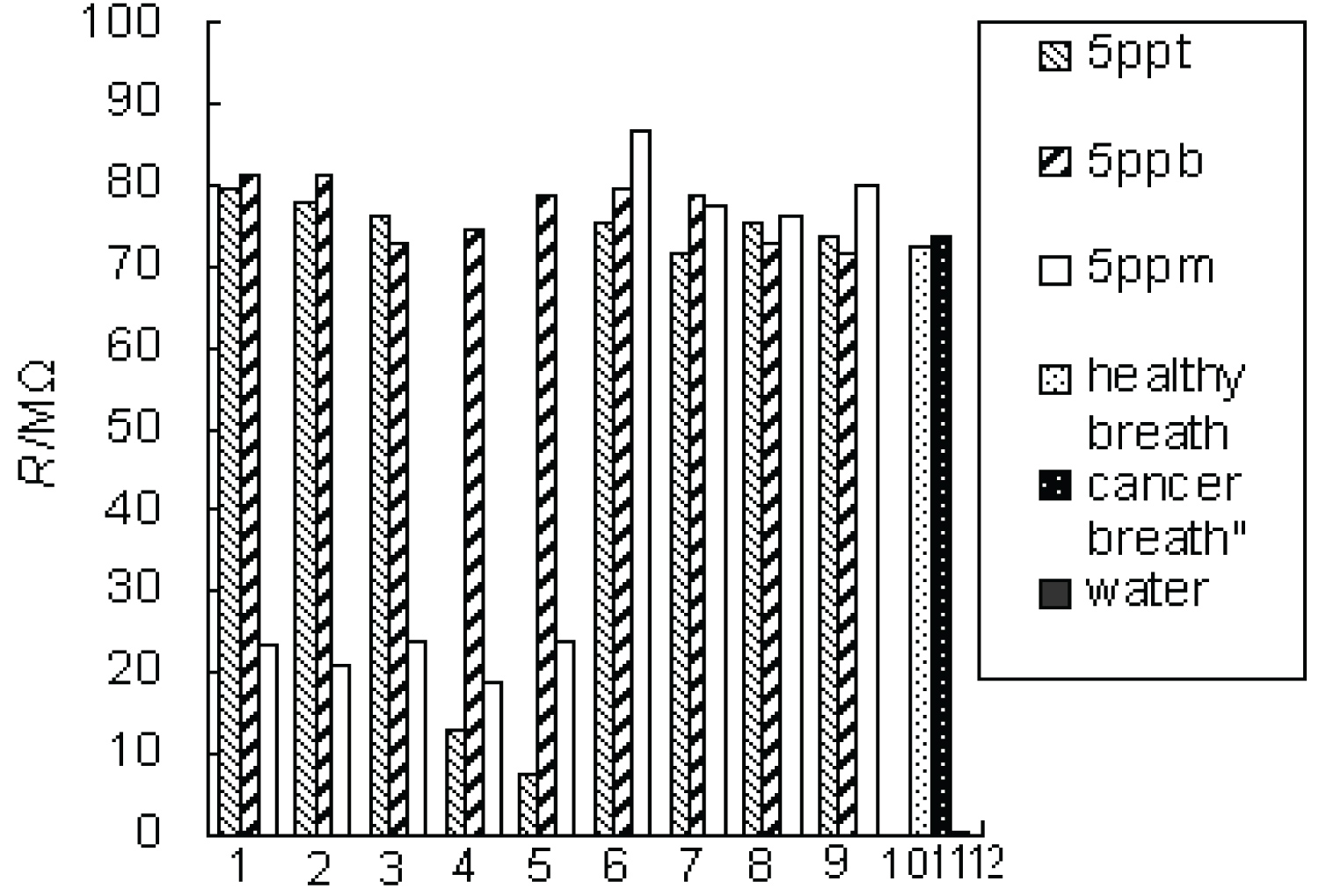
The responses of the Sr0.6Ba0.4TiO3 sensor to nine representative cancer biomarkers at different concentrations were investigated in Figure 4. The results show that the detection limit of sensor can arrive at ppt level, much below the concentration levels of these VOCs in the exhaled breath of cancerous patients. The responses are hardly affected by the presence of the water molecules. The sensor to methylhydrazine and toluene show a very different response at different concentration, but to other biomarkers or simulated breath of cancers and breath of healthy individuals, there is no clear difference.
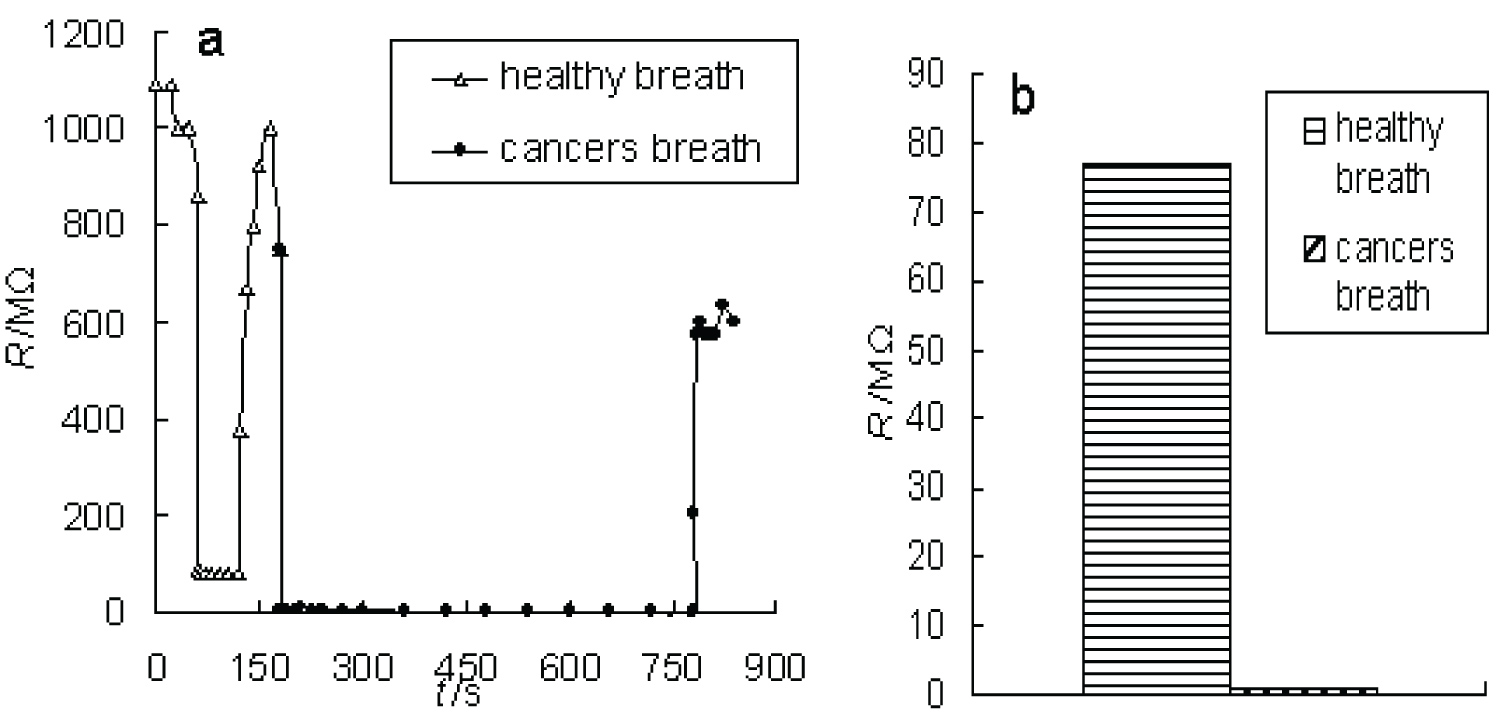
In order to improve the sensitivity of the sensor, an array of sensors was designed by combing Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 sensors in series. The results in Figure 5a show that the response is rapid and reversible. Figure 5b shows a very big difference and no overlap between the simulated cancers and healthy patterns, accounts for > 98% variance. It can be seen that the array of sensors can distinguish the breath of cancers (lung, liver, kidney, breast and diabetes cancers) from the breath of healthy without the need for pre-concentration and dehumidification. The array of sensors was designed just by Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 nanowires without any organic modifier, pH adjustment, organic template or precursors, high temperature, much simpler and lower detection limit than the gold nanoparticle sensors [25] and colorimetric sensor [26]. The results demonstrate the array of the sensors is a low-cost, simple, rapid, sensitivity and non-invasive sensing technology for predicting the cancers.
Conclusion
Sr0.6Ba0.4TiO3, SrTiO3, BaTiO3 nanowires were synthesized by hydrothermal method involves all-inorganic raw materials, without pH adjustment, organic template or precursors, high temperature. The width of nanowires is 25-150 nm, the length is ten to hundred of micrometers.
The array of sensors based on Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 nanowires is a simple, inexpensive, rapid, sensitive and non-invasive sensing technology for predicting the cancers. It can rapidly distinguish the simulated breath of cancers patients (lung, liver, kidney, diabetes, breast cancers) from the breath of healthy individuals, accounts for > 98% variance, without the need for pre-concentration and dehumidification. The detection limit of the array of sensors is low to ppt level. The array of sensors was designed just by Sr0.6Ba0.4TiO3, SrTiO3 and BaTiO3 nanowires without any organic modifier, pH adjustment, organic template or precursors, high temperature, much simpler and lower detection limit than the recent report.
Acknowlegement
The authors thank David Paul for helpful instructions and suppling the SR810 DSP Lock-In Amplifier. This work was supported by China Scholarship Council.
References
- Turner C, Spanel P, Smith D (2006) A longitudinal study of ammonia, acetone and propanol in the exhaled breath of 30 subjects using selected ion flow tube mass spectrometry, SIFT-MS. Physiological Measurement 27: 321-337.
- Ruzsanyi V, Baumbach JI (2005) Analysis of human breath using IMS. International Journal for Ion Mobility Spectrometry 8: 5-7.
- Turner C, Spanel P, Smith D (2006) A longitudinal study of breath isoprene in healthy volunteers using selected ion flow tube mass spectrometry (SIFT-MS). Physiological Measurement 27: 13-22.
- Phillips M, Renee NC, Beth AD, et al. (2006) Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Research and Treatment 99: 19-21.
- Kneepkens FCM, Lepage G, Roy CC (1994) The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radical Biology & Medicine 17: 127-160.
- Phillips M, Renee NC, Beth AD, et al. (2003) Volatile markers of breast cancer in the breath. The Breast Journal 9: 184-191.
- Phillips M, Gleeson K, Hughes JM, et al. (1999) Volatile organic compounds in breath as markers of lung cancer: A cross-sectional study. Lancet 353: 1930-1933.
- Abbott SM, Elder JB, Spanel P, et al. (2003) Quantification of acetonitrile in exhaled breath and urinary headspace using selected ion flow tube mass spectrometry. International Journal of Mass Spectrometry 228: 655-665.
- Francesco Di F, Fuoco R, Trivella MG, et al. (2005) Breath analysis: Trends in techniques and clinical applications. Microchemical Journal 79: 405-410.
- Spinhirne JP, Koziel JA, Chirase NK (2003) A device for non-invasive on-site sampling of cattle breath with solid-phase microextraction. Biosystems Engineering 84: 239-246.
- Buszewski B, Kesy M, Ligor T, et al. (2007) Human exhaled air analytics: Biomarkers of diseases. Biomed Chromatogr 21: 553-566.
- Buszewski B, Ulanowska A, Ligor T, et al. (2008) Identification of volatile organic compounds secreted from cancer tissues and bacterial cultures. Journal of Chromatography B Analyt Technol Biomed Life Sci 868: 88-94.
- Dumitras DC, Giubileo G, Puiu A (2005) Investigation of human biomarkers in exhaled breath by leaser photoacoustic spectroscopy. Proc SPIE 5850: 111-121.
- Giubileo G (2002) Medical diagnostics by laser-based analysis of exhaled breath. Proceedings of SPIE 4762: 318-325.
- Groves WA, Grey AB, Shaughnessy OPT (2006) Surface acoustic wave (SAW) microsensor array for measuring VOCs in drinking water. Journal of Environmental Monitoring 8: 932-941.
- Hao Y, Liang X, Mingfu C, et al. (2003) Detection of volatile organic compounds in breath as markers of lung cancer using a novel electronic nose. Proceedings of IEEE Sensors 2: 1333-1337.
- Natale C, Macagnano A, Martinelli E, et al. (2003) Lung cancer identification by the analysis of breath by means of an array of non-selective gas sensors. Biosensors & Bioelectronics 18: 1209-1218.
- Xie J, Ji T, Ou-Yang X, et al. (2008) Preparation of SrTiO3 nanomaterial from layered titanate nanotubes or nanowires. Solid State Communications 147: 226-229.
- Joshi UA, Lee JS (2005) Template-free hydrothermal synthesis of single-crystalline barium titanate and strontium titanate nanowires. Small 1: 1172-1176.
- Ji T, Tang M, Liu G, et al. (2006) Preparation of ultrafine BaTiO3 and SrTiO3 powders from Na2-xHxTi3O7 nanowires via hydrothermal processing. Advanced materials research 11-12: 603-606.
- Armstrong AR, Armstrong G, Canales J, et al. (2004) TiO2-B nanowires. Angewandte Chemie 43: 2286-2288.
- Sun X, Li Y (2003) Synthesis and characterization of ion-exchangeable titanate nanotubes. Chemistry A Europe Journal 9: 2229-2238.
- Zhu X, Wang J, Zhang Z, et al. (2008) Perovskite nanoparticles and nanowires: Microwave-hydrothermal synthesis and structural characterization by high-resolution transmission electron microscopy. Journal of the American Ceramic Society 91: 2683-2689.
- Yun WS, Urban JJ, Gu Q, et al. (2002) ferroelectric properties of individual barium titanate nanowires investigated by scanned probe microscopy. Nano Lett 2: 447-450.
- Peng G, Tisch U, Adams O, et al. (2009) Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nature Nanotechnology 4: 669-673.
- Mazzone PJ, Hammel J, Dweik R, et al. (2007) Diagnosis of lung cancer by the analysis of exhaled breath with a colorimetric sensor array. Thorax 62: 565-568.
Corresponding Author
Kun Xing, Association of Ecology and Environment of Zibo, Zibo, Shandong 255003, China, Tel: +861-7605336791.
Copyright
© 2021 Xing K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.