The Impact of Enhanced Recovery Pathways in Head and Neck Surgery: A Systematic Review
Abstract
Background: Enhanced recovery after surgery (ERAS) pathways have been implemented across multiple surgical specialties and have been found to be successful in reducing post-operative complications and length of stay (LOS) in hospital. They have only more recently been adopted by Head and Neck surgery but there is now a sufficient body of evidence which would benefit from consolidation and review. The purpose of this review is to determine what impact ERAS pathways have on patient outcomes and post-operative recovery following Head and Neck surgery.
Methods: A literature search of Pubmed, CINAHL and Google Scholar was conducted. Results were limited to publication between 2013-2021 and those written in English. The search terms used were "enhanced recovery" and "head and neck surgery" or "ERAS" and "head and neck surgery" or "head and neck surgery" and "clinical pathway" or "head and neck surgery" and "fast track".
Results: The search yielded 17 papers for inclusion in the review. 16 of the studies were cohort studies (50% prospective and 50% retrospective) with sample sizes ranging from 31-445. Five studies observed a statistically significant reduction in length of stay (LOS) in intensive care for those in the ERAS cohort, with an average reduction of 3.42 days. Additionally, 73% of studies (n = 11) reported a statistically significant reduction in overall LOS for ERAS patients. Lower analgesic requirements as measured by morphine equivalent dosing (MED) were reported (17.5 mg ± 46 gmg ERAS vs. 82.7 ± 116 mg in the control (p =< 0.001) in combination with lower average pain scores (2.6 ± 1.8 ERAS vs. 3.6 ± 1.9 control (p =< 0.001)). Only one study identified a statistically significant reduction in post-operative complications (pulmonary) of 30% in the ERAS cohort vs. 63% in the control (p =< 0.001).
Conclusions: There is evidence to suggest that ERAS pathways can impact positively on post-operative recovery following Head and Neck surgery by reducing overall LOS, LOS in ITU and opioid requirements. However, current evidence is limited and does not give insight into long-term outcomes or the patient experience of ERAS.
Keywords
Enhanced recovery after surgery, ERAS, head and neck surgery
Introduction
Enhanced recovery after surgery (ERAS) is a multi-modal, multi-disciplinary approach aimed at facilitating patient recovery by mitigating the effects of the surgical stress response [1]. ERAS pathways are underpinned by the ERAS principles (Figure 1) and are designed to create consistency of care based on the best-available research evidence. ERAS has been adopted by a range of surgical specialties over the last 30 years and has been found to reduce post-operative complication rates and length of stay (LOS) in hospital [2]. However, the application of ERAS to Head and Neck surgery has only emerged more recently, with guidelines published by the ERAS society in 2017 [3] outlining optimal perioperative care in Head and Neck surgery. This review aims to consolidate and critique all relevant literature to gain an understanding of the impact of ERAS in Head and Neck surgery.
Methods
A computerised literature search of Pubmed, Google Scholar and CINAHL was conducted. The following key words/search terms were used to identify papers for review: "enhanced recovery" and "head and neck surgery" or "ERAS" and "head and neck surgery" or "head and neck surgery" and "clinical pathway" or "head and neck surgery" and "fast track".
Inclusion and exclusion
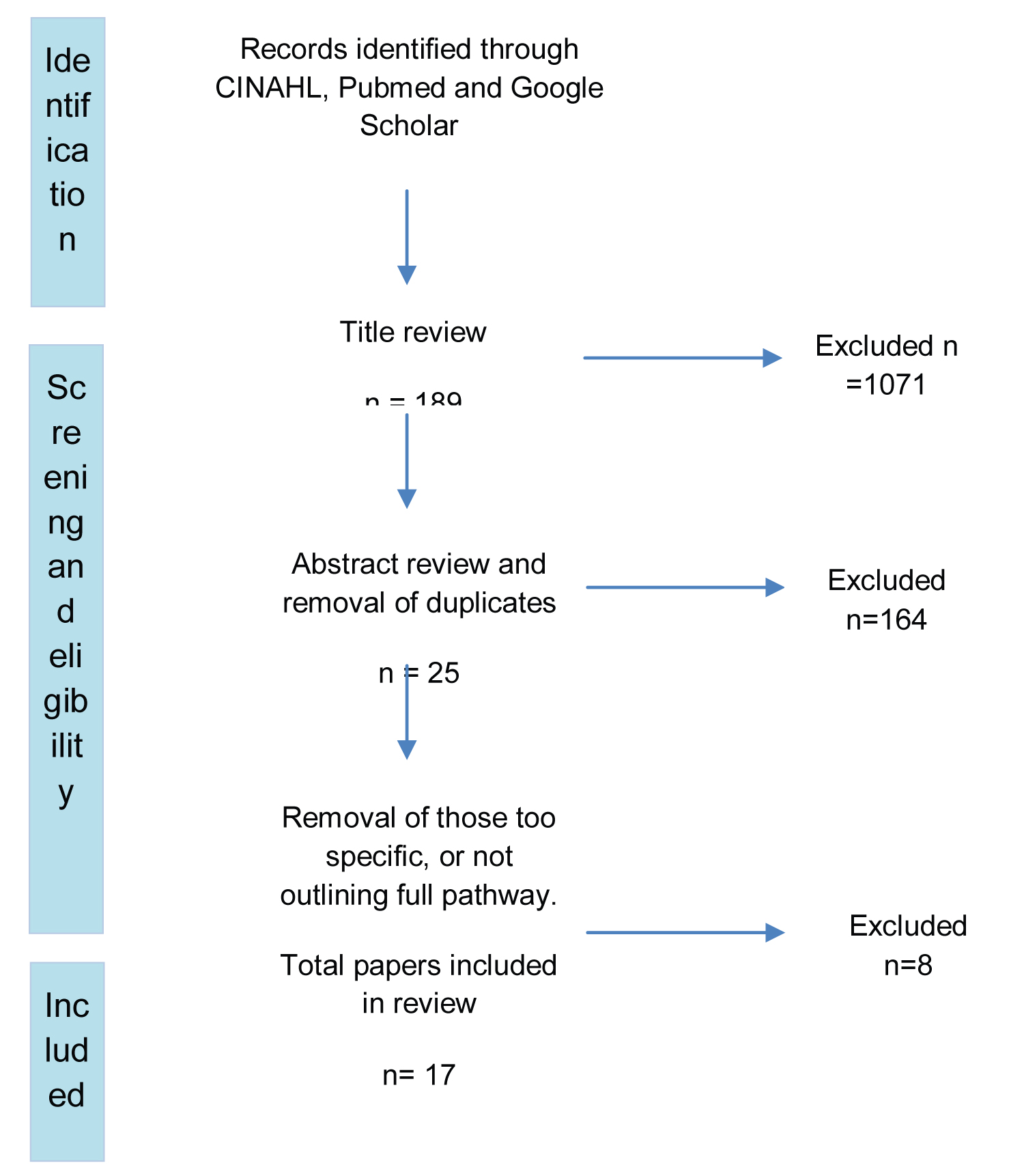
Papers written in English were eligible for inclusion. The timeframe for publication was set from 2013-2021 to ensure that the evidence is relevant to current clinical practice. It should be noted that a prior review was conducted by Bannister, et al. [4] who reviewed papers between 1994-2013, since which time more recent evidence has been published. Another review was also conducted by Watson, et al. [5]; however, the authors focused on the impact of early feeding only. A number of studies have implemented new clinical care pathways but these were not labelled as 'ERAS' pathways. Further review of each of these papers was conducted to ensure that there was a clear description of the components of the pathway and that these components aligned with the ERAS principles. Studies which did not include elements of ERAS were excluded. Removal of duplicates resulted in 17 papers for inclusion in this review. The inclusion/ exclusion process is outlined in the PRISMA flow diagram (Figure 2).
Critical appraisal methods and rating of evidence
To ensure a consistent and structured approach to the critique of each paper, the Critical Appraisal Skills Programme (CASP) tool for cohort studies was applied. Each paper was also ranked from 1-5 according to a quality of evidence rating scheme (see Table 1).
Results
Hospital length of stay (LOS) and LOS in intensive care (ITU)
Five studies observed a reduction in LOS in ITU for those on ERAS pathways in comparison to those on traditional care pathways; 2.1 days vs. 3.4 days [6], 1.2 days vs. 9.5 days [7], 2.1 days vs. 3.8 days [8], 0.2 days vs. 5.0 days [9] and 1 day vs. 2 days [10]. All reductions in LOS on ITU were deemed to be statistically significant. Across these five studies there was an average reduction of LOS in ITU of 3.4 days for those on ERAS pathways. Jandali, et al. [11] observed no difference in ITU LOS between the ERAS and control cohorts and Kiong, et al. [12] and Morse, et al. [13] did not report on ITU LOS; however, the authors did record fewer routine post-op admissions to ITU for those on ERAS pathways.
Total LOS in hospital was a primary outcome measure in all studies. A statistically significant reduction in hospital LOS was observed in the ERAS cohorts in 73% of the studies (n = 13). Although improvement by way of reduction in LOS was seen in ERAS cohorts, it varied considerably between centers, ranging from 7-31 days. This highlights that, despite each study following a pathway which is guided by the ERAS ethos, clinical practice differs widely between centers (as shown by a wide range in hospital LOS).
Post-operative pain scores and analgesic requirements
Kiong, et al. [12] identified that the ERAS group spent a shorter time on the post-anaesthetic care unit (PACU) with lower pain scores immediately post-op; however, pain scores from 24-72 hours post-op were comparable between the groups. Despite this, the ERAS group had lower analgesic requirements as measured by mean morphine equivalent dose (MED) (138.8 mg ± 181.5 mg vs. 207.9 mg ± 205.5 mg (p =< 0.001)), indicating that fewer opioids were required to achieve the same level of pain relief. The requirement of patient-controlled analgesia (PCA) was also lower in the ERAS group (31% vs. 74% (p =< 0.001)) and fewer patients required opioids on discharge in comparison to the control (64.5% vs. 81.5% (p =< 0.001)). These findings were echoed by Jandali, et al. [11] who described the ERAS cohort as having a significantly lower MED (17.5 mg ± 46 gmg vs. 82.7 ± 116 mg (p =< 0.001), with lower average pain scores (2.6 ± 1.8 vs. 3.6 ± 1.9 (p =< 0.001)), fewer ERAS patients requiring a PCA (6.5% vs. 18.3% (p =< 0.028)) and the proportion of ERAS patients discharged home with opioids was also significantly lower (21.7% vs. 90.3% (p =< 0.001)). However, in this study a higher proportion of patients in the control group were taking opioids pre-operatively, so this may have impacted on their opioid requirements, both immediately post-operatively and on discharge. The findings of Clark, et al. [10] support those of Kiong, et al. [12] and Jandali, et al. [11] in demonstrating lower post-operative peak pain scores (4.6 ± 3.6 vs. 6.5 ± 3.5 (p = 0.004)) and lower morphine milligram equivalents in the ERAS group in comparison to the non-ERAS cohort (6.0 ± 9.8 vs. 10.3 ± 10.8 (p = 0.010)). Hinther, et al. [14] compared patients on a multi-modal analgesia (MMA) protocol with those on a 'pre-MMA' pathway. Average daily opioid consumption was lower in the MMA group (29.7 mg vs. 43.3 mg (p = 0.04)) and pain was managed more effectively for the first 6 days post-operatively using an MMA approach. From days 7-10 post-operatively, however, the pre-MMA group reported better pain control which the authors attributed to a change in prescribing in the MMA group from regular intervals to 'as required' on Day 3. They comment that this was perhaps too early and have since changed their pathway to 'as required' MMA from Day 6. Patients in the MMA group were mobilized on average 1.2 days earlier than those in the pre-MMA cohort and usage of the MMA protocol did not have an impact on overall LOS nor complication rates.
Post-operative complication rates, rates of return to theatre and readmission to hospital
Following implementation of ERAS pathways, Bertelson, et al. [6], Won, et al. [7], Kiong, et al. [12], Bater, et al. [15] and Yetzer, et al. [16] reported no difference in complication rates (e.g. pneumonia, delirium, wound infections and flap complications, including flap complications requiring return to theatre) in comparison to patients on 'traditional' or historic pathways. Bertelson, et al. [6] recorded 30-day readmission to hospital rates of 6.4% ERAS vs. 13.1% (p = 0.828) of the control cohort, Morse, et al. [13], Bater, et al. [15], and Dautremont, et al. [17] also reported no difference in complication rates, and their findings were not statistically significant. Comparatively, Dort, et al. [18] demonstrated a statistically significant reduction in post-operative pneumonia and delirium and Yeung, et al. [19] identified a significant reduction in pulmonary complications (30% in ERAS cohort vs. 63% in the control (p = 0.0001)) which were attributed to early extubation and avoidance of prolonged mechanical ventilation.
Post-operative progression and ERAS goals
Airway management: Moreno, et al. [9] achieved a reduction in the number of tracheostomies performed (75.7% vs. 12.2% (p = 0.04)), without an increase in post-operative complications nor increase in readmission rates to ITU. Yeung, et al. [19] reported earlier decannulation in their ERAS cohort (8.2 ± 3.1 vs. 13.8 ± 9.4 days (p =< 0.001)) and reduced time spent on mechanical ventilation (25.0 ± 14.6 hours vs. 36.2 ± 22.7 hours (p = 0.05)), in conjunction with a reduction in pulmonary complications, as outlined above. These findings are also reflected in the studies by Dautremont, et al. [17] and Dort, et al. [18] who performed decannulation on average 5.4 days earlier for those on the ERAS pathway (p = 0.001), with no adverse effects to patient outcomes.
Mobilisation: Timing to first mobilisation post-operatively was reported in four studies. Jandali, et al. [11] described a reduction in time to first mobilisation of 1.4 ± 1.3 days in the ERAS group vs. 2.0 ± 1.6 days in the control group. Bater, et al. [15] also saw a reduction from 3 days to first mobilisation in the 'traditional' pathway group vs. 1 day to mobilisation in the ERAS group (p =< 0.001). Won, et al. [7] demonstrated a notable reduction; however, timing to first mobilisation occurred much later in the patient pathways in their study for both the ERAS and the control group (23.78 ± 20.25 days vs. 6.65 ± 3.27 days (p = 0.006)) in comparison to the other two studies. The study by Twomey, et al. [20] demonstrated that patients who did not mobilise within 24 hours post-operatively were at higher risk of both minor (OR = 1.76, 95% CI, p = 0.049) and major complications (OR = 1.76, 95% CI, p = 0.005). Mobilisation after 48 hours was associated with an increased incidence of pneumonia of 13% and increased LOS by 4 days (p = 0.001). The main predictors of delayed mobilisation in this study were prolonged ITU stay and tracheostomy.
Enteral and oral nutrition: Won, et al. [7] commenced ERAS patients on enteral feeding on post-operative day (POD) one, progressing to sips of water orally on POD five whilst continuing enteral feed, and starting an oral diet on POD seven. The authors found that there was a significant correlation between the start time of both oral feeding (p =< 0.001) and normal mobilisation (p = 0.003) with hospital and ITU LOS. Sharkh, et al. [8] identified that in patients with a prolonged LOS, 73% of discharges were delayed as a result of a failed swallow test, consequently requiring percutaneous endoscopic gastrostomy (PEG) placement. The authors state that progression to oral intake was a key factor in preventing delayed discharge in their study, with 77% of patients on the new clinical care pathway successfully commencing oral intake on POD five.
Compliance to ERAS pathways
Coyle, et al. [21] looked at the compliance to each element of the ERAS pathway which had been implemented. Just over half (55%) of patients received education pre-operatively about ERAS and the ERAS pathway, 97% of patients were admitted on the same day of surgery and 74% took carbohydrate drinks pre-operatively. Intra-operatively, long-acting sedatives were avoided in 97% of patients, 10% received goal directed fluid therapy and 100% received hypothermia prevention through active warming. Post-operatively, no patients were fluid overloaded, 90% received nutrition either enterally or orally within 24 hours of surgery and 7% of patients mobilised within 24 hours. Despite there being low compliance (< 55%) in many areas of the pathway in the study, there was a reduction in overall hospital LOS of 3.5 days in comparison to patients at the centre treated pre-ERAS implementation. However, the results of this paper should be interpreted with some caution as data collection was performed as a quality improvement project with no control cohort for comparison. It is therefore not possible to determine whether the reduction in LOS was seen as a result of the ERAS pathway or extraneous variables. Dort, et al. [18] classified the pathway goals as key performance indicators (KPIs) and looked at compliance to each over time. The authors compared pre-ERAS pathway implementation with 'early' stages of pathway implementation and 'current'. They were able to demonstrate a reduction in total LOS (21.6 days vs. 14 days (p = 0.001)) and earlier decannulation (13.8 days vs. 8.6 days (p = 0.001)) whilst also demonstrating improvement to pathway compliance over time, achieving ~60-80% compliance for the majority of KPIs.
Discussion
The reviewed research indicates that ERAS pathways in Head and Neck surgery can be successful in reducing both overall LOS [7-9,11,12,15-19,21] and LOS in ITU [6-10]. There is convincing evidence that an opioid sparing/multi-modal analgesic approach is effective, as demonstrated by lower opioid requirements with no increase in pain scores in the ERAS cohorts [10-12,20]. There is limited evidence demonstrating a reduction in post-operative complications [12,18,19]; one study demonstrated a higher risk of both minor and major complications in patients who aren't mobilised within 24 hours of surgery [20].
Sixteen of the studies included in this review are retrospective or prospective cohort studies. Although randomised-controlled trials (RCTs) are typically considered the gold standard in research design, they are often not practical or lack suitability due to ethical constraints. When looking to assess the impact of a new clinical pathway, cohort studies are appropriate as they enable assessment of associations between multiple exposures and outcomes. Cohort studies also have broader and less restrictive inclusion/ exclusion criteria which can generate results that are more generalisable to the wider population [22]. However, due to a lack of randomisation, causal effects of an intervention cannot be established when using cohort study methods. Retrospective data collection is typically more straightforward than prospective and allows researchers to answer current questions with pre-existing or historic data; however, by the nature of this, the researcher is restricted by what was deemed important at the time of data collection.
Prospective cohort studies are ranked higher than retrospective methods in terms of the hierarchy or research evidence, and each study included in this review has been ranked according to a quality of evidence rating scheme (see table). However, some epidemiologists argue [22,23] that all follow-up data is prospective as follow-up data collection always goes forward in time, and, therefore, one should not be considered superior over the other by default, without taking a closer look at the research methodology. A historic cohort or 'pre-pathway' group was used as a control group in 14 of the 17 studies. To correct for potential confounders, three authors used matched control groups. Bertelsen, et al. [6] matched the ERAS and historic cohorts for sex, tumour histology and comorbidities, whereas Kiong, et al. [12] matched for age (within a five-year range), surgery type and type of free flap. In a bid to reduce the risk of selection bias, Morse, et al. [13] performed matched analysis that was blinded to all outcomes and Clark, et al. [10] reported that data entry for both the ERAS and the control cohort was double-blinded. Bater, et al. [15] were the only authors to use propensity score matching as a way of mimicking the effect of randomisation, thus reducing the risk of treatment assignment bias.
The weakest piece of evidence identified in this review is the study by Coyle, et al. [21]; as it was a service improvement project with no control population for comparison and thus the results should be interpreted with caution. However, the paper gives good insight into the implementation of an ERAS pathway and outlines compliance to each area of the pathway, which is useful to readers who are looking to identify potential barriers to ERAS pathway implementation. Another factor that limits generalisability of the results of these studies is that they are all single-centre studies; often single centres are only able to recruit small sample sizes. Sample sizes for the studies reviewed ranged from 31 to 445 participants (see summary table).
A crucial consideration of this review was ensuring that only pathways which aligned with the ERAS principles were included. Not all studies labelled their pathways as ERAS pathways, instead opting for clinical care pathways. It was therefore important that pathway components were scrutinised (see Table 2) to ensure only appropriate studies were included. There is a level of subjectivity of interpretation of ERAS and the ERAS guidelines [3], which, when combined with local policy, can result in pathways which differ, and this is therefore identified as a limitation of this review. In addition, tumour types and the extent of resection required also differ widely. Surgery involving reconstruction with a microvascular free flap is indicative of tumour resection which has resulted in a major defect; however, the extent of the resection and the size of the defect can vary significantly between patients. As a result, post-operative recovery will look different for each patient, even for those who have all undergone free flap surgery. This variation in the extent of surgery may in turn impact the level at which an ERAS pathway can be applied to improve post-operative recovery. ERAS pathways are made up of multiple components which means that it is important to take pathway compliance into consideration. For those patients in ERAS cohorts, it is unlikely that all POD benchmarks will be achieved (i.e., 100% compliance) as ERAS pathways do not negate the usage of clinical judgement; for example, the pathway may state to aim for decannulation on POD 3; however this will not be performed if the patient has extensive swelling and is not an appropriate candidate for decannulation. Compliance to the pathway is not reported on in 15 of the 17 studies and it could be argued that this is essential in order to understand the 'dose-response' relationship between the achievement of ERAS benchmarks (such as early decannulation, early oral intake etc.) and any observed reduction in overall LOS, ITU LOS and the development of post-operative complications.
The aim of this review was to identify evidence of ERAS pathways in Head and Neck surgery; however, 15 of the 17 studies focused solely on free flap surgery in Head and Neck. The principles of ERAS are applicable to all surgical specialties and to procedures of ranging complexity (from minor to major), but this review highlights a gap in the literature. There is currently no evidence to either support or refute ERAS in Head and Neck surgery in procedures other than those involving free flap reconstructions. In addition to improving clinical outcomes, the purpose of ERAS is to also improve patient satisfaction; however, this is not addressed by any of the studies included in this review. It is hypothesised that by successfully implementing ERAS (e.g., by reducing complication rates and shortening LOS in hospital) that patient-experience will also improve as a result, but these studies provide no evidence to substantiate this. Usage of patient reported outcome measures (PROMs) would enable better understanding of the patient facing benefits of ERAS and could be measured concurrently with the commonly recorded clinical outcome measures. Nunns, et al. [24] conducted a review into PROMS and ERAS across multiple surgical specialties and highlighted that not only is research into patient-experience lacking, but long-term outcomes are almost entirely unmeasured. Qualitative research into the impact of ERAS in Head and Neck surgery is undoubtedly required in order to better understand both the patient experience and that of the clinicians involved in ERAS pathway implementation.
Conclusions
There is evidence to suggest that ERAS pathway implementation in Head and Neck surgery can be successful in reducing overall LOS, LOS in ITU and opioid requirements. However, the evidence is limited predominantly to free flap surgery only and does not give insight into the long-term outcomes nor the patient experience of ERAS.
References
- Varadhan KK, Lobo DN, Ljungqvist O (2010) Enhanced recovery after surgery: The future of improving surgical care. Critical Care Clinics 26: 527-547.
- Greenshields N, Mythen M (2020) Enhanced recovery after surgery. Current Anesthesiology Reports 10: 49-55.
- Dort JC, Farwell DG, Findlay M, et al. (2017) Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction. JAMA Otolaryngology Head Neck Surg 143: 292-303.
- Bannister M, Ah-See KW (2015) Enhanced recovery programmes in head and neck surgery: Systematic review. The J Laryngol Otol 129: 416-420.
- Watson LJ, Ewers C (2020) Enhanced recovery after head and neck cancer surgery: A review of current literature. Curr Opin Otolaryngol Head Neck Surg 28: 161-164.
- Bertelsen C, Hur K, Nurimba M, et al. (2020) Enhanced recovery after surgery-based perioperative protocol for head and neck free flap reconstruction. OTO Open 4: 2473974X20931037.
- Won HR, An JY, Lee JJ, et al. (2019) The effectiveness of an enhanced recovery after surgery protocol in head and neck cancer surgery with free-flap reconstruction. Ann Surg Treat Res 97: 239.
- Abo Sharkh H, Madathil S, Al-Ghamdi O, et al. (2019) A comprehensive clinical care pathway for microvascular maxillofacial reconstructive surgery. J Oral Maxillofac Surg 77: 2347-2354.
- Moreno MA, Bonilla-Velez J (2019) Clinical pathway for abbreviated postoperative hospital stay in free tissue transfer to the head and neck: Impact in resource utilization and surgical outcomes. Head Neck 41: 982-992.
- Clark BS, Swanson M, Widjaja W, et al. (2021) ERAS for head and neck tissue transfer reduces opioid usage, peak pain scores, and blood utilization. Laryngoscope 131: E792-E799.
- Jandali DB, Vaughan D, Eggerstedt M, et al. (2020) Enhanced recovery after surgery in head and neck surgery: Reduced opioid use and length of stay. Laryngoscope 130: 1227-1232.
- Kiong KL, Vu CN, Yao CMKL, et al. (2021) Enhanced recovery after surgery (ERAS) in head and neck oncologic surgery: A case-matched analysis of perioperative and pain outcomes. Ann Surg Oncol 28: 867-876.
- Morse E, Henderson C, Carafeno T, et al. (2019) A clinical care pathway to reduce ICU usage in head and neck microvascular reconstruction. Otolaryngology Head Neck Surg 160: 783-790.
- Hinther A, Nakoneshny SC, Chandarana SP, et al. (2021) Efficacy of multimodal analgesia for postoperative pain management in head and neck cancer patients. Cancers (Basel) 13: 1266.
- Bater M, King W, Teare J, et al. (2017) Enhanced recovery in patients having free tissue transfer for head and neck cancer: Does it make a difference? Br J Oral Maxillofac Surg 55: 1024-1029.
- Yetzer JG, Pirgousis P, Li Z, et al. (2017) Clinical pathway implementation improves efficiency of care in a maxillofacial head and neck surgery unit. J Oral Maxillofac Surg 75: 190-196.
- Dautremont JF, Rudmik LR, Yeung J, et al. (2013) Cost-effectiveness analysis of a postoperative clinical care pathway in head and neck surgery with microvascular reconstruction. J Otolaryngol Head Neck Surg 42: 59.
- Dort JC, Sauro KM, Chandarana S, et al. (2020) The impact of a quality management program for patients undergoing head and neck resection with free-flap reconstruction: Longitudinal study examining sustainability. J Otolaryngol Head Neck Surg 49: 42.
- Yeung JK, Dautremont JF, Harrop AR, et al. (2014) Reduction of pulmonary complications and hospital length of stay with a clinical care pathway after head and neck reconstruction. Plast Reconstr Surg 133: 1477-1484.
- Twomey R, Matthews TW, Nakoneshny S, et al. (2021) Impact of early mobilization on recovery after major head and neck surgery with free flap reconstruction. Cancers (Basel) 13: 2852.
- Coyle MJ, Main B, Hughes C, et al. (2016) Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin Otolaryngology 41: 118-126.
- Euser AM, Zoccali C, Jager KJ, et al. (2009) Cohort studies: Prospective versus retrospective. Nephron Clinical Practice 113: c214-c217.
- Vandenbroucke JP (2008) Observational research, randomised trials, and two views of medical science. PLoS Medicine 5: e67.
- Nunns M, John JB, McGrath JS, et al. (2020) Evaluating enhanced recovery after surgery: Time to cover new ground and discover the missing patient voice. Perioper Med (Lond) 9: 27.
Corresponding Author
Ursula Mackie-Savage, University College London Hospital, Ground Floor Central, 250 Euston Road, London, NW1 2PG, UK.
Copyright
© 2022 Savage UM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.