Impact of Acute Kidney Injury in Hospitalizations with Non-Variceal Upper Gastrointestinal Bleeding
Abstract
Background: Acute Kidney Injury (AKI) is a commonly occurring comorbidity in hospitalized patients. Our objective was to identify the role of AKI in relation to the morbidity and mortality in Non-Variceal Upper Gastrointestinal Bleeding (NVUGIB) patients.
Study: Nationwide Inpatient Sample (NIS) database was used from the year 2000 to 2014 and queried for patients with NVUGIB. Patients were further stratified with and without the presence of concomitant AKI. Weighed multivariate regression analyses were performed to determine the final conclusions of the study.
Results: Total of 4,889,410 patients with NVUGIB were identified during the study period. AKI was a comorbid condition in 6.37% of all patients. Incidence of AKI in NVUGIB patients has increased over five-fold over the study period. After adjusting for confounders through multivariate regression model, patients in the AKI group had higher odds of mortality (OR 2.84, p < 0.001). Additionally, patients with AKI were more likely to receive blood transfusion (OR 1.30, p < 0.001), had longer length of stay (+ 1.73 days, p < 0.001), had higher hospital charges (+ $16,221, p < 0.001), and had higher disposition to intermediate care facility (OR 1.84, p < 0.001).
Conclusions: Incidence of AKI in hospitalized NVUGIB patients has increased dramatically. Interestingly, AKI is also associated with higher morbidity and mortality in NVUGIB patients. Consequently, AKI should be used as a marker for disease severity in hospitalized NVUGIB patients.
Keywords
Acute Kidney Injury, Non-Variceal Upper Gastrointestinal Bleeding, Nationwide Inpatient Sample
Abbreviations
AKI: Acute Kidney Injury; GI: Gastrointestinal; NVUGIB: Non-Variceal Upper Gastrointestinal Bleed; NIS: Nationwide Inpatient Sample; OR: Odds Ratio; aOR: Adjusted Odds Ratio; VUGIB: Variceal upper gastrointestinal bleeding; CKD: Chronic kidney disease; ESRD: End-stage renal disease; HCUP: Healthcare Cost and Utilization Project; AHRQ: Agency for Healthcare Research and Quality; LOS: Length of stay
Introduction
Upper gastrointestinal bleeding (UGIB) is a common gastrointestinal emergency which carries a mortality rate of 5-14% [1]. The causes of UGIB have been broadly classified into variceal upper gastrointestinal bleeding (VUGIB) due to esophageal and gastric varices, and non-variceal upper gastrointestinal bleeding (NVUGIB) due to peptic ulcer disease, erosive gastroduodenitis, reflux esophagitis, etc. Due to advances in endoscopic treatments and the advent of proton pump inhibitors, mortality attributed to NVUGIB has decreased significantly over the past two decades [2]. However, a recent meta-analysis concluded that patients admitted with all-cause UGIB with three or more co-morbid conditions had a four-fold increase in mortality than those with one or two co-morbid conditions [3]. Specifically, conditions such as advanced age, chronic kidney disease, heart diseases, and malignancies have been implicated [3,4].
Acute Kidney Injury (AKI) appears in 5-7% of all hospitalized patients and up to 50% in intensive care patients as a co-morbid condition [5-7]. The acronym "RIFLE" classifies acute renal dysfunction based on severity and outcomes. According to the RIFLE criteria, AKI is defined as an increase of serum creatinine 1.5-fold from baseline or decrease in glomerular filtration rate by greater than 25% [8]. The incidence of AKI in patients admitted with NVUGIB has been poorly defined in the literature. Further, presence of AKI as a co-morbid condition is not usually used in the common risk-stratification systems to predict outcomes in hospitalized patients with UGIB [9-11]. Interestingly, chronic kidney disease (CKD) and end-stage renal disease (ESRD) have a known association with increased mortality and longer hospital stays in patients with all-cause UGIB [12], and have been incorporated in the Rockall scoring system [10]. In regards to AKI, small single centered studies have previously shown worsening of patient outcomes in intensive care unit patients [13] and elderly patients [14] admitted for all cause UGIB. The role and pathophysiology of AKI in VUGIB bleeding has also been previously defined [15,16]. However, there is no current large-or small-scale population-based study evaluating the independent role of AKI as a prognostic marker among hospitalized NVUGIB patients. In our study, we hypothesized that AKI, independent of CKD, adversely affects the outcomes in patients admitted with NVUGIB, such as mortality, length of stay, hospital charges, and disposition status.
Materials and Methods
Design and data source
This is a retrospective, longitudinal study of patients who presented to acute care hospitals in the United States with a principal diagnosis of NVUGIB. We extracted our study cohort from the National Inpatient Sample (NIS) of the Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality (AHRQ) [17]. NIS is the largest and most widely used source of inpatient data in the United States. The database is a stratified sample of approximately 20% of all hospital discharges in the United States, excluding rehabilitation hospitals and long-term care facilities. HCUP discharge weights were used to obtain national estimates [18]. The discharge records contain primary diagnosis and secondary diagnosis fields as well as patient, hospital, and outcome related variables. The sample averages 35 million weighed discharges every year, representing 95% of the US population [18]. The validity of NIS in studying UGIB has been previously shown [19,20]. We used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to identify diagnoses (Supplementary Data).
Study population
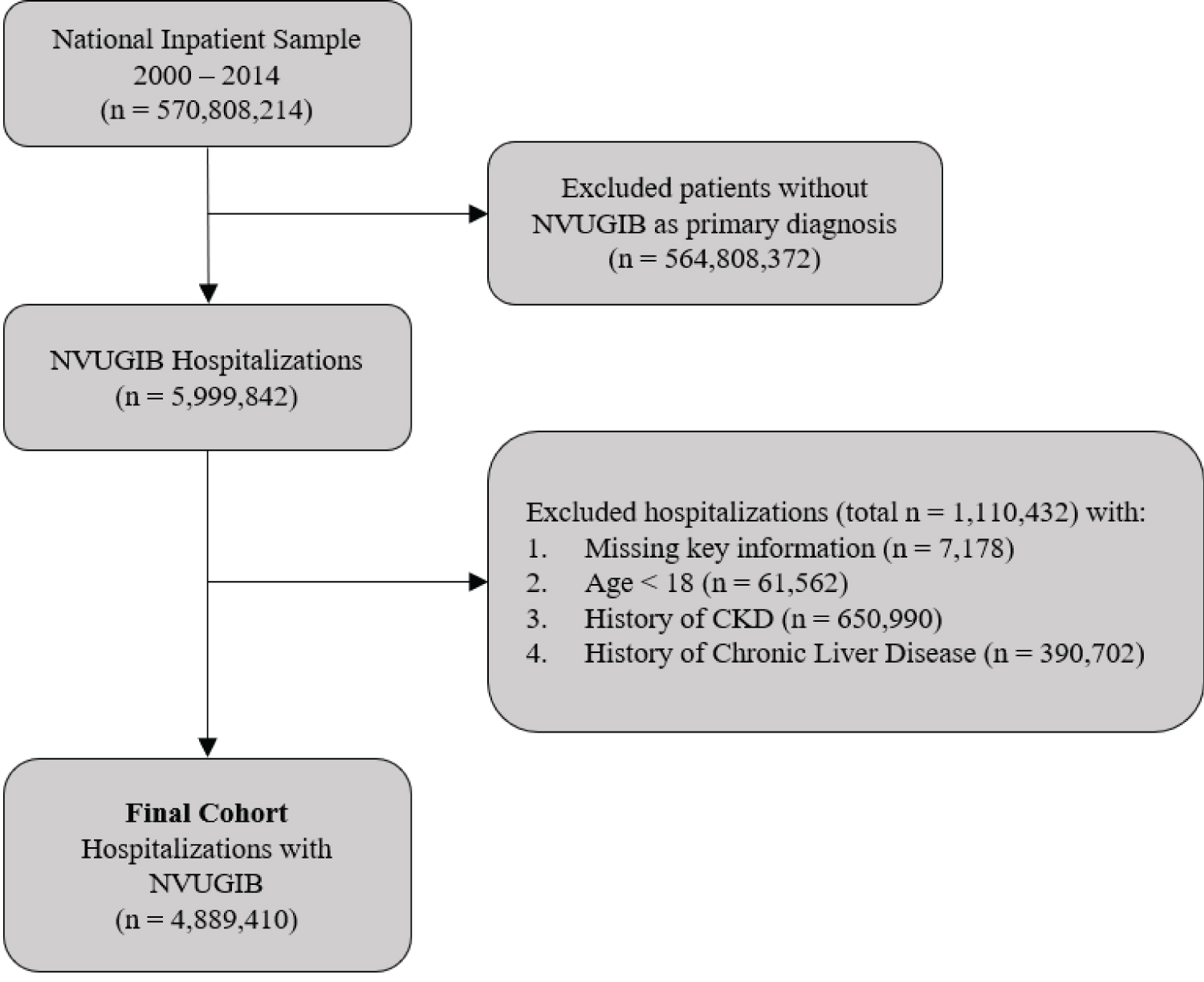
We chose to evaluate the time period from 2000 to 2014 based on availability of complete data and adequate sample size for modeling time trends. For data cleansing purposes, we eliminated discharge records with missing information for age, sex, year, mortality, or primary diagnosis according to a scheme suggested by the AHRQ [21]. Inclusion criteria included all adults age ≥ 18-years, with a primary diagnosis of NVUGIB who required inpatient endoscopy procedure. Exclusion criteria included patients with history of CKD, ESRD or chronic liver disease. Patients with history of CKD or ESRD were excluded as they are known risk factors for developing AKI and hence would act as a confounding factor. Chronic liver disease patients were excluded to remove patients with possible confounding of VUGIB. Because the data was unidentified and publicly available, institutional board review approval was not required.
Definition of variables
The NIS carries demographic variables that include age, gender, race, household income per patient zip-code, and hospitalization-related variables of insurance, bed-size, region, and teaching status and location. We also extracted information regarding various comorbid conditions and procedures. We derived information regarding length of stay (LOS), hospitalization charges, disposition, and in-hospital mortality. Patient's comorbidities were measured by Deyo adaptation of the Charlson Comorbidity Index for administrative data [22]. Hospitalization charges were calculated, after being adjusted for annual inflation specific for healthcare (Bureau of Labor Statistics: http:// data.bls.gov/), with the reference year 2014.
Outcomes
The primary outcome was the independent association of AKI with in-hospital mortality of NVUGIB patients. The secondary outcomes were the proportion of admissions in which blood transfusion services were required, length of stay, hospital charges and disposition at the end of hospitalization. Finally, the annualized AKI incidence trend in NVUGIB-related hospitalizations and its associated mortality were also examined.
Statistical analyses
We compared the baseline characteristics of NVUGIB hospitalized adults in 2 groups: With and without AKI. We utilized the chi-squared test for categorical variables, Student t test for normally distributed continuous variables, and Wilcoxon rank-sum test for non-normally distributed continuous variables. We performed a survey regression analysis to explore potential reasons for temporal trends and patient outcomes attributable to AKI by fitting a series of sequential models. In the unadjusted model we included only calendar year as the predictor and AKI as the outcome. Additional patient level demographic covariates (age, sex, race, income, insurance type) and hospital characteristics (bed size, geographical region, location, teaching status) were added in the second model to determine the degree to which they explained temporal trends. Finally, the significant covariates from models 1 and 2 along with concurrent acute/chronic comorbidities (acute myocardial infarction, atrial fibrillation, hypertension, diabetes, coagulation disorders, gastrointestinal malignant neoplasm's, HIV infection, sepsis) and procedures (ventilator use, total parenteral nutrition) were included in a third and final model. Logistic and Linear regression modelling with aforementioned covariates were used for binary variables (mortality, blood transfusion, disposition status) and continuous variables (length of stay, hospital charges), respectively.
Analyses were performed by using Stata, version 13.0 (Stata Corp, College Station, TX). NIS is based on a complex sampling design that includes stratification, clustering, and weighing. This software facilitates analysis to produce nationally representative unbiased results, variance estimates, and p values.
Results
Proportion and Trends of NVUGIB hospitalizations complicated by AKI
A total of 4,889,410 weighed discharges with NVUGIB as the primary diagnosis were included in the analysis (Figure 1) over the 15-year study period. Overall, the mean age of the study population was 68.5 years with 50.46% of the patients being women, and 73.88% of the patients being Caucasian. The mean LOS was 4.4 days and the mean hospital charges were $29,677.
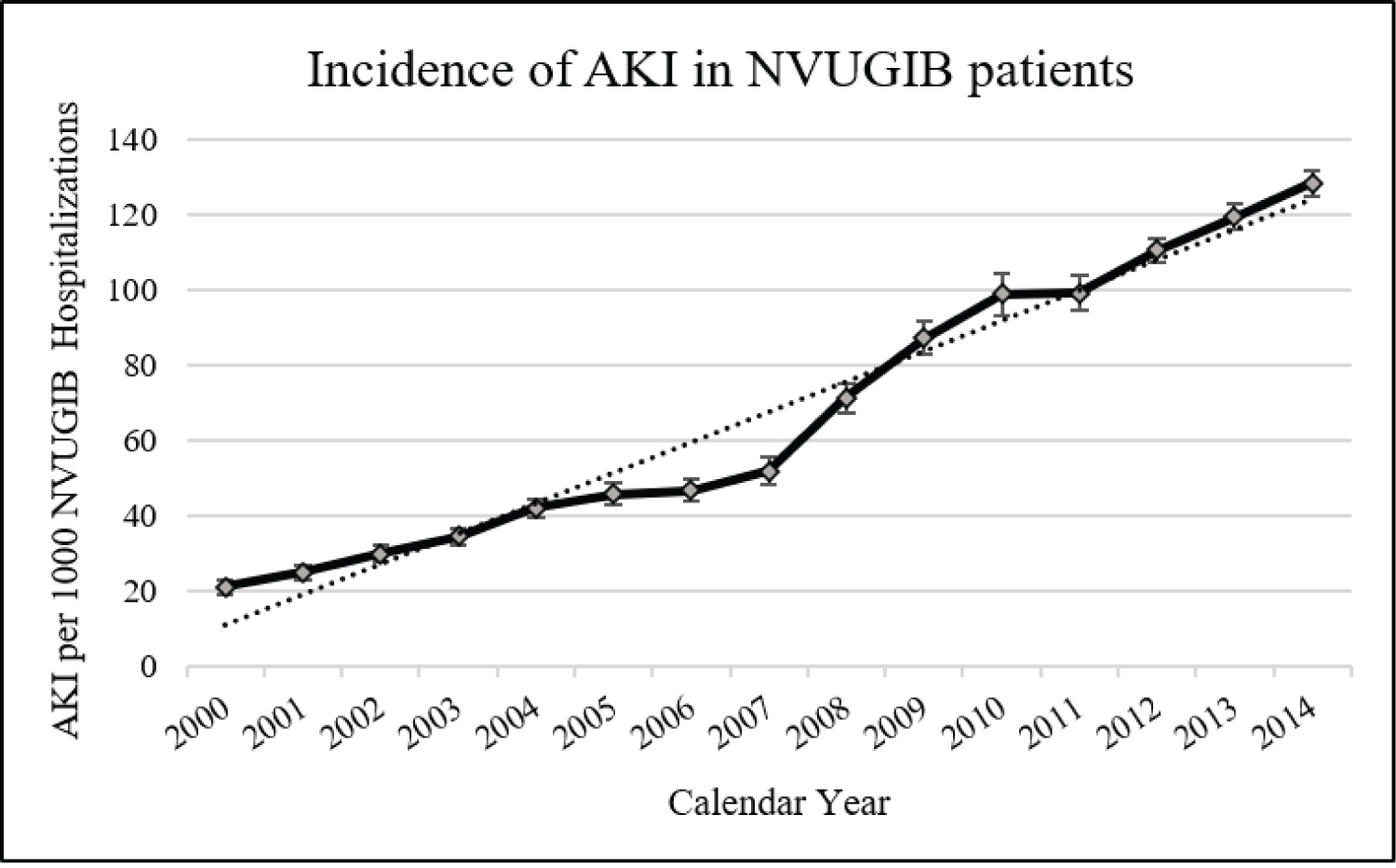
Overall, the incidence of AKI was 63.7 for every 1,000 NVUGIB hospitalizations (n = 311,455). As shown in Figure 2, the incidence of AKI in NVUGIB patients has steadily increased from 21 to 128 for every 1,000 hospitalizations from 2000 to 2014 (p-trend < 0.001), representing 5.74-fold increase in incidence in our regression model, after adjusting for patient demographics, co-morbidities, and hospital characteristics. In unadjusted analysis, for every 1-year increase, there was an associated increase of 15.5% in the risk of developing AKI (unadjusted OR 1.155, p-trend < 0.001) (Table 1). When adjusted for variations in patient characteristics, co-morbidities, and hospital characteristics, the increase in risk of developing AKI in NVUGIB patients remained unchanged at 15.4% each year (adjusted Odds Ratio or aOR 1.154, p-trend < 0.001).
Characteristics of AKI versus non-AKI patients
Demographic differences between NVUGIB patients with and without AKI are represented in Table 2. NVUGIB patients with AKI were significantly older (73.2-years vs. 68.2-years, p < 0.001), were more likely to be a male (54.15% vs. 49.23%, p < 0.001) (Table 2), were represented by lowest quartile of income (27.65% vs. 24.06%, p < 0.001), were more likely to have Medicaid as their insurance (76.24% vs. 65.57%, p < 0.001) and less likely to have private insurance (14.13% vs. 21.89%, p < 0.001). With regards to the hospital characteristics, there were modest hospital bed size differences between the two groups and no differences in the geographical hospital region (Table 2). Further, NVUGIB patients with AKI were much more likely to present to a teaching hospital (48.63% vs. 38.81%, p < 0.001) and in an urban location (89.55% vs. 82.22%, p < 0.001). Additionally, NVUGIB patients had increased rate of disposition to short term facility (43.18% vs. 26.74%, p < 0.001). There were also higher rates of individual co-morbid conditions such as diabetes, hypertension, HIV infection, chronic liver disease, sepsis, myocardial infarction and ventilator use in the AKI cohort of NVUGIB patients (individually described in Table 3) and had an overall higher comorbidity burden as measured by Charlson Comorbidity Index (Table 3).
Significant associations of AKI
Table 4 determines the associations of comorbidities and procedures with AKI, in order of strongest association. The strongest predictors for the presence of AKI were associated diagnosis of sepsis (aOR 6.91, p < 0.001), ventilator use (aOR 6.05, p < 0.001), total parenteral nutrition use (aOR 3.85, p < 0.001) and acute myocardial infarction (aOR 3.17, p < 0.001). Additionally, NVUGIB patients with AKI had a modest association with diabetes (aOR 1.21, p < 0.001) and a decreased association with hypertension (aOR 0.91, p < 0.001).
Association of AKI on patient outcomes
Table 5 shows the association of AKI with patient outcomes such as mortality, requirement for blood transfusion, length of stay and cost of hospitalization. The overall unadjusted mortality rate in NVUGIB hospitalizations with AKI was 10.34% compared to 2.13% in patients without AKI. After adjustments for significant patient demographics, co-morbidities and hospital characteristics, the adjusted mortality was 2.84-fold higher in those with AKI (p < 0.001). Similarly, NVUGIB patients with AKI had a higher rate of blood transfusions (aOR 1.30, p < 0.001) (Table 5).
Additionally, NVUGIB patients with AKI had significantly longer LOS (+ 1.73 days, p < 0.001), higher cost of hospitalizations (+ $16,221, p < 0.001), and higher likelihood of disposition to an intermediate care facility (aOR 1.84, p < 0.001) after adjusting for confounding factors.
Trends of mortality and attributable mortality due to AKI
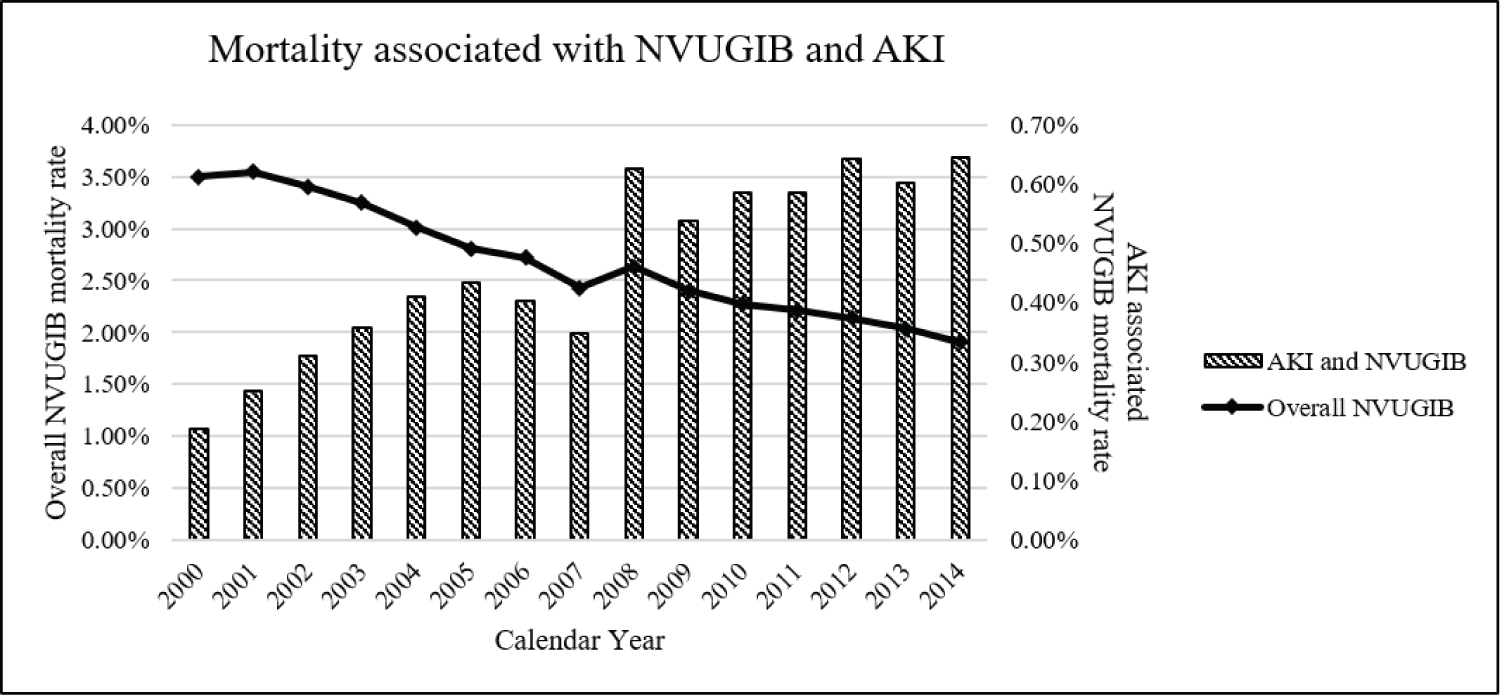
The overall adjusted mortality rate among NVUGIB hospitalizations has decreased significantly over the study period (Figure 3 - line), representing a 45.6 % decrease from mortality rate of 3.50% in 2000 to 1.91% in 2014 (p < 0.001). However, the absolute mortality rate among NVUGIB patients admitted with concomitant AKI has increased significantly from 0.19% in 2000 to 0.65% in 2014, representing a 2.92-fold increase in adjusted mortality.
Discussion
We utilized a large nationally representative sample of hospitalizations to assess trends, predictors, and outcomes for AKI in NVUGIB hospitalizations. In our analyses, we determined that the proportion of AKI in this patient population has increased almost six-fold over the fifteen-year time period. Additionally, AKI was associated with almost three-fold higher mortality rate. Further, these patients had higher blood transfusion rates and were more likely to be transferred to an intermediate care facility following their hospitalization. These patients also required additional resources in regards to longer hospital stay and higher hospitalization charges. Although, there has been a dramatic decrease of NVUGIB related mortality over this time period, mortality associated with AKI has increased significantly.
The detrimental role of AKI in cohorts with undifferentiated upper gastrointestinal bleeding has been previously reported [13,14]. However, these studies remain small single center studies and fail to distinguish patients with non-variceal upper gastrointestinal bleeding. One study retrospectively examined 245 intensive care unit admission with upper gastrointestinal bleeding. Patients with concurrent AKI had significantly longer length of stay and hospitalization costs as well as a four-fold increase in mortality compared to patients without AKI [13]. In another study of 113 elderly patients with upper gastrointestinal bleeding, patients with AKI had increased length of stay and hospitalization costs and presented more often with altered mental status and weakness [14]. Retrospective studies in cirrhotic patients with variceal bleeding have demonstrated similar results [15,16]. However, the segregation of variceal and non-variceal bleeding is important as the pathophysiology of acute renal dysfunction is distinct in patients with chronic liver disease. Well-established mechanisms for renal injury in these patients include hyper-dynamic circulation, hepatorenal syndrome, intravascular volume depletion secondary to diuretic and lactulose use, as well as increased susceptibility to nephrotoxic agent exposure such as nonsteroidal anti-inflammatory drugs, contrast agents, and aminoglycosides [16,23]. Consequently, we isolated patients with AKI and non-variceal upper gastrointestinal bleeding in our cohort, as there are no large-scale studies analyzing this phenomenon.
Our findings of increasing incidence of AKI in NVUGIB patients coincides with the results of a recent study establishing an increasing trend of AKI at the unadjusted rate of 11% in the general population [24]. In our analysis, after adjusting for multiple covariates, the incidence of AKI in NVUGIB patients has been increasing at a rate of 15% each year from 2000 to 2014, suggesting a modest elevation from the general population. One possible explanation for this difference includes a higher absolute incidence of AKI in NVUGIB patients secondary to volume depletion or hypo perfusion. Additionally, we expect the rate of AKI in NVUGIB patients to be even higher by inclusion of CKD patients in this cohort. Our rational for this exclusion was to avoid confounding role of CKD, as it is an independent risk factor for developing AKI. Additionally, previous reports have already shown that CKD and ESRD are significant independent risk factors for worsening outcomes in NVUGIB hospitalized patients and have been incorporated in various risk measuring scales for inpatient intervention and prognosis [3,10,12,25].
The most meaningful independent demographic factor leading to development of AKI in our study was advanced age, which is consistent with the finding in the general population [26-28]. Anatomic and physiologic changes related to aging coupled with increased co-morbidities have been primarily implicated in this phenomenon. Additionally, co-morbid conditions during the hospital stay such as sepsis, ventilator use, and TPN administration, all of which can be attributed to admission to an intensive care unit, were highly associated with the development of AKI. This finding was consistent with a higher overall incidence of AKI in intensive care unit patients ranging from 20% to 50% in previous studies [5-7]. Consequently, these conditions, along with patient demographics and hospital characteristics, were included in our logistic and linear regression modeling to account for their confounding effects in the development of AKI.
Several studies that have established risk assessment scoring system have discounted the significance of AKI, and consequently it is rarely used in clinical assessment scales such as Glasgow-Blatchford Bleeding Score [9], Rockall Score for Upper GI bleeding [10], or AIMS65 Score [11]. Based on our trend analysis, AKI incidence has increased from 2.1% in 2000 to almost 12.8% in 2014. It is likely that the early versions of the risk-stratification tools failed to address the role of AKI as a prognostic marker based on the lower incidence rate of AKI in these patients at the time of the studies, largely occurring between 1990-2005.
With the incidence of AKI increasing six-fold in the fifteen years study period and an increase in mortality of three-fold within NVUGIB patients, it is important for clinicians to devise strategies for AKI screening, formulate appropriate risk-stratification tools and institute preventative therapies to avoid the detrimental role of AKI and its consequences in NVUGIB patients. Further studies should identify specific trends of creatinine rise from baseline and its incorporation into risk-stratification tools to further prognosticate the morbidity and mortality in NVUGIB patients.
Grant Support
None.
Disclosures
None.
Writing Assistance
Augustine Tawadros, MD, Laura Rotundo, MD, Kalpesh G Patel, MD, Pavan Patel, MD, Sushil Ahlawat, MD.
Author Contribution
All authors were involved in gathering, analyzing the data and writing of the manuscript. Each author has approved the final draft for submission.
The manuscript has not been previously published in any language and is not currently being considered elsewhere for publication.
References
- Leerdam MEV (2008) Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol 22: 209-224.
- Abougergi MS, Travis AC, Saltzman JR (2015) The in-hospital mortality rate for upper GI hemorrhage has decreased over 2 decades in the United States: A nationwide analysis. Gastrointest Endosc 81: 882-888.e1.
- Leontiadis GI, Michael MB, Moayyedi P, et al. (2013) Effect of comorbidity on mortality in patients with peptic ulcer bleeding: Systematic review and meta-analysis. Am J Gastroenterol 108: 331-345.
- Longstreth GF (1995) Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: A population-based study. Am J Gastroenterol 90: 206-210.
- Case J, Khan S, Khalid R, et al. (2013) Epidemiology of acute kidney injury in the intensive care unit. Crit Care Res Pract.
- Koeze J, Keus F, Dieperink W, et al. (2017) Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol 18: 70.
- Singbartl K, Kellum JA (2012) AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int 81: 819-825.
- Bellomo R, Ronco C, Kellum JA, et al. (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care 8: 204-212.
- Blatchford O, Murray WR, Blatchford M (2000) A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 356: 1318-1321.
- Rockall TA, Logan RF, Devlin HB, et al. (1996) Risk assessment after acute upper gastrointestinal haemorrhage. Gut 38: 316-321.
- Saltzman JR, Tabak YP, Hyett BH, et al. (2011) A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc 74: 1215-1224.
- Sood P, Kumar G, Nanchal R, et al. (2012) Chronic kidney disease and end-stage renal disease predict higher risk of mortality in patients with primary upper gastrointestinal bleeding. Am J Nephrol 35: 216-224.
- Cakmak U, Merhametsiz O, Oguz EG, et al. (2016) Effects of acute kidney injury on clinical outcomes in patients with upper gastrointestinal bleeding. Ren Fail 38: 176-184.
- Alkhatib AA, Lam A, Shihab F, et al. (2009) RIFLE criteria accurately identifies renal dysfunction and renal failure in elderly patients with upper gastrointestinal hemorrhage: A pilot study. South Med J 102: 580-584.
- Hsieh YC, Lee KC, Chen PH, et al. (2017) Acute kidney injury predicts mortality in cirrhotic patients with gastric variceal bleeding. J Gastroenterol Hepatol 32: 1859-1866.
- Shetty S, Nagaraju SP, Shenoy S, et al. (2018) Acute kidney injury in patients with cirrhosis of liver: Clinical profile and predictors of outcome. Indian J Gastroenterol 37: 248-254.
- HCUP National Inpatient Sample (NIS) (2012) Healthcare cost and utilization project (HCUP).
- Go JT, Vaughan SM, Auerbach A, et al. (2010) Do hospitalists affect clinical outcomes and efficiency for patients with acute upper gastrointestinal hemorrhage (UGIH)? J Hosp Med 5: 133-139.
- Wolf AT, Wasan SK, Saltzman JR (2007) Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol 102: 290-296.
- Houchens R (2015) Missing data methods for the NIS and the SID. H-CUP.
- Deyo RA, Cherkin DC, Ciol MA (1992) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: 613-619.
- Garcia TG, Parikh CR, Viola A (2008) Acute kidney injury in cirrhosis. Hepatology 48: 2064-2077.
- Hsu RK, McCulloch CE, Heung M, et al. (2016) Exploring potential reasons for the temporal trend in dialysis-requiring AKI in the United States. Clin J Am Soc Nephrol 11: 14-20.
- Cheung J, Yu A, LaBossiere J, et al. (2010) Peptic ulcer bleeding outcomes adversely affected by end-stage renal disease. Gastrointest Endosc 71: 44-49.
- Fliser D (2005) Ren sanus in corpore sano: The myth of the inexorable decline of renal function with senescence. Nephrol Dial Transplant 20: 482-485.
- McLachlan MS, Guthrie JC, Anderson CK, et al. (1977) Vascular and glomerular changes in the ageing kidney. J Pathol 121: 65-78.
- Kaplan C, Pasternack B, Shah H, et al. (1975) Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 80: 227-234.
Corresponding Author
Savan Kabaria MD, Internal Medicine, Robert Wood Johnson Medical School, Rutgers Biomedical and Health Sciences (RBHS), Rutgers University, 125 Paterson St, New Brunswick, NJ 08901, Tel: 973-619-4509, Fax: 779-213-2036.
Copyright
© 2021 Kabaria S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.