Phantom Assessment of Unshielded Magnetocardiography Repeatability, Precision and Accuracy in Electric Sources Localization
Abstract
Background
Pre-interventional knowledge of arrhythmogenic substrate location may reduce interventional time and arrhythmias ablation risks. Magnetocardiographic Mapping (MCG) is a contactless method for non-invasive localization of intracardiac sources used for Three-Dimensional (3D) Electro-Anatomical Imaging (EAI) of arrhythmogenic substrates. The aim of this study was to assess the repeatability, precision, and accuracy of MCG in localizing dipolar sources in an unshielded environment.
Methods
The phantom consisted of a rectangular plastic box filled with 0.9% NaCl saline solution. Multiple artificial current dipoles (6 mm length, 10 mA, 50 Hz) were induced with two a magnetic electro-catheters. The distance between two dipoles was constant. 30 seconds MCG recordings (bandwidth DC-200 Hz, 1 KHz sampling rate) were performed with 36 DC-SQUID sensors at distances between Sensors plane and Dipole Sources (SSD) decreasing from 18 to 9 cm. MCG localization was assessed by inverse solution based on the Equivalent Magnetic Dipole (EMD) model.
Orthogonal fluoroscopic imaging, employing lead markers to correct for x-ray divergence effect, was used to define the 3D physical relative position of each dipole. MCG repeatability, precision and accuracy were evaluated. The correlation between precision, Goodness of Fit (GOF) of the EMD model and SSD was also analyzed.
Results
Overall, optimal repeatability (Coefficient of Variation ± Standard Error of the Mean = 0.79 ± 0.43%, 3D absolute error = 0.26 ± 0.25 cm), average localization precision (1.13 ± 0.42 cm) and average accuracy (0.2 ± 0.13 cm) were found. Localization precision improved (0.87 ± 0.3 cm) with GOF of the model increasing above 73% and SSD lower than 14 cm.
Conclusion
Contactless MCG provides optimal precision and accuracy in localizing dipolar sources, even when performed in an unshielded environment. By integrating source localization into cardiac 3D imaging by cardiac magnetic resonance, MCG is foreseen to provide both pre-interventional and intraoperative 3D-EAI of arrhythmogenic substrates.
Keywords
Unshielded magnetocardiography, Phantom validation, Localization accuracy.
Introduction
Cardiac arrhythmias ablation is currently performed with the aid of invasive Three-Dimensional Electro Anatomical Imaging (3D-EAI) and localization methods such as Carto®, EnSite Navx® or MediGuide®. Such methods use electric or magnetic fields to reconstruct cardiac anatomy and visualize intracardiac catheter(s), employing fluoroscopy to assess the correct initial catheter position. Though their precision and accuracy are excellent, ranging from 0 to 0.5 cm in phantom studies [1-3], interventional 3D-EAI still requires the use of radiation with consequent risks for both patients and operators [4] and, most important implies cardiac catheterization.
Especially when dealing with otherwise healthy subjects, precise pre-interventional knowledge of arrhythmogenic substrate location may be useful for appropriate patients' selection and to minimize interventional radiation time, risk of failure and can be performed with Body Surface Potential Mapping (BSPM) or Magnetocardiography (MCG).
Non-invasive 3D-EAI has been recently attempted with the solution of the inverse-problem in terms of epicardial and/or endocardial electrogram reconstruction from Body Surface Potential Mapping (BSPM) [5]. A faster alternative to BSPM can be contactless Magnetocardiographic Mapping (MCG) a contactless method which passively records the very weak magnetic field (10 pT) produced by the electric activity of the heart [6]. MCG electric source localization is based on the inverse solution problem, which is the derivation of the maximum amount of information about the electrical sources associated with the measured magnetic field distribution, by analyzing the magnetic field recorded outside the body. Since inverse problem hasn't univocal solution, in a simplified approach two approximations are needed: firstly a semi-infinite half-space with homogenous conductivity has to be considered as torso model in which magnetic field variations happen, secondly the entire electromagnetic field has to be parameterized as a single Equivalent Current (ECD) or Magnetic (EMD) Dipole [7].
MCG can provide quantitative and qualitative parameters regarding electric source localization, and it is proposed to obtain non-invasive Three-Dimensional (3D) Electro-Anatomical Imaging (EAI) when integrated with 3D rendering cardiac anatomical images obtained with Cardiac Magnetic Resonance (CMR) or CT scan [8]. Since their beginning, the majority of MCG instrumentations was constructed to be operational in magnetically shielded rooms only [9-13]. Although shielded MCG is demonstrated to provide excellent precision and accuracy in phantom studies [11], magnetic shielded rooms are still expensive and limit the widespread clinical application of MCG, especially when dealing with critical cardiac patients. To favor clinical ambulatory use of MCG, multichannel instrumentation operational without any electromagnetic shielding have been used since the early 2000 [14,15].
The aim of this study was to evaluate the repeatability, precision, and accuracy of 3D artificial dipolar source localization achievable with MCG in an unshielded catheterization laboratory for interventional electrophysiology, using a geometrically simplified phantom and a magnetic catheter.
Methods
Experimental instrumentation
The phantom was a simple 50 × 60 cm rectangular box filled with 0.9% sodium chloride saline solution, simulating a semi-infinite half-space of homogeneous conductivity (0.21 S/m).
The amagnetic catheters, used as an artificial source, have been previously described in detail [16]. Briefly, it featured multiple electrodes for clinical recording of multiple monophasic action potentials, pacing, and, at the same time, generation of geometrically-known electromagnetic dipoles adequate for MCG source localization (length 6 mm, current 10 mA, duration 20 ms, and variable pulse rate, usually 3 Hz). The catheters were introduced in the box through watertight accesses. The support was positioned on the same amagnetic bed used for patient recordings, exactly at the center of the 36-point recording grid. The appropriate positioning of the sensors on the phantom was controlled with the aid of three laser beams solid to the Dewar and a specially designed grid placed on the phantom.
Two-projection (posterior-anterior and lateral) fluoroscopic imaging was used to correctly localize the physical position of each catheter. Lead markers were used as radiopaque references, corresponding to the center of each MCG sensor pick-up coil, inserted into a Plexiglas 36-point grid. Further lateral markers were used to normalize the fluoroscopic localization per x-ray divergence.
MCG mapping
MCG was performed with a 36-channel system (CardioMag Imaging, Inc., Schenectady, NY, USA) in an unshielded laboratory equipped for interventional electrophysiology, measuring the z-component of magnetic field with Direct Current Superconducting Quantum Interference Device (DC-SQUID) sensors coupled to second order axial gradiometers with a 50-mm baseline, enclosed in a cylindrical cryostat (the second order axial gradiometers configuration intrinsically reduces the magnetic environmental noise, since it is insensitive to uniform magnetic fields and environmental gradients fields and records only gradients magnetic fields under the sensors).
Moreover, the instrumentation features a real-time Electronic Noise Subtraction System (ENSS) which automatically eliminates from the MCG signals a significant part of the three-dimensional background noise components (X,Y,Z) detected by three additional SQUID sensors located in the dewar far from the measuring pick-up coils.
The intrinsic sensitivity of the system was about 30 fT/√Hz in the frequency range of clinical interest (1-250 Hz). Signals were digitally recorded (bandwidth DC-250 Hz, 1 kHz sampling frequency, 24-bit resolution) from and area of 20 × 20 cm. The MCG recording time was 30 seconds.
MCG recordings were repeated at different Source-Sensors Distance (SSD), from 180 to 90 mm and vice versa (to check for reproducibility), which was changed by rising the Dewar in Z direction (height), while the X-Y plane position was kept unchanged.
Post-processing of MCG signals consisted of digital filtering (low-pass at 100 Hz and selective COMB filter of power line 50 Hz noise) and time averaging, to optimize the signal-to-noise ratio, and magnetic field reconstruction. The time-averaging window was selected starting 200 msec before and ending 200 msec after the signal of interest.
The reference baseline was automatically selected before the averaged squared wave by MCG software (40 msec) and the inverse solution was calculated from the beginning to the end of the squared wave (duration 20 msec, time resolution 1 msec).
The mean values of the three coordinates during the time interval were used to assess the Three-Dimensional (3D) position of EMD.
For each registration, the GOF of the model was estimated by calculating the ratio between the intensity of the negative and positive magnetic field components.
Parameters analyzed
To check for MCG localization consistency, 5 features were analyzed:
1. Repeatability of Localization, considered as the MCG error in localizing a motionless dipole twice.
2. MCG precision which was the 3D difference between the fluoroscopy-dependent physical single dipole localization and the localization of the same dipole provided by MCG.
3. MCG accuracy which was the fluoroscopy-independent MCG capability to estimate the 3D distance between two constant-spaced dipoles on the same catheter.
4. Correlation between precision and electric field dipolar pattern, expressed as GOF.
5. Correlation between precision and SSD.
Statistical analysis
All statistical calculations were performed with SPSS software, version 21.0 (SPSS Inc., Chicago, Illinois). Continuous variables were expressed by mean ± SD and were compared using Mann-Whitney U test. A p value < 0.05 was considered as statistically significant.
Repeatability of MCG recordings were evaluated with the Coefficient of Variation (CV) and the Standard Error of the Mean (SEM) for each parameter.
Relationship measurements were obtained by Spearman's rank correlation coefficient (Spearman's Rho) and coefficient of determination (R2) calculation.
Results
Noise levels and noise subtraction
Typically, in the frequency range of interest (DC-100 Hz), the peak-to peak magnetic noise was about 1 picotesla (MCG) and less than 100 microvolts (µV ECG). After adaptive digital filtering of 50 Hz (power line) noise and time averaging, the sensitivity was better than 30 fT/√Hz and the MCG signal quality was adequate to detect magnetic fields above 50 fT.
Repeatability analysis
Average repeatability between two subsequent MCG recordings, evaluated by calculating the CV between first and second measurements and expressed as averaged value for the entire cohort ± SEM, was 0.79% (CV) and 0.43% (SEM) with a mean 3D overall absolute error of 0.26 ± 0.3 cm. Three coordinates showed comparable results, as considered alone. 90% of 3D error derived by Z coordinate error (R2 = 0.903, p < 0.0001) and was considerably correlated to it (Spearman's Rho = 0.925, p < 0.0001) (Table 1).
Overall precision and accuracy assessment
Overall precision, estimated by comparing MCG localization with that provided by fluoroscopic imaging of the physical position of the catheter dipole in respect of the recording grid, was 1.13 ± 0.42 cm. Instead the localization accuracy, calculated as the MCG spatial localization difference of two dipoles of fixed known distance, embedded in the same electro-catheter, thus independently from fluoroscopic measurements, was as small as 0.20 ± 0.13 cm (Table 2).
GOF and SSD influence
A Change with "statistically significant" difference was found between precision measurements with GOF value higher or lower than 73%. Indeed, precision was 0.87 ± 0.30 cm with GOF > 73% and 1.39 ± 0.36 cm with GOF < 73% (p < 0.0001). No statistical significance was found for GOF improvement up to 80% (p = 0.8).
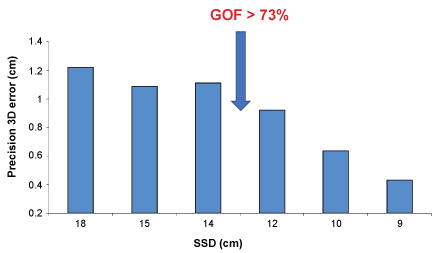
SSD showed an important role in precision assessment: 3D localization error was almost constant for SSD ranging between 18 and 14 cm, while linearly decreased for SSD < 14 cm (Figure 1). Precision decreased from 1.01 ± 0.21 cm (SSD > 14 cm) to 0.78 ± 0.33 cm (SSD < 14 cm).
Discussion
In clinical practice, MCG is increasingly used for non-invasive functional cardiac imaging of electrophysiological phenomena and for the localization of the site of origin of cardiac arrhythmias [8,14,15,17,18]. Such pre-interventional non-invasive approach is useful for a better understanding of arrhythmias' mechanisms and, if catheter ablation is needed, could reduce the risks associated with invasive procedures. Earlier phantom studies have demonstrated the accuracy and precision superiority of MCG over BSPM in localizing artificial electric sources [11,19]. Magnetic-based technique superiority over electric-based methods has been also recently confirmed with a novel instrumentation for invasive 3D-EAI [2]. However, although the accuracy of contactless MCG has been previously assessed in a magnetically shielded room [11,19], the experience in an unshielded clinical environment was limited [20]. The present study confirms the results of the earlier study [20], but adds information about high repeatability (overall CV of 0.79%) and accuracy of the MCG method.
On the other hand, the results demonstrate also the limitation of present MCG technology, in that the range of precision of the MCG localization with first approximation models depends on the GOF of the model used and its correlation with the SSD. In fact, the localization precision is correlated with GOF which is limited by the SSD (GOF > 73% and SSD < 14 cm). It was also observed that the 3D localization error, at least in our unshielded laboratory, increased (was in the order of 12 mm) for sources deeper that 14 cm and could not be better than 3-4 mm even for shallow sources.
It must be noted however, that the most relevant component of the 3D error was due to the Z component of the magnetic field. A possible explanation (for higher uncertainty in depth localization) could be that typically the environmental electromagnetic noise of our unshielded laboratory is characterized by very stable X and Y components, but wide oscillation of the Z component.
On the contrary, accuracy evaluation shows that MCG is able to localize the reciprocal position of two different dipoles embedded in the same catheter with an overall error of only 0.2 ± 0.13 cm. Since this evaluation was independent from fluoroscopic imaging, it represents the real MCG accuracy. Interestingly it is noted that the use of fluoroscopy imaging as the gold-standard to assess the precision of MCG localization is somehow misleading. In fact, the x-ray divergence effect, although care was paid to minimize and correct it by using multiple radiopaque markers, induces estimation uncertainties higher than the intrinsic accuracy of MCG localization experimentally assessed in this study.
Overall the results of the present study provide experimental evidence that, even with present technological limitations and when performed in unshielded environment, precision and accuracy of MCG localization are adequate for clinically reliable 3D-EAI.
Limitation of the Study
The most important limitation of this study is linked to fluoroscopic-based measurements. Although planar lead markers were used to delete the x-ray divergence, manually-taken measurements are consistently operator-dependent.
Another limitation is that EMD model and semi-infinite half-space with homogeneous conductivity are approximations in respect of human reality, where the variable conductivity of tissues interposed between the heart and the sensors could impair the precision and accuracy of cardiac sources localization. On the other hand, this is only partially true. In fact, previous studies carried out in the Helsinki magnetically shielded room have shown very similar localization accuracy of the same amagnetic catheters in patients and in phantoms [10,19-22]. Furthermore, the intrinsic limitation of solution of the inverse problem based on a single-dipole model in semi-infinite space with homogeneous conductivity may be overcome with the implementation of more realistic models, nowadays available and applicable with acceptable computing time.
Conclusion
This phantom study confirms previous evidence that MCG-based inverse solution is highly repeatable and accurate in localizing the site of origin of dipolar sources, even when performed in an unshielded environment, and using a simple model based of the source (single dipole) and of the volume conductor (semi-infinite space with homogeneous conductivity).
Since contactless MCG source localization can be MERGED into 3D rendering of cardiac anatomy obtained with cardiac magnetic resonance or CT scan, MCG provides accurate non-invasive pre-interventional 3D-EAI of arrhythmogenic substrates, without radiation and with less patient's discomfort compared with BSPM and CT scan. Moreover, since MCG can also localize intracardiac catheters MCG 3D-EAI is foreseen as a novel method to combine in a single package pre-interventional and intraoperative arrhythmogenic source imaging to guide catheter ablation. However, further investments, engineering and software developments are still required to reach that target [18].
References
- Gaita F, Guerra PG, Battaglia A, et al. (2016) The dream of near-zero X-rays ablation comes true. Eur Heart J 37: 2749-2755.
- Bourier F, Reents T, Ammar-Busch S, et al. (2015) Sensor-based electromagnetic navigation (Mediguide®): how accurate is it? A phantom model study. J Cardiovasc Electrophysiol 26: 1140-1145.
- Sy RW, Thiagalingam A, Stiles MK (2012) Modern electrophysiology mapping techniques. Heart Lung Circ 21: 364-375.
- Carpeggiani C, Rossi G, Landi P, et al. (2015) Long-term outcome and medical radiation exposure in patients hospitalized for cardiovascular disease. Int J Cardiol 195: 30-36.
- Dubois R, Shah AJ, Hocini M, et al. (2015) Non-invasive cardiac mapping in clinical practice: Application to the ablation of cardiac arrhythmias. J Electrocardiol 48: 966-974.
- Baule G, Mcfee R (1963) Detection of the magnetic field of the heart. Am Heart J 66: 95-96.
- Tavarozzi I, Comani S, Del Gratta C, et al. (2002) Magnetocardiography: current status and perspectives. Part I: Physical principles and instrumentation. Ital Heart J 3: 75-85.
- Fenici R, Brisinda D (2006) Magnetocardiography provides non-invasive three-dimensional electroanatomical imaging of cardiac electrophysiology. Int J Cardiovasc Imaging 22: 595-597.
- Cohen D, Edelsack EA, Zimmerman JE (1970) Magnetocardiograms taken inside a shielded room with a superconducting point-contact magnetometer. Appl Phys Lett 16: 278-280.
- Weis A, Wynands R, Fenici R, et al. (2004) Dynamical MCG mapping with an atomic vapor magnetometer. Neurol Clin Neurophysiol 2004: 38.
- Fenici R, Pesola K, Makijarvi M, et al. (1998) Nonfluoroscopic localization of an amagnetic catheter in a realistic torso phantom by magnetocardiographic and body surface potential mapping. Pacing Clin Electrophysiol 21: 2485-2491.
- Lim HK, Kwon H, Chung N, et al. (2009) Usefulness of magnetocardiogram to detect unstable angina pectoris and non-ST elevation myocardial infarction. Am J Cardiol 103: 448-454.
- Kleiner R, Koelle D, Ludwig F, et al. (2004) Superconducting quantum interference devices: State of the art and applications. Proc IEEE 92: 1534-1548.
- Fenici R, Brisinda D, Meloni AM (2005) Clinical application of magnetocardiography. Expert Rev Mol Diagn 5: 291-313.
- Fenici R, Brisinda D, Nenonen J, et al. (2003) Noninvasive study of ventricular preexcitation using multichannel magnetocardiography. Pacing Clin Electrophysiol 26: 431-435.
- Fenici R, Melillo G (1991) Biomagnetically localizable multipurpose catheter and method for MCG guided intracardiac electrophysiology, biopsy and ablation of cardiac arrhythmias. Int J Card Imaging 7: 207-215.
- Earley MJ, Showkathali R, Alzetani M, et al. (2006) Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J 27: 1223-1229.
- Fenici R, Brisinda D (2007) Bridging noninvasive and interventional electroanatomical imaging: role of magnetocardiography. Journal of Electrocardiology 40: S47-S52.
- Pesola K, Nenonen J, Fenici R, et al. (1999) Bioelectromagnetic localization of a pacing catheter in the heart. Phys Med Biol 44: 2565-2578.
- Fenici R, Brisinda D, Nenonen J, et al. (2003) Phantom validation of multichannel magnetocardiography source localization. Pacing Clin Electrophysiol 26: 426-430.
- Fenici R, Pesola K, Korhonen P, et al. (1998) Magnetocardiographic pacemapping for nonfluoroscopic localization of intracardiac electrophysiology catheters. Pacing Clin Electrophysiol 21: 2492-2499.
- Fenici R, Nenonen J, Pesola K, et al. (1999) Nonfluoroscopic localization of an amagnetic stimulation catheter by multichannel magnetocardiography. Pacing Clin Electrophysiol 22: 1210-1220.
Corresponding Author
Prof. Riccardo Fenici, Biomagnetism and Clinical Physiology International Center, Catholic University of Sacred Heart, Largo Agostino Gemelli 8, 00168 Rome, Italy, Tel: +390630154138.
Copyright
© 2017 Lombardi G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.