Mitochondrial Dysfunction Partially Explains Macrocytic Anemia in HIV-Exposed Uninfected Infants
Abstract
Objectives
Antiretroviral drugs are successfully used to prevent mother-to-child transmission of HIV worldwide. Hematologic and mitochondrial disturbances have been extensively reported among HIV-exposed uninfected (HEU) infants, but their pathogenic mechanisms remain largely unexplained. We aimed to analyze the relationship between mitochondrial markers and hematologic values in a large cohort of HEU infants.
Methods
A single-center prospective observational study was conducted among a cohort of HEU patients. Hematologic values and mitochondrial markers (peripheral blood mononuclear cells, mitochondrial DNA [mtDNA] and mitochondrial respiratory chain complex IV [CIV] function, corrected with citrate synthase [CS] activity) were collected at 6 weeks and 3, 6 and 12 months of age; the former were classified according to Division of AIDS toxicity (DAIDS) Grades. Joint analysis of mitochondrial and hematological data was performed.
Results
Overall, 118 HEU infants were included. Macrocytic, mild and transitory anemia affected 63.6%, 47.5% and 17.8% of infants at 3, 6 weeks and 3 months of life, respectively. Significantly lower mtDNA/CS levels were observed among patients exposed to ZDV during gestation at 3 (0.008 vs. 0.014 mtDN2/nRPII ratio normalized by CS, p = 0.043) and 6 months of life (0.007 vs. 0.013, p = 0.023). Patients with anemia DAIDS grade ≥ 2 had a lower CIV/CS activity at 6 weeks of life (0.05 vs. 0.11, p = 0.002) with no differences in mtDNA/CS content.
Conclusions
Mitochondrial dysfunction may partially explain ZDV-related macrocytic anemia observed in HEU infants. Other pathogenic pathways associated with ZDV exposure, but also with other HIV-related and unrelated variables, are probably involved.
Keywords
Anemia, Antiretrovirals, HIV-exposed uninfected infants, Mitochondrial DNA, Mitochondrial function, Neutropenia
Introduction
The prevention of mother-to-child transmission (MTCT) of HIV remains one of the major successes in the period since the beginning of the HIV/AIDS pandemic. It is mainly driven by maternal use of highly active antiretroviral (ARV) therapy that leads to suppression of viral replication throughout pregnancy and delivery. However, the potential toxicity of ARV exposure in the HIV-exposed uninfected (HEU) infant remains of concern, especially in the long-term [1,2]. Hematological and mitochondrial toxicity are by far the most common adverse effects observed to date in the first months of life [1,2].
Early self-limited macrocytic anemia was first described in AIDS Clinical Trial Group 076 study as a consequence of zidovudine (ZDV) exposure [3], this was later confirmed by other authors [4-6]. Other hematologic cell lines can be affected as well, although less commonly and to a lesser extent [6]. Mitochondrial toxicity is mainlycaused by the inhibition of DNA-γ-polymerase by nucleoside analogue reverse transcriptase inhibitors (NRTIs), which interferes with mitochondrial DNA replication and repair [7]. Mitochondrial toxicity is thought to be very rarely symptomatic, but severe and even fatal cases have been described [8], and the potential for its long-term consequences remains uncertain.
We aimed to analyze the relationship between mitochondrial markers and hematologic values in a large cohort of HEU infants followed-up in the first year of life.
Methods
A single-center prospective observational study was conducted in a tertiary care hospital in Barcelona (Spain) among a cohort of healthy infants who were exposed to HIV and ARV during gestation, birth and the neonatal period. From January 2000 to December 2014, 320 mother-child pairs were included in the cohort, the current MTCT rate being 1.88% (95% CI: 0.91-3.98). Informed consent is obtained from all mothers at enrollment and local ethics committee approved the study protocol. Demographic, clinical and laboratory data of both mother and infant are routinely collected at enrollment, including ARV history during pregnancy, delivery and neonatal period. Complete clinical assessment is performed at every visit (at birth, 2-3 and 6 weeks, 3, 6 and 12 months, and yearly thereafter) as are blood tests per protocol to determine HIV infection status and to detect potential toxicity of ARV prophylaxis. Lab tests consist of complete blood count, serum biochemistry and plasma proviral HIV-DNA (Amplicor HIV; Roche, Basel, Switzerland) until 2004, and HIV-RNA load quantification (CA HIV-1 Monitor, Roche, Basel, Switzerland; limit of < 50 copies/mL) afterwards. HIV infection is ruled out as per National Guidelines [1].
For this particular study, all infants born from January 2000 to May 2005 were eligible. Exclusion criteria were MTCT of HIV or HCV infection, gestational age at birth < 36 weeks and any other clinical condition that could lead to mitochondrial or hematological dysfunction. Peripheral blood mononuclear cells (PBMC) were obtained from 3-5 mL of venous blood to assess mitochondrial function at 6 weeks, and 3, 6 and 12 months. Cytochrome c oxidase activity (complex IV or CIV, in nmol/min per mg of protein) and mitochondrial DNA (mtDNA, as the ND2 mitochondrial-encoded [mtND2] gene/nuclear-encoded RNA polymerase II [nRPII] generatio) were measured and corrected with citrate synthase (CS) activity (in nmol/min per mg of protein), representative of mitochondrial mass; extensive methods and results on mitochondrial genetics and function have been previously reported [9].
The following hematological variables were collected at 3 and 6 weeks, and at 3, 6 and 12 months of age: hemoglobin (Hb; g/L), mean corpuscular volume (MCV; fL) and leukocyte, neutrophil, lymphocyte cells (109/mm3) and platelet counts (109/L), and these were classified according to toxicity grades established by the division of acquired immunodeficiency syndrome (DAIDS), recently updated [10].
Statistical analysis
Categorical and continuous variables were described as percentages and mean/median values and standard deviation/ranges, respectively. To compare unrelated variables, Student's t-test was used for normally distributed data; the Mann-Whitney U test was applied to non-normally distributed data. Pearson's and Spearman test were used to identify correlations between quantitative variables. A multivariate analysis to identify factors related to both mitochondrial and hematologic parameters was performed; factors examined included those showing a significant association in the bivariate analysis and/or having clinical relevance. The analysis was carried out using SPSS 17.0 Software, and statistical significance was set at p ≤ 0.05.
Results
Between January 2000 and May 2005, 138 mother-infant pairs were enrolled in the cohort, of whom 20 were excluded (gestational age at birth < 36 weeks, n = 17; congenital heart defects, n = 2; and MTCT HIV infection, n = 1). Finally, 118 ARV-exposed infants (4 sets of twins and 8 pairs of siblings) born to 106 HIV-infected mothers were included in the study. Mother-infant pairs, gestation and main birth variables are summarized in Table 1. Most mothers (87.7%) received HAART, 63% of them from the first trimester of pregnancy. The most commonly used ARV drugs were lamivudine (n = 88%, 77.2%), ZDV (n = 84%, 73.7%) and stavudine (n = 28%, 24.6%) in combination with either nevirapine (n = 56%, 49.1%) or nelfinavir (n = 39%, 34.2%). Only 8 mothers received ZDV monotherapy starting at 28 weeks of pregnancy, all of them before the year 2003. All newborns received ARV prophylaxis, mostly ZDV monotherapy. No infant showed clinical symptoms consistent with mitochondrial dysfunction in the first year of life.
With regard to hematological parameters, macrocytic anemia was observed in 63.6%, 47.5% and 17.8% of infants at 3 and 6 weeks, and 3 months of life, respectively, of whom only 21.6%, 11.9% and 2% were DAIDS grade ≥ 2 (Table 2). No transfusion therapy or early discontinuation of ARV prophylaxis was required. A highly significant trend (p < 0.001) towards normalization of both Hb and MCV levels was observed after 6 weeks of age, attaining normal values at 6 months of life in all cases.
The highest neutropenia prevalence (10.9%) was observed at 6 weeks of age. Taking into account all time points, self-limited grade 2 neutropenia was observed in 4 different infants, and was not associated with clinically relevant infections; no grade 3-4 neutropenia was detected. No other relevant results were identified in the rest of the cell lines (Table 2). No significant correlation was identified between ARV exposure and Hb concentration, MCV, leukocyte, neutrophil, lymphocyte or platelet counts during follow-up.
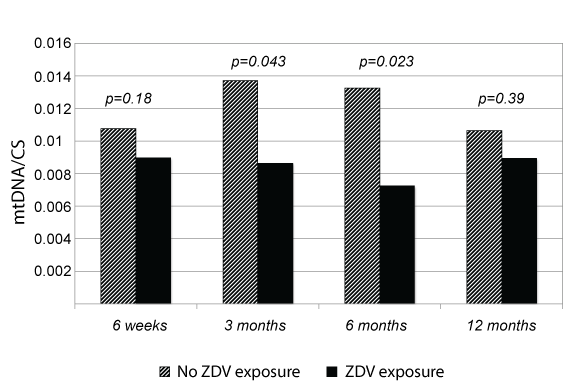
Overall, CIV/CS enzymatic activity was measured in 73, 55, 61 and 39 infants and mtDNA/CS in 52, 42, 50 and 27 children at 6 weeks and 3, 6 and 12 months of age, respectively. Patients whose mother received ZDV had lower mtDNA/CS levels at all time points, although statistical significance after adjusting for confounding variables (maternal age, ethnicity, HIV viral load, CD4+ cell count and drug use, and Apgar score below 8 at 1 and/or 5 minutes of life) was found only at 3 (mean values 0.008 vs. 0.014 mtDN2/nRPII ratio normalized by CS; p = 0.043) and 6 months of age (0.007 vs. 0.013; p = 0.023) (Figure 1). CIV/CS enzymatic activity was not associated with maternal use of ZDV at any of the time points that we analyzed.
Lower mean CIV/CS activity was observed at 6 weeks of life among infants with grade ≥ 2 anemia (0.05 vs. 0.11; p = 0.002). This finding remained statistically significant (p = 0.042) after logistic regression. No other relationships between anemia DAIDS toxicity grades and CIV/CS or mtDNA/CS values were found during follow-up (data not shown). Continuous hematologic values, including Hb concentration, white cell and platelet counts, were also not associated with mitochondrial parameters.
Discussion
Hematologic toxicity remains the most common adverse effect of ARV in HEU infants, although it is usually non-symptomatic and self-limited. According to the recently updated version of DAIDS toxicity grades [10], with noticeably higher thresholds for anemia and neutropenia, the prevalence rates we observed for anemia and neutropenia in our series were lower than those previously described by other authors (25.8%-53.8% for DAIDS grade ≥ 2 anemia and 14.6%-48.0% for neutropenia), although Hb and neutrophil absolute values were very similar [4-6].
With regard to mitochondrial toxicity, mtDNA depletion, general impairment of mitochondrial respiratory chain, and an increase in apoptosis and oxidative stress have been demonstrated in the HIV-infected ARV-treated child [11]. Mitochondrial disturbances have also been observed in the HEU infant. We recently reported consistently lower CIV enzymatic activity during the first 12 months of life among HEU infants as compared to unexposed controls, but no difference in mtDNA levels, which inversely correlated with CIV activity [9]. These findings are in line with the results of other groups reporting biochemical and genetic findings consistent with mitochondrial impairment in placenta, cord and neonatal PBMCs of HEU infants [12].
In the HEU infant, mainly fetal and neonatal exposure to ZDV have been blamed for the development of macrocytic anemia, with a nadir in Hb levels at 6 weeks of age, which normalizes in some weeks upon interruption of the neonatal prophylactic treatment [3-6]. In contrast to this, mitochondrial toxicity has been associated with different NRTIs, but especially with stavudine and/or didanosine exposure, and even with HIV itself [7]. Mitochondrial markers (lactate levels or CIV enzymatic activity) steadily normalize over the first year of life in the HEU patient and associated symptoms, albeit very rare, have been described beyond the age of 12 months in most cases [8]. The differing natural histories of mitochondrial and hematologic toxicities in the HEU infant suggests different pathogenic pathways [9,12].
This is the first study to longitudinally analyze the relationship between hematological toxicity and mitochondrial function markers in a large cohort of HEU infants during the first year of life. Interestingly, C4/CS activity was lower in those infants with DAIDS grade ≥ 2 anemia at 6 weeks of age. No other associations between mitochondrial parameters or specific ARV drug use and hematologic toxicity grades or continuous hematologic values were observed.
Zidovudine is known to have a potent inhibitor effect on the replication of hematopoietic precursors, the erythroid line being the most sensitive [13], especially during fetal life. Some mitochondrial-related mechanisms have been hypothesized for this toxicity: depletion in mtDNA/nuclear DNA ratio and increased lactate levels were reported in ZDV-exposed hematopoietic cell cultures [13], and impairment in the mitochondrial production of heme group has been described in an animal model as well, with secondary transferrin receptor overexpression and cellular iron overload [14], mimicking sideroblastic anemias. In contrast, other potential pathogenic mechanisms may be independent of mitochondrial metabolism, including a disruption in globin synthesis, a decrease in the levels of the transcription factors involved in erythroid cells differentiation [15] and a ZDV concentration-dependent decrease in erythropoietin receptor expression and its mRNA levels [16]. We observed lower mtDNA levels at 3 and 6 months of age in HEU infants exposed to ZDV during gestation, but found no association between ZDV and anemia. Although very preliminary, these results also point to mitochondrial dysfunction as one of the causes of hematologic toxicity in the HEU infant.
In our study, mitochondrial markers were not associated with neutropenia or abnormal findings in the rest of the hematological cell lines. Several studies have reported subtle long-term abnormalities on platelet and neutrophil counts and lymphocyte subsets and function in HEU children, which have been associated with exposure to ARV [6], but also to maternal CD4+ count and HIV viral load [17]. Importantly, these findings have shown no clinical relevance to date, and in industrialized countries, the increased incidence of bacterial infections in the first year of life in HEU infants has been attributed rather to weakened humoral immunity [18], possibly due to an altered mother-to-child IgG transfer through the placenta [19].
Our observational study has several limitations, including lack of hematological and mitochondrial maternal data, a lack of baseline neonatal birth values, the absence of a control group of HEU ARV-unexposed infants for obvious ethical reasons, and the low number of samples available for mitochondrial analysis at some time points. Besides these, some of the ARVs the mothers in the study received (stavudine, didanosine, nelfinavir) have been replaced by new drugs, including several NRTIs with a lower propensity to mitochondrial toxicity [20]. Nonetheless, ZDV and lamivudine remain first-line options for the treatment of HIV-infected pregnant women. Finally, we were not able to perform a complete mitochondrial genetic and functional study, as large volumes of blood are required.
In summary, our results suggest that mitochondrial toxicity may partially explain ZDV-related macrocytic anemia observed in HEU infants. Most probably, this toxicity is the result of several additive pathogenic pathways associated with ZDV exposure, but also with other HIV-related and unrelated variables (i.e. maternal and neonatal use of other ARVs, other MTCT infections, or gestational age and weight at birth). While daily ARV use prevents hundreds of new MTCT HIV infections worldwide, further studies are needed to better characterize ARV-related toxicities in HEU children, with special attention paid to long-term follow-up of this population into adulthood.
Acknowledgements
This work was supported by the Fundación para la Investigación y la Prevención del SIDA en España [grant numbers FIPSE 36612/06, FIPSE 360982/10]; Fundació Cellex, Fondo de Investigación Sanitaria [grant numbers FIS 00462/11, FIS 01199/12, FIS01738/13, FIS 01455/13]; Suports a Grups de Recerca de la Generalitat de Catalunya [grant numbers SGR 14/376 and 14/505] and CIBER de Enfermedades Raras (CIBERER, an initiative of ISCIII).
References
- Documento de consenso para el seguimiento de la infección por el VIH con relación a la reproducción, el embarazo y la prevención de la transmisión vertical. Grupo de expertos de la Secretaría del Plan Nacional sobre el Sida (SPNS), Grupo de Estudio de Sida (GeSIDA), Sociedad Española de Ginecología y Obstetricia (SEGO) y Sociedad Española de Infectología Pediátrica (SEIP).
- (2016) Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health andInterventions to Reduce Perinatal HIV Transmission in the United States. Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission.
- Sperling RS, Shapiro DE, McSherry GD, et al. (1998) Safety of the maternal-infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. AIDS 12: 1805-1813.
- Le Chenadec J, Mayaux MJ, Guihenneuc-Joyaux CH, et al. (2003) Perinatal antiretroviral treatment and hematopoyesis in HIV-uninfected infants. AIDS 17: 2053-2061.
- Bunders MJ, Bekker V, Scherpbier HJ, et al. (2005) Haematological parameters of HIV-1-uninfected infants born to HIV-1-infected mothers. Acta Paediatr 94: 1571-1577.
- Pacheco SE, McIntosh K, Lu M, et al. (2006) Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: an analysis of the women and infants transmission study. J Infect Dis 194: 1089-1097.
- Brinkman K, ter Hofstede Hj, Burger DM, et al. (1998) Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12: 1735-1744.
- Blanche S, Tardieu M, Rustin P, et al. (1999) Persistent mitochondrial dysfunction and perinatal exposure to antirretroviral nucleoside analogues. Lancet 354: 1084-1089.
- Noguera-Julian A, Morén C, Rovira N, et al. (2015) Decreased mitochondrial function among healthy infants exposed to antiretrovirals during gestation, delivery and the neonatal period. Pediatr Infect Dis J 34: 1349-1354.
- (2014) Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Version 2.0. DAIDS RSC.
- Moren C, Garrabou G, Noguera-Julian A, et al. (2013) Study of oxidative, enzymatic mitochondrial respiratory chain function and apoptosis in perinatally HIV-infected pediatric patients. Drug Chem Toxicol 36: 496-500.
- Ross AC, Leong T, Avery A, et al. (2012) Effects of in utero antiretroviral exposure on mitocondrial DNA levels, mitochondrial function and oxidative stress. HIV Med 13: 98-106.
- Lewis LD, Amin S, Civin CI, et al. (2004) Ex vivozidovudine (AZT) treatment of CD34+ bone marrow progenitors causes decreased steady state mitochondrial DNA (mtDNA) and increased lactate production. Hum Exp Toxicol 23: 173-185.
- Pollack S, Weaver J (1993) Azidothymidine (AZT)-induced siderosis. Am J Hematol 43: 230-233.
- Bridges EG, Trentesaux C, Lahlil R, et al. (1996) 3'-azido-3'-deoxythymidine inhibits erytroid-specifictranscription factors in humanerythroid K562 leukemia cells. Eur J Haematol 56: 62-67.
- Gogu SR, Malter JS, Agrawal KC (1992) Zidovudine-induced blockade of the expresión and function of the erythropoietin receptor. Biochem Pharmacol 44: 1009-1012.
- Kakkar F, Lamarre V, Ducruet T, et al. (2014) Impact of maternal HIV-1 viremia on lymphocyte subsets among HIV-exposed uninfected infants: protective mechanism or immunodeficiency. BMC Infect Dis 14: 236.
- Taron-Brocard C, Le Chenadec J, Faye A, et al. (2014) Increased risk of serious bacterial infections due to maternal immunosuppression in HIV-exposed uninfected infants in a European country. Clin Infect Dis 59: 1332-1345.
- Jones CE, Naidoo S, De Beer C, et al. (2011) Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 305: 576-584.
- Curran A, Ribera E (2011) From old to new nucleoside reverse transcriptase inhibitors: changes in body fat composition, metabolic parameters and mitochondrial toxicity after the switch from thymidine analogs to tenofovir or abacavir. Expert Opin Drug Saf 10: 389-406.
Corresponding Author
Dr. Antoni Noguera-Julian, MD, PhD, Infectious Diseases Unit, Pediatrics Department, Hospital Sant Joan de Déu, Universitat de Barcelona, Passeig Sant Joan de Déu 2, 08950 Esplugues, Spain, Tel: +34-93-280-40-00, Fax: +34-93-203-39-59.
Copyright
© 2017 Rovira N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.