Hematological Malignancy Diagnosed on Cytological Examination of Cavity Fluid: Pleural Effusion as First Manifestation of Acute Myeloid Leukemia
Keywords
Leukemia, Pleural effusion, Malignant, Bone marrow
Dear editors
Approximately 10% of pleural effusions have malignant etiology. Among malignancies, carcinomas represent more than three-quarters of the cases. Hematopoietic neoplasms correspond to 15% of the causes and non-Hodgkin's lymphomas are the most frequently involved. Acute Myeloid Leukemia (AML) rarely manifest primarily with pleural effusion [1].
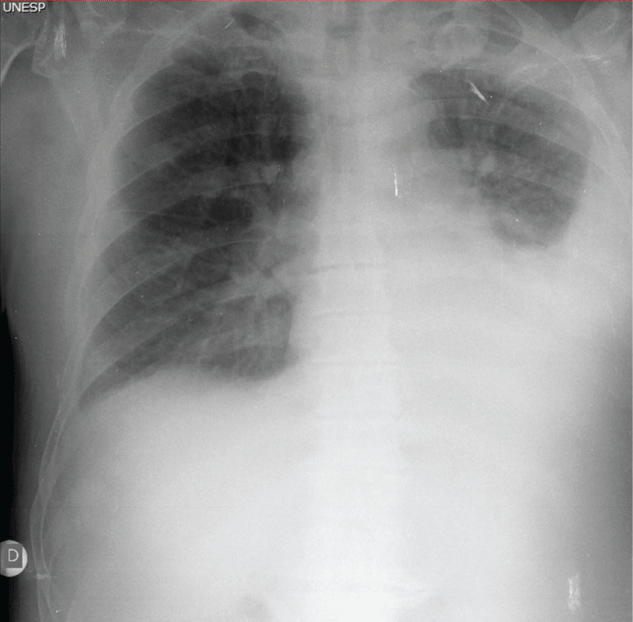
We would like to present in this letter the case of a 74-year-old man, with historical of controlled systemic arterial hypertension, admitted to the emergency of our hospital with clinical symptoms of diffuse bone pain and respiratory failure. The physical examination revealed an important decrease in the vesicular murmur, considering the hypothesis of pleural effusion. Chest radiography confirmed the initial clinical suspicion (Figure 1). Blood count reveals anemia (hemoglobin level 7.6 g/dL, reference value 14.00-18.00 g/dL), thrombocytopenia (114,000 cells/mm3, reference value 140,000-440,000 cells/mm3) and leukocytosis (115,600 cells/mm3, reference value 4,000-11,000 cells/mm3) with 25% of immature cells compatible with myeloid blasts.
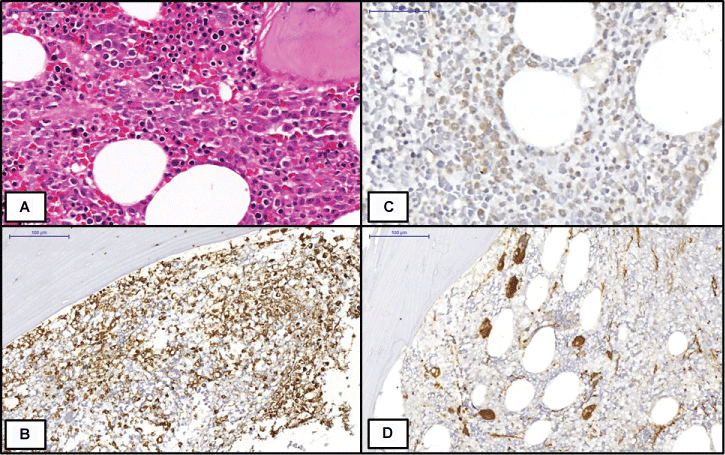
Bone marrow biopsy, performed seven months before that admission, demonstrated increased global cellularity for age with granulocytic hyperplasia characterized by immature myeloid cells, in an expansive process from the peritrabecular niche toward the center of the intertrabecular space (Figure 2A). Immunohistochemistry detected these immature myeloid elements by the immunopositivity to myeloperoxidase (Figure 2B) and CD34 (20% of the cells, Figure 2C), emphasizing the clusters of these cells in the central regions of the intertrabecular spaces (In addition, the bone marrow presented hypercellularity of the megakaryocytic series, with dysplastic and ectopic elements (Figure 2D). Reticulin staining also evidenced moderate increase in the reticulinic scaffold. This set of findings was consistent with Myelodysplastic Syndrome, AREB II (refractory anemia with excess type II blasts). The karyotype study, at the time, revealed no alterations. Blood count of that moment reveled anemia (hemoglobin level 13.7 g/dL, reference value 14.00-18.00 g/dL) and leukopenia (3,500/mm3, reference value 4,000-11,000 cells/mm3), without blasts. At that moment, the clinical follow-up was with blood counts and blood transfusions.
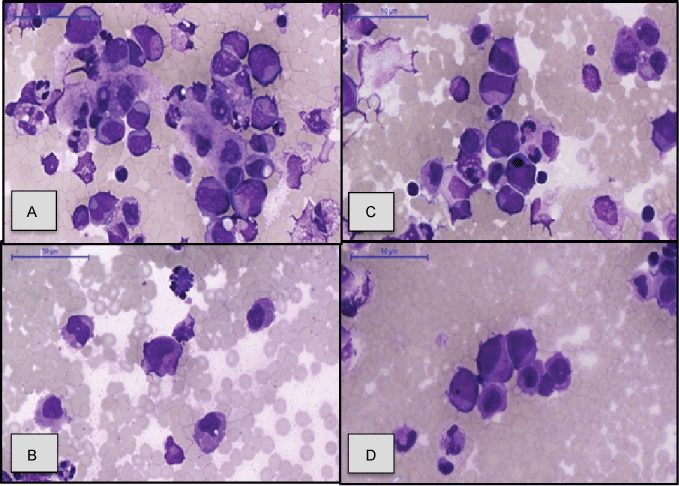
At his new admission, the patient underwent thoracocentesis, and the cytological analysis showed the presence of numerous atypical neoplastic cells compatible with leukemia. Cells were large, with large nuclei, nuclear membranes with central irregularity and cytoplasm with loss of normal granulation (Figure 3). That context favored the diagnosis of AML, resulting from the progression of Myelo Dysplastic Syndrome (MDS). Blood cultures and pleural fluid were negatives for microorganisms. Biochemistry and differential cell counts in pleural fluid were not availed because the material received for these exams was hemorrhagic. Lactic dehydrogenase levels in blood was 1066 U/L (reference value 300 U/L) and in pleural effusion is 1278 U/L. Even with chemotherapy, which was cytarabine (Ara-C low dose), the patient evolved to death, after five days of the beginning of the treatment, and after ten days of the pleural effusion/AML diagnosis, and no necroscopic study was performed. The patient needed five day for the beginning of the treatment because he had to wait the restoration of renal function and blood pressure control.
Our group would like to alert pathologists and cytopathologists to hematopoietic diseases in the evaluation of cavity fluid smears, such as pleural fluid and ascitic fluid. Hematopoietic etiologies, especially myeloproliferative and myelodysplastic diseases, which are not frequently diagnosed in these topographies, thus representing a differential diagnosis that is not very well remembered in the diagnostic routine [2]. Sahu, et al. in a review from 1977 to 2013 found only 13 cases of pleural effusion as first manifestation of AML [3].
AML is defined by clonal proliferation of neoplastic myeloid precursors with impaired maturation capacity. Approximately 80% of AML carriers are adults [4]. Clinically, this patient has fatigue, hemorrhage, severe infections and manifestations related to extramedullary disorders, which, although not the typical form of presentation, may be the first manifestation of the disease [5]. The presence of pleural effusion due to AML is related to a worse prognosis, especially if it occurs within the first six months after diagnosis of the neoplasia [4,5].
The diagnosis of pleural effusion in a patient with AML, however, has important differential conditions such as infections secondary to treatment or to the evolution of the disease and drug toxicity [6-10]. It is also important to emphasize that pleural effusion in patients with AML is only one example of respiratory complications due to hematopoietic malignancies, including parenchymal infiltration, pneumonias, hypereosinophilia, alveolar proteinsin and pulmonary arterial hypertension [9,10]. Depending on the context, cytological evaluation alone may be difficult, since neoplastic cells may not represent a very exuberant cellularity. For these cases, the individual's clinical history is important, including previous diagnoses, hematological or not, and sometimes immunophenotyping studies, by immunohistochemistry or flow cytometry is fundamental [6].
The case presented here exposes the evolution of a patient with myelodysplastic syndrome that is a disease characterized by ineffective hematopoiesis with a paradox between the bone marrow hypercellularity and peripheral blood cytopenia. The morphology is most often of hypercellularity in bone marrow with cytological/architectural megakaryocytic and granulocytic dysplasia, including immature precursors of Abnormal Localization (ALIP). Reticulin is often increased. The main function of immunohistochemistry is the detection of ALIPs by the CD34 marker. Posivities above 5% indicate the diagnosis of MDS. Approximately one quarter of AMLs is the result of progression of MDSs. This type of AML is more common in the elderly and has a poor prognosis [11]. Pleural effusion may be one of the clinical evidences of disease evolution and therefore, in the clinical context of MDS, hematologists and pathologists should consider this hypothesis. Unfortunately, immunohistochemistry, flow cytometry and cytogenetic techniques, which exams that may confirm with high specificity these king of diagnoses, are not always available. In these contexts, the recognition of the cytological characteristics of immature myeloid cells allows the suspicion and represents a fundamental diagnosis resource for pathologists and clinicians.
References
- Agarwal M, Purohit AHL, Mahapatra M, et al. (2014) Pleural Effusion as an Unusual Initial Presentation of Acute Myeloid Leukemia. Indian J Hematol Blood Transfus 30: 195-196.
- Suharti C, Santosa, Setiawan B (2015) Malignant pleural effusion in acute myeloid leukemia with hepatitis B virus infection. Acta Med Indones 47: 153-156.
- Sahu KK, Tyagi R, Law AD, et al. (2015) Myeloid Sarcoma: An Unusual Case of Mediastinal Mass and Malignant Pleural Effusion with Review of Literature. Indian J Hematol Blood Transfus 31: 466-471.
- Faiz SA, Sahay S, Jimenez CA (2014) Pleural effusions in acute and chronic leukemia and myelodysplastic syndrome. Curr Opin Pulm Med 20: 340-346.
- Duhan A, Kalra R, Kamra HT, et al. (2014) Leukaemic pleural effusion as a manifestation of acute myeloid leukaemia: a case report and review of literature. Ecancer 8: 397.
- Pemmaraju N, Chang E, Daver N, et al. (2014) Extramedullary acute myeloid leukemia: leukemic pleural effusion, case report and review of the literature. Front Oncol 4: 130.
- Rawal R (2013) Acute Myeloid Leukaemia (AML) Presenting as a Bilateral Pleural Effusion. J Clin Diagn Res 7: 187.
- Lokireddy P, Bloxam D, Hodson A, et al. (2015) Extramedullary acute myeloid leukaemia: isolated leukaemic pleural and pericardial effusions without marrow disease. British Journal of Haematology 171: 295.
- Lamour C, Bergeron A (2011) Non-infectious pulmonary complications of myelodysplastic syndromes and chronic myeloproliferative disorders. Rev Mal Respir 28: e18-e27.
- Alexandrakis MG, Passam FH, Kyriakou DS, et al. (2004) Pleural effusions in hematologic malignancies. Chest 125: 1546-1555.
- Barbara J Bain, David M Clark, Bridget S Wilkins (2010) Bone Marrow Pathology. (4th edn), Wiley-Blackwell, London, 153.
Corresponding Author
Cristiano Claudino Oliveira, Department of Pathology, Botucatu School of Medicine, São Paulo State University, Distrito de Rubião Junior, Botucatu, São Paulo, Brazil, Zip code: 18618897.
Copyright
© 2017 Oliveira CC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.