Assessment of Tenofovir-Induced Nephrotoxicity Development and Recovery in HIV Patients on TDF Based Regimens at Kenyatta National Hospital Comprehensive Care Clinic
Abstract
Background: TDF containing HAART is currently the most approved global HIV first-line treatment. Despite its accessibility as a mixture single-tablet regimen for once daily dosing, favorable safety profile and resistance, and effective antiviral activity, it is associated with renal impairment among patients with concurrent use of protease inhibitors and those with advanced HIV virus. Several studies have been done investigating the risk factors for occurrence of kidney disease among HIV positive patients on TDF regimens but few have specifically investigated the time to recovery from this disease.
Objective: To assess Tenofovir-induced nephrotoxicity development and recovery among patients on TDF based regimen at KNH CCC between 2010 and 2015.
Study design and study population: The study was retrospective cohort that used HIV care follow-up data for patients (n ≥ 528) started on TDF based regimens between 2009 and 2012. Information on the baseline distinctiveness of the patients at start of treatment and dates of change of regimen for patients that developed Tenofovir-induced nephrotoxicity was collected from the database using a structured data collection tool.
Data analysis: The two outcomes of interest were the time to development and time to recovery from Tenofovir-induced nephrotoxicity. Conditional Presmoothed Kaplan-Meier Weighted estimator was used to estimate the survival functions (time to development & recovery). Multivariate Log-rank test was utilized to evaluate the endurance functions based on the three TDF based regimens. Conditional risk set model was used to evaluate prognostic factors for time to recovery TDF-induced nephrotoxicity adjusted for time to diagnosis.
Results: Of the 534 patients followed, 324 were diagnosed with TDF-induced nephrotoxicity with only 88 reported recovered. The median time to diagnosis was estimated at 43.7months after initiation of ART (IQR = 24.3-59.9 months); three quarters had recovered by the 16th month upon withdrawal of TDF. Patient gender (Male-HR = 0.68, P-value = 0.013), age group (Adults-HR = 0.66, P-value = 0.029) and ALT/GPT levels (HR = 1.01, P-value = 0.044) were found to significantly affect the expected hazard of patient recovery.
Conclusion: Prolonged use of TDF in first line ART regimen is associated with nephrotoxicity which is reversible upon withdrawal. Males and older patients are at a high risk of taking longer to recover from the disease once diagnosed. Regular monitoring of creatinine authorization during follow-up with TDF uses is paramount to prevent nephrotoxicity especially in this high-risk group of patients.
Abbreviations
AKI: Acute Kidney Infection; CCC: Comprehensive Care Center; HAART: Highly Active Antiretroviral Therapy; TDF: Tenofovir Disoproxil Fumarate; KNH: Kenyatta National Hospital; SCOLTA: Surveillance Cohort Long-term Toxicity Antivirals
Introduction
Background of the study
Tenofovir disoproxil fumarate (TDF) became available in 2001 and it was the first nucleotide inhibitor of HIV reverse transcript. Since then, it has been expansively used globally and now it has become the most prescribed antiretroviral (ARV) drug. Its success has been attributed to its high antiviral activity and positive metabolic profile.
Generally antiretroviral therapy works by inhibiting HIV replication stages. HAART which is the standard management of choice for HIV patients includes a combination of at least three antiviral drugs, usually from two different classes Dybul, et al. [1]. The use of HAART has helped in reducing morbidity and mortality resulting from HIV. There are different types of HAART combination of available treatment that is determined by the therapeutic objectives, the cost and the tolerability, Ngondi, et al. [2].
Tenofovir disoproxil fumarate (TDF) containing HAART regimen is the most preferred antiretrovirals (ARVs) among young people and adults due to its better pharmacokinetic (P.K.) profile and potency that allows daily dosage, Chapmanet, al., Lyseng-Williamson, et al. [3,4]. Despite all the above, renal toxicity is associated with TDF containing antiretroviral regimen. The use of TDF in clinical practice is linked to proximal tubular dysfunction with or without decreased renal function causing acute renal failure, acute kidney injury and Fanconis syndrome Mouss, et al. [5].
When they are detected early appearance of nephrotoxicity commonly improves due to discontinuation of the TDF drug. Herlitz, et al. [6] indicated that approximately 50% of infected persons improved renal function to baseline levels following 20 +/- 26 months of tenofovir discontinuation after diagnosis of Acute Kidney Injury and others were reported to have partial recovery of renal function. Late detection of nephrotoxicity is reported to lead to irreversible tubule interstitial damage. There's no study that has assessed the dependency of the recovery time on the time to development of the disease. This information would be very useful for the clinicians when monitoring the patients put on TDF drugs, and would serve as an indicator for when to change the course of treatment so as to lessen the occurrence of TDF-induced nephrotoxicity.
Problem statement
Chronic Kidney Disease (CKD) is still a stern snag in HIV-infected patients on ART; this is according to findings done in Sub-Saharan African Countries. A decreased anticipated glomerular filtration rate (eGFR) is seen in 25% of these patients when they were started on ART, while 72% have microalbuminuria Msango, et al. [7]. Despite TDF being the most approved antiretroviral globally for the first-line treatment of HIV infection because they are available as a combination single-tablet regimen and can be taken once daily, favorable safety profile and resistance, and effective antiviral activity, it is associated with CKD among patients with coexisting use of protease inhibitors and those with complex HIV infection.
TDF is also associated with severe kidney injury and proximal tubular dysfunction in patients in developed countries, those with lower body mass index, or those with preexisting kidney disease [8]. However, despite all the above factors, no significant work has been done at KNH on patients on TDF based regimen to determine the time to diagnosis and recovery from kidney disease. Therefore, this study will not only focus on the risk factors for developing and/or recovery from kidney disease but also capture the time to these events.
Justification
Several studies focus on connectivity between exposure to TDF and occurrence of kidney disease and few have specifically investigated the recovery from this disease. In addition, no study has been done to evaluate the relationship between diagnosis of and recovery from nephrotoxicity among patients on TDF based regimen. The main endeavor of this study is to address this gap by assessing the dependency of time to recovery from TDF associated nephrotoxicity on the time to diagnosis using a multivariate failure time for ordered events. This information will be very useful for clinicians to monitor the duration of treatment for patients at risk of kidney disease based on their profile at the start of treatment.
Broad Objective
To assess Tenofovir-induced nephrotoxicity development and recovery among patients on TDF based regimen at KNH CCC between 2009 and 2015.
Specific objectives
1. To describe the baseline profile of HIV patients at the time of initiation into TDF based regimen at KNH-CCC
2. To compare the time to diagnosis of Tenofovir-induced nephrotoxicity among HIV patients onTDF+3TC+EFV, TDF+3TC+NVP, and TDF+3TC+LPV\r regimens at KNH- CCC
3. To compare the time to recovery from Tenofovir-induced nephrotoxicity among HIV patients on TDF+3TC+EFV, TDF+3TC+NVP, and TDF+3TC+LPV\r regimens at KNH- CCC
4. To determine the effect of factors associated with time to development and recovery from Tenofovir-induced nephrotoxicity among HIV patients on TDF-based regimens at KNH-CCC
Research hypotheses
1. The time to diagnose Tenofovir-induced nephrotoxicity among HIV patients does not depend on the TDF-based ART regimen.
2. The time to recovery from Tenofovir-induced nephrotoxicity among HIV patients does not depend on the TDF-based ART regimen.
3. The time to development and recovery from Tenofovir-induced nephrotoxicity among HIV patients on TDF-based regimens at KNH-CCC is completely random.
Methodology
Study design
The study aimed to assess the effect of baseline characteristics (e.g., presence of co-morbidities like hypertension and creatinine levels) of the patient when TDF-based ART treatment was introduced during the development of TDF-induced nephrotoxicity. In achieving this, a retrospective cohort study design was adopted to investigate the characteristics associated with time to diagnosis of TDF-induced nephrotoxicity and recovery among HIV patients on TDF based regimen. Therefore, patients put on TDF-based ART between 1st January 2009 and 31st December 2012 were sampled from the KNH-CCC database; Information on their baseline characteristics was retrieved from the screening records of the patients before initiation of treatment, and dates when the diagnosis of TDF-induced nephrotoxicity was made and when the patients were declared to have recovered from the nephrotoxicity.
Study site
This study was done at Kenyatta National Hospital Comprehensive Care Centre Clinic in Upper hill Nairobi, which is an ongoing HIV clinic supported by the CDC and working with the collaboration of the University of Nairobi, running from Monday to Friday serving all patients who can follow set national guideline for HIV clinic services. This site started prescribing TDF-based regimens in January 2009. Patients registered in this sight benefit from services such as nursing care, clinical care, psychosocial support, and nutritional and physiotherapy services.
Study population
The population for this study consisted of HIV-positive patients initiated into TDF-based regimens between 1st January 2009 and 31st December 2012 at KNH-CC. The TDF-based regimens prescribed at the facility are; TDF + 3TC + EFV, TDF + 3TC + NVP, and TDF + 3TC + LPV\r. A sum of 1852 patients was put on TDF based regimen between 2009 and 2012. By 2015, 177 patients had developed TDF-induced nephrotoxicity.
Inclusion criteria
◼ Must be an HIV patient put on TDF-based ART at KNH-CCC between 15th January 2009 and 31st December 2012.
◼ Must have complete patient records' screening results at the commencement of TDF based on ART treatment
Exclusion criteria
◼ Patients that developed TDF-induced nephrotoxicity but whose dates of change of ART regimen are not available in the database.
Sampling method
Stratification was done based on the type of TDF-based regimen to have a representative sample for each type of TDF regimen (TDF + 3TC + EFV, TDF + 3TC + NVP, and TDF + 3TC + LPV\r). TDF + 3TC + EFV is a fixed combined dose regimen and is, therefore, the preferred regimen to TDF + 3TC + NVP. TDF + 3TC + LPV\r is a second-line ART regimen. Therefore the number of patients initiated on this regimen depends on the number of patients failing on the first line. Therefore, there are more patients on TDF + 3TC + EFV at any given point in time compared to TDF + 3TC + NVP and TDF + 3TC + LPV\r. A list of patients in each regimen was obtained from the records. N-random numbers were generated using a Microsoft Excel random function based on the number of patients in each list. Simple random sampling with proportional size allocation was used to select patients in each stratum (regimen type).
Sample size determination
A multivariate log-rank (Wei-Lachin) test was used to compare the effect of different TDF-based regimens on time to diagnosis and recovery from TDF-induced nephrotoxicity. The sample size was determined based on this test using the formula, Lachin [9];
Where; n represents the minimum sample size required
Represents the standard normal distribution critical value at α-level of significance for one sided test (α = 0.05; =?) Represents the standard normal distribution critical value at β-type II error (β = 0.2; Z1- α =?)
Represents a vector of coefficients of covariates for event a (diagnosis of TDF-induced nephrotoxicity)
Represents a vector of coefficients of covariates for event b (recovery from TDF-induced nephrotoxicity)
Represents variance of
Let Sa = SD ( ); Sb = SD ( ) and Corrab = Corr ( )
where k is a constant.
Let r represent Corrab
Assuming sample size allocation ratio is one with no missing observations, then;
With expected difference in the coefficients of 0.25SD in each event, then
For a one-sided test at 0.05, the sample size required to give a power of at least 0.9;
Because we are interested in the minimum sample size;
The estimated minimum sample size is 550 patients using the formula and defined parameters.
Data collection and analysis
Data were retrieved from KNH-CCC electronic records for patients initiated into TDF-based regimens between 2009 and 2011 and stored in the M.S. Access database. Data cleaning, coding, and analysis will be done using STATA version 13 S.E.
Exploratory data analysis was done to summarize the data. First, histograms were plotted to show the distribution of quantitative variables and measures of central tendency (mean/median) and dispersion (standard deviation/inter-quartile range) reported in tables. For categorical variables, bar/pie charts were plotted to show the distribution, frequencies, and proportions reported in tables.
A conditional Kaplan-Meier Weighted estimator was used to estimate the survival functions;
Let (T1, T2) represent a pair of successive event times corresponding to two ordered consecutive events (such as diagnosis and recovery from Kidney nephrotoxicity) measured from the start of the follow-up (T1 < T2 = T).
The conditional survival probabilities can be estimated as follows;
P (T2 > y|T 1> x) or P(T2 > y|T1 ≤ x)
Let S1 and S represent the marginal survival functions of T1 and T that is;
S1(y) = P(T1>y) and S(y) = P(T>y) with conditional probabilities P (T > y|T1 > x) and P (T>y|T1 ≤ x)
Since S (y|x) can be expressed as;
S (y|x) = P (T > y|T1 > x) = 1-P (T ≤ y|T1>x) =
then the conditional survival function is estimated as;
Where denotes the ordered -sample and Wi
is the Kaplan-Meier weight attached to .
Median times to the development of kidney disease and median time to recovery were reported. A multivariate Log-rank (Wei-Lachin) test was applied to compare the continued existence functions based on the different TDF-based regimens. As a result, Chi-square omnibus statistic and corresponding p-value were reported.
Consider the case where each subject can experience one or both of two events A and B, with log hazard ratios βa and βb, respectively, for groups x and y
Where J = (1 1)' and ~N(0,1) under the null hypothesis (H0:βa = 0 and βb = 0) from Slutsky's theorem. is the is the Wei Lachin variance computed as Var .
The test rejects the test rejects H0 in favor of H1 when ZS ≤ Zα at level α one-sided.
According to Prentice, et al. [10], a provisional risk set model was used to evaluate prognostic factors for the expansion of kidney disease and recovery. Development of kidney disease and recovery were considered ordered events because recovery cannot occur before a diagnosis of the disease. In this model, the provisional risk set at time t for event k is made up of all subjects under inspection at time t that have had event k - 1. Hazard ratios and corresponding confidence intervals were reported.
Conditional risk set model
Where; λik represents the hazard role for the kth event of the ith subject at time t
β' represents a vector p-dimensional regressions coefficients for the p-covariates
λk (t)represents the baseline hazard function for the kth event
Zk represents a matrix of p-covariates dim (1, p) for the kth event
Under this model, the patient enters the process upon initiation of a TDF-based ART regimen and is at risk of developing TDF-induced nephrotoxicity (first event). The patient enters the second risk set upon diagnosis and treatment changes. The effect of covariate p on the risk of recovery of a patient at time t is eβ conditional on the history of diagnosis at time t-1.
Ethical considerations
Ethical consent was required from the University of Nairobi/Kenyatta National Hospital Research and Ethics Committee. Further approvals were sought from the KNH departmental head of Health Information Services and also from the head of the unit at Kenyatta National Hospital comprehensive care center to use the electronic medical records servers was granted.
Results
Baseline characteristics of the patients
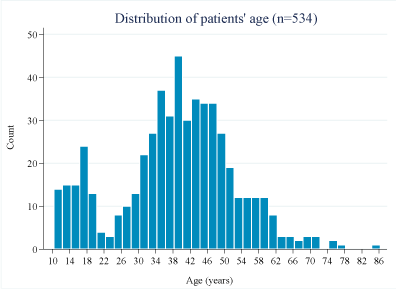
We retrieved a total of 534 complete patient records for this study. Close to three quarters were female (73.0%). Their age ranged between 10 years and 85.3 years, with a median of 39.6 years (IQR = 47.3-32.3). The age distribution was bimodal, with peaks at 17 and 39-years (Figure 1). The patients were classified as pediatric (≤ 15-years) or adults (> 15-years) depending on the age at the time of initiation into ART.
Most (70.4%) of the patients were started on the TDF + 3TC + EFV regimen, and about a quarter (24.4%) were taking the TDF + 3TC + NVP regimen. The hemoglobin level was discretized at normal for patients with at least 10g/dl (male) and 12 g/dl (female), below which the patient was regarded as having a low hemoglobin level. One-third (33.7%) of the patients were found to have low hemoglobin levels. Regarding CD4 count, more than half (58.6%) had less than 500cells/l of blood. The majority (77.7%) had undetectable viral load by the second visit after ART initiation (Table 1).
Diagnosis and recovery from TDF-induced nephrotoxicity
A patient was considered to have TDF-induced nephrotoxicity following a creatinine clearance rate of less than 50 mL/min during the clinic follow-up visits after initiation into a TDF-based ART regimen. Of the 534 patients, 324 were diagnosed with TDF-induced nephrotoxicity, and only 88 had recovered by the end of the study follow-up period. Therefore, the overall incidence rate for TDF-induced nephrotoxicity was estimated at 2 cases per 100 person-years (534 patients/18079.3 times at risk).
The risk of a patient being diagnosed with TDF-induced nephrotoxicity increased gradually with a change in time/continuous exposure to the drug. The median time to diagnose TDF-induced nephrotoxicity was 43.7 months (IQR = 59.9-24.3 months). After the 70th month, the risk of being diagnosed with the disease rose drastically to about the 80th month (Figure 2).
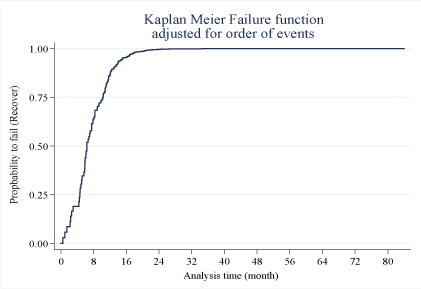
Adjusting for the time to development of TDF-induced nephrotoxicity, the patients were found to recover fast, with the majority recovering within 16 months (Figure 3) after withdrawal of TDF as a part of the ART regimen.
Comparison of survival functions based on ART start regimen
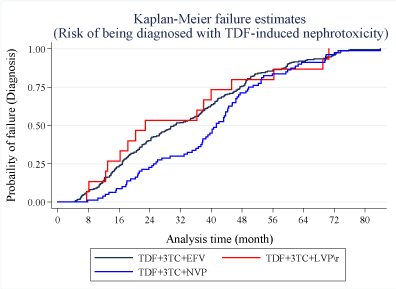
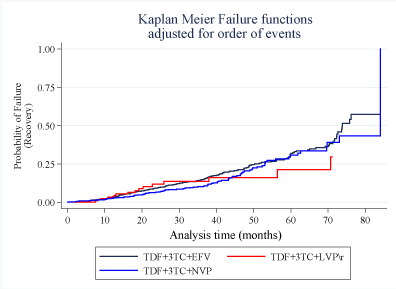
Figures 4 and Figure 5 show the failure functions estimated using the Kaplan Meier method corresponding to the risk of a patient being diagnosed with TDF-induced nephrotoxicity and recovery, respectively. Within the first 24 months, the risk of being diagnosed with TDF-induced nephrotoxicity at any given time among those who started on the LVP\r regimen was equivalent to the risk among those on the EFV regimen. The risk of TDF-induced nephrotoxicity among patients on NVP appeared to increase gradually after the first eight months of initiation up to the 28th month. The slope of the NVP failure curve was steeper after the 34th month up until the 50th month. After the 48th month, the risk of being diagnosed with the disease was based on the start regimen was equivalent.
Following the withdrawal of the TDF drug, the risk of a patient recovering from the disease increased gradually concerning time. The patients who previously started on LVP\r portrayed a better recovery experience than those who started on EFV and NVP (Figure 5).
Log-rank tests were done to evaluate the overall survival functions of patients following the diagnosis of TDF-induced nephrotoxicity concerning the patient characteristics and treatment regimen. There was significant difference in the survival functions with respect to patient's gender (p-value = 0.006), age group (p-value = 0.003) and CD4 count (p-value = 0.028). Regarding the ART start regimen, there was no significant difference (P-value = 0.915) in the overall recovery experience between the three treatment regimens conditional on the time to diagnosis of the disease (Table 2).
Factors associated with time to development and recovery from tenofovir-induced nephrotoxicity
The conditional risk set model was used to explore the effect of ART start regimen, CD4 count, hemoglobin level, ALT/GPT level, viral load, gender, and age group of the patient on time to recovery from the disease. The time to recovery was measured from the time of a patient's diagnosis with TDF-induced nephrotoxicity and not from the time of entry into the study.
A change in the patient's ALT/GPT level was positively associated (HR = 1.01, P-value = 0.044) with the time taken for a patient to recover from TDF-induced nephrotoxicity. In addition, the male patients were found to take longer to recover compared to female patients (HR = 0.68, P-value = 0.013), adjusting for the effect of other covariates in the model. An adult patient had a 34% (HR = 0.66, P-value = 0.029) decline in the expected hazard relative to a pediatric patient, holding other factors constant. The ART start regimen, CD4 count, hemoglobin level, and patient viral load did not significantly affect the expected hazard (Table 3).
Discussion
The use of TDF in first-line ART among HIV-infected patients has been widely documented [11-14] to have nephrotoxic potential resulting in Kidney injury, CKD or Fanconi Syndrome. The current study finding supports these reports, considering the high number of patients reported to have decreased creatinine clearance rate (below 50mL/min) following initiation of TDF-based ART regimens. Considering the undisputed benefits of TDF in HIV care, safe-regular monitoring of its use is essential to prolong the patient's life.
In estimating the probability of a patient on a TDF-based regimen being diagnosed with nephrotoxicity, we noted that the risk increased positively with time; the longer a patient stays on TDF-based ART, the higher the chances of that patient presenting signs of nephrotoxicity. Beyond two years of TDF-based regimen use, patients had more than a 25% chance of developing the disease and more than 50% risk after three and a half years. This result is useful for clinicians managing newly diagnosed HIV patients and starting on first-line ART in determining the appropriate time to consider a change of regimen to prevent the adverse events associated with renal failure as a consequence of using TDF.
Regarding recovery from TDF-induced nephrotoxicity, the creatinine clearance rate was found to resolve fast, with 75% of the patients reported to have recovered within the first 16months after withdrawal of TDF. This finding is consistent with [6,15], which reported that half of the patients recovered from TDF-induced renal impairment to baseline creatinine levels within 13-49.5 months and 20 +/- 26 months, respectively.
The study further sought to explore factors affecting the expected hazard of a patient recovering from TDF-induced nephrotoxicity. ALT/GPT was positively associated with the time to recovery from TDF-induced nephrotoxicity. This has not been reported in the previous studies evaluating the risk factors for TDF- induced renal impairment. Furthermore, ALT/GPT, a marker for the liver function, would be quite useful in informing the clinicians in case of drug-induced liver damage, which would further complicate the recovery of a patient from TDF-induced renal failure.
In this study, the CD4 cell count did not affect the expected hazard of a patient recovering from TDF-induced nephrotoxicity. This finding agrees with the results by Ojeh, et al. [16] in a study conducted in Nigeria evaluating the Incidence and predictors of TDF-induced renal injury in HIV-infected patients in Nigeria. Previous studies by Tourret, et al. [17] and Wantakisha, et al. [18], however, indicate baseline CD4 cell count is an important risk factor for the disease, with patients having > 350 CD4 cell count having a higher threat of TDF-induced renal dysfunction among HIV patients. Therefore, it is possible that the CD4 cell count at the time of diagnosis of the disease among the cases with reduced creatinine clearance rate was comparable to patients with a normal rate.
A male HIV-infected patient diagnosed with TDF-induced nephrotoxicity was found to have a significant decrease in the expected hazard relative to a female patient after adjusting for the effect of other covariates. Consequently, male patients are likely to suffer from the disease for longer despite TDF withdrawal compared to female patients. Considering previous reports indicating male HIV-infected patients being at a higher threat of renal impairment [19,20], special attention should be accorded to those receiving TDF-based ART regimen to determine when to change the course of treatment as a precaution to prevent this adverse event.
Older patients, usually above 50 years, are considered to have an increased risk of TDF-induced nephrotoxicity [18,21]. In this study, adult patients had a reduced expected hazard of recovery from TDF-induced nephrotoxicity relative to pediatric patients, implying that they take longer to recover. The sole purpose of ART is to improve the quality of life of an HIV-infected patient, and to achieve this, patients at risk of drug-induced adverse events that further complicate the management need to be prevented through intensified safety monitoring.
Conclusion
Kenya is one of the countries with a higher Incidence of HIV infections; the use of TDF in first-line ART regimens is invaluable. However, this study has highlighted the differential effect of age group, gender, and ALT/GPT levels on time to diagnosis and recovery from the TDF-induced nephrotoxicity. Whereas factors such as gender and age group cannot be changed, the findings of this study inform on the high-risk group of patients that require special attention in terms of safety monitoring to ensure sustained improved health.
References
- Dybul M, Dybul M, Fauci A, et al. (2002) Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med 137: 381-433.
- Ngondi J, Oben J, Forkah D, et al. (2006). The effect of different combination therapies on oxidative stress markers in HIV infected patients in Cameroon. AIDS Res Ther 3: 1-7.
- Chapman TM, McGavin JK, Noble S (2003) Tenofovir disoproxil fumarate. Drugs 63: 1597-1608.
- Lyseng Williamson KA, Reynolds NA, Plosker GL (2005) Tenofovir disoproxil fumarate: A review of its use in the management of hiv infection. Drugs, 65: 413-432.
- Mouss S, Berger F, Schmutz G (2005) Antiretroviral therapy with tenofovir is associated with mild renal dysfunction. AIDS 19: 93-95.
- Herlitz LC, Mohan S, Stokes MB, et al. (2010) Tenofovir nephrotoxicity: Acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 78: 1171-1177.
- Msango L, Downs Ja, Kalluvya SE, et al. (2013). Renal dysfunction among HIV-infected patients starting antiretroviral therapy in Mwanza, Tanzania. Aids: 1421-1425.
- Scherzer R, Estrella M, Li Y, et al. (2012) Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 26: 867-875.
- Lachin JM (2014) Applications of the wei-lachin multivariate one-sided test for multiple outcomes on possibly different scales. PLoS ONE 9: e108784.
- Prentice RL, Williams BJ, Peterson AV (1981) On the regression analysis of multivariate failure time data. Biometrika 68: 373-379.
- Pere Soler-Palacín, Susana Melendo, Antoni Noguera-Julian, et al. (2011) Prospective study of renal function in HIV-infected pediatric patients receiving tenofovir-containing HAART regimens. AIDS 25: 171-176.
- Leonardo Calza, Filippo Trapani, Caterina Salvadori, et al. (2013) Incidence of renal toxicity in HIV-infected, antiretroviral-naive patients starting tenofovir/emtricitabine associated with efavirenz, atazanavir/ritonavir or lopinavir/ritonavir. Scand J Infect Dis 45: 147-154.
- Boswell MT, Rossouw TM (2017) Approach to acute kidney injury in HIV-infected patients in South Africa. S Afr J HIV Med 18: 714.
- Venter WDF, Fabian J, Feldman C (2018) An overview of tenofovir and renal disease for the HIV-treating clinician. S Afr J HIV Med 19: 817.
- Bonjoch A, Echeverría P, Perez Alvarez N, et al. (2012) High rate of reversibility of renal damage in a cohort of HIV-infected patients receiving tenofovir-containing antiretroviral therapy. Antiviral Res 96: 65-69.
- Ojeh BV, Abah IO, Ugoagwu P, et al. (2018) Incidence and predictors of tenofovir disoproxil fumarate-induced renal impairment in HIV infected Nigerian patients. Germs 8: 67-76.
- Tourret J, Deray G, Isnard-Bagnis C (2013) Tenofovir effect on the kidneys of HIV-infected patients: A double-edged sword? J Am Soc Nephrol 24: 1519-1527.
- Wantakisha E, Chongwe G, Munkombwe D, et al. (2017) Renal dysfunction among HIV-infected Patients on tenofovir-based antiretroviral therapy at ronald ross hospital in Zambia. J AIDS Clin Res 8: 651.
- Nelson MR, Katlama C, Montaner JS, et al. (2007) The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: The first 4 years. AIDS 21: 1273-1281.
- Madeddu G, Bonfanti P, De Socio GV, et al. (2008) Tenofovir renal safety in HIV-infected patients: Results from the SCOLTA Project. Biomed Pharmacother 62: 6-11
- Fernandez Fernandez B, Montoya Ferrer A, Sanz AB, et al. (2011) Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat 2011: 354908.
Corresponding Author
Walter Odongo Owako, University of Nairobi, Kenya
Copyright
© 2022 Owako WO. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.