Prognostic Value of Probe-Based Confocal Laser Endomicroscopy in Pediatric Inflammatory Bowel Diseases
Abstract
The incidence of inflammatory bowel diseases (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is increasing in children. We recently demonstrated increased epithelial gaps in the non-inflamed duodenum of CD and UC patients, and high capillary flow rates in the duodenum of UC patients using probe-based confocal laser endomicroscopy (pCLE). The aim of the present study was to determine if increased epithelial gap density or capillary flow could predict the clinical course of IBD patients and response to therapy.
A total of 26 patients (16 CD and 10 UC cases for the epithelial gap study and 9 UC patients for the vascular flow study - all also included in the gap study) were followed from the time of initial pCLE imaging for a minimum of 24 months. Clinical outcomes analysed included the occurrence of significant clinical events (hospitalizations, surgeries, disease flares), alterations in inflammatory parameters, and disease activity indices.
High epithelial gaps and capillary flow rates did not predict the risk of clinical events or alterations in inflammatory parameters in IBD patients. CD patients with high gap density who were treated with infliximab had a significant reduction in ESR levels at 12 months.
The study did not support an ability for pCLE to predict hospitalizations or flares. Future studies evaluating epithelial gap density and vascular changes in pediatric IBD patients and their relation with inflammatory parameters and sub-clinical molecular changes might be helpful in determining disease pathogenesis and better defining treatment options.
Keywords
Crohn disease, Ulcerative colitis, Erythrocyte sedimentation rate, Infliximab
Introduction
Inflammatory bowel diseases (IBD), including both Crohn disease (CD) and ulcerative colitis (UC) are common, debilitating, intestinal disorders that frequently present in childhood [1]. The causes of IBD remain unknown; theories support a model where unremitting inflammation, likely triggered by environmental exposures, leads to intestinal damage in genetically susceptible hosts [2]. This is accompanied by gut barrier dysfunction, although it remains unclear whether this is the cause or result of intestinal inflammation. The gut barrier is comprised of a single layer of epithelial cells, enhanced by a mucus layer, antimicrobial proteins, immunoglobulin A (IgA), and resident bacteria, which all separate the luminal bacteria from the submucosal immune system. Epithelial cells originate from stem cells in the crypt base and migrate to the villi (in the small bowel) until they are shed from the villus tip into the lumen. Increased intestinal epithelial cell shedding has been reported as a feature of IBD [3,4] and has been shown to be predictive of disease relapse and hospitalization in adult patients [5].
Probe-based confocal laser endomicroscopy (pCLE) enables live imaging of the intestinal mucosa during endoscopy and allows visualization of epithelial cell gaps in vivo [3,6]. Interestingly, in IBD, increased epithelial cell shedding is not only limited to the colon and terminal ileum. Two independent studies have demonstrated this phenomenon in the duodenum in UC. Increased epithelial gaps in the duodenum of adult IBD patients were reported by Lim, et al. [7]. We also found increased epithelial gap density, defined as the number of epithelial gaps per 1000 epithelial cells, in the unaffected duodenum of both CD and UC patients [8]. Epithelial gaps were unrelated to local inflammation or disease activity in our study, suggesting that these gaps could represent a baseline defect in the gut barrier in pediatric IBD patients and that they are not secondary to inflammation. In another study, we reported increased capillary flow rates in the non-inflamed duodenum of UC patients, also measured with pCLE [9].
Further probing the outcomes of these significant features in IBD patients, we wanted to analyze if the presence of increased epithelial gap density and vascular flow could predict disease course and/or response to therapy, especially since they were found in unaffected areas. Thus, we conducted a follow up of the patients enrolled in our previous studies [8,9], in order to determine the clinical course of patients who had increased epithelial gaps and capillary flow rates in the duodenum, as measured using pCLE. Our objective was to analyze if patients with higher epithelial gaps and/or capillary flow had increased incidences of clinical complications (described as IBD-related hospitalization, surgeries, or flares), changes in disease activity indices, or altered inflammatory markers (CRP and ESR) over a period of at least 24 months. In a subanalysis, we also aimed to see if gaps and capillary flow could predict response to therapy.
Materials and Methods
Patients
Patients included in the study were both established and newly diagnosed IBD patients (3-18 years of age), who had epithelial gaps and/or capillary flow rates quantified in the duodenum using pCLE during esophagogastroduodenoscopy conducted as part of previous studies [8,9]. A total of 26 IBD patients (16 CD and 10 UC patients) of the epithelial gap analysis, and 9 UC patients of the capillary flow study were included. All patients included had consented to participate in both the confocal study and subsequent follow up, as approved by the University of Alberta Research Ethics Board (Study ID Pro00023820). The initial studies were conducted from 2012-2015, and the prospective follow up ranged between 24-47 months.
Clinical outcomes
The clinical follow-up of the patients included systematic documentation of significant clinical complications (described as hospitalization, surgeries, or disease flares, using a prospective patient registry), inflammatory parameters [(erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)], disease indices [Pediatric Crohn's Disease Activity Index (PCDAI), Pediatric Ulcerative Colitis Activity Index (PUCAI)] [10], and medication use. All data for the study were systematically and prospectively obtained through routine clinic visits at the Edmonton Pediatric IBD Clinic (EPIC) and extracted from our database, confirmed by Alberta Netcare and eClinician (provincial electronic medical records managed by Alberta Health Services, which store patients' complete medical records, including hospitalizations, medications, and laboratory investigations). Laboratory investigations were performed following a clinic protocol by regional Alberta laboratories. All clinical data including events (flares, hospitalizations, etc) and disease scoring indices were documented prospectively in the patients' health charts by IBD physicians who were blinded to the pCLE results. Flares were defined as PCDAI [11] or PUCAI [12] absolute scores of > 10.
Statistical analysis
The cut-off point for normal gap density and capillary flow was determined based on a similar previous study [5]. Epithelial gap densities and capillary flow rates above the estimated 94th percentile of the non-IBD group were considered to be abnormal (this cutoff has been used previously for the gaps [5], but there are no published papers on normal cutoffs for capillary flows, as we were the first to describe this method [9]; therefore, we decided to use the same methods for this analysis). Kaplan-Meier plots were used to determine event-free survival probabilities, with log-rank tests to determine the differences between study groups. Cox proportional hazard models were used to determine if gap density and vascular flow were predictors of risk of clinical events or inflammatory parameters. Comparison between the inflammatory/disease activity parameters (ESR, CRP, PCDAI, and PUCAI) were done with Student's t-tests and Mann-Whitney test. Correlation of epithelial gap density and capillary flow with clinical events and inflammatory parameters was done using Spearman's test. The differences in inflammatory markers of patients on various treatments were analyzed with Student's t-test and Mann-Whitney test. Statistical analysis was done with Graph Pad (San Diego, CA, USA) and XLStat (New York, NY, USA). A P value of < 0.05 was considered to be significant.
Results
Patients characteristics
Of the CD cohort included in the epithelial gap study, 8 patients were newly diagnosed and 8 were previously diagnosed and on active therapy at the time of epithelial gap calculation. All UC patients included carried a previous diagnosis at the time of recruitment and measurement. Of the CD group, 10 patients were determined to have normal gap density, whereas 6 had high gap density. In the UC group, 6 patients had normal and 4 had high epithelial gaps in the duodenum. In the UC cohort of the capillary flow rate study, all patients were established cases. Five (5) were determined to have normal flow, whereas, 4 had high capillary flow rates. All patients had normal-appearing mucosa in the duodenum, validated with both histological and endoscopic assessment. The pCLE study was conducted between 2012-2015 and the follow-up period continued until the middle of 2017. For the entire group, the median for follow-up was 31 months, IQR 17.25. Patient characteristics are detailed in Table 1.
Epithelial gap density follow up
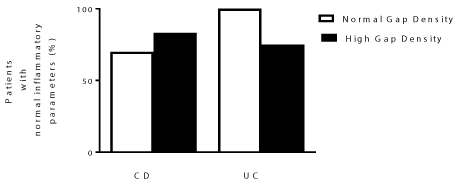
Proportion of IBD patients with normal inflammatory parameters in the follow-up period varied between CD and UC patients
Analysis of clinical outcomes was conducted at 24 months after pCLE testing. The 24 month time-point was chosen as the duration of the follow-up period varied amongst patients, but data were available for all patients from baseline to at least 24 months. From the time of endoscopy, when the epithelial gaps were initially analyzed, until 24 months later, the percent of patients with continuously normal inflammatory parameters (PCDAI/PUCAI < 10, lab values of CRP < 8 mg/L, and ESR < 15 mm/hr) was 70 and 84% in CD patients with normal and high epithelial gaps, respectively, and 100 and 75% in UC patients with normal and high epithelial gaps, as shown in Figure 1. These high clinical and biochemical remission rates may have limited our ability to predict events during the follow-up period, as flares were uncommon in our cohort. To minimize the bias associated with compounding factors, such as medications, we divided the patient groups into 'newly diagnosed' and 'established' patients for statistical analysis. However, this subdivision was not possible in our UC cohort, as all 10 UC patients were established cases who were also being actively treated. Of the epithelial gap study cohort, in the CD group 8 patients were newly diagnosed and 8 were established cases on active therapy. Of these, 2 patients with high epithelial gaps and 6 with normal epithelial gaps were newly diagnosed with active disease. As our patient population was very heterogeneous, particularly with all patients of the UC group on active therapy prior to the study, we looked at their treatment regimens as well (Supplemental Digital Content 1, Supplemental Digital Content 2, Supplemental Digital Content 3, Supplemental Digital Content 4 and Supplemental Digital Content 5).
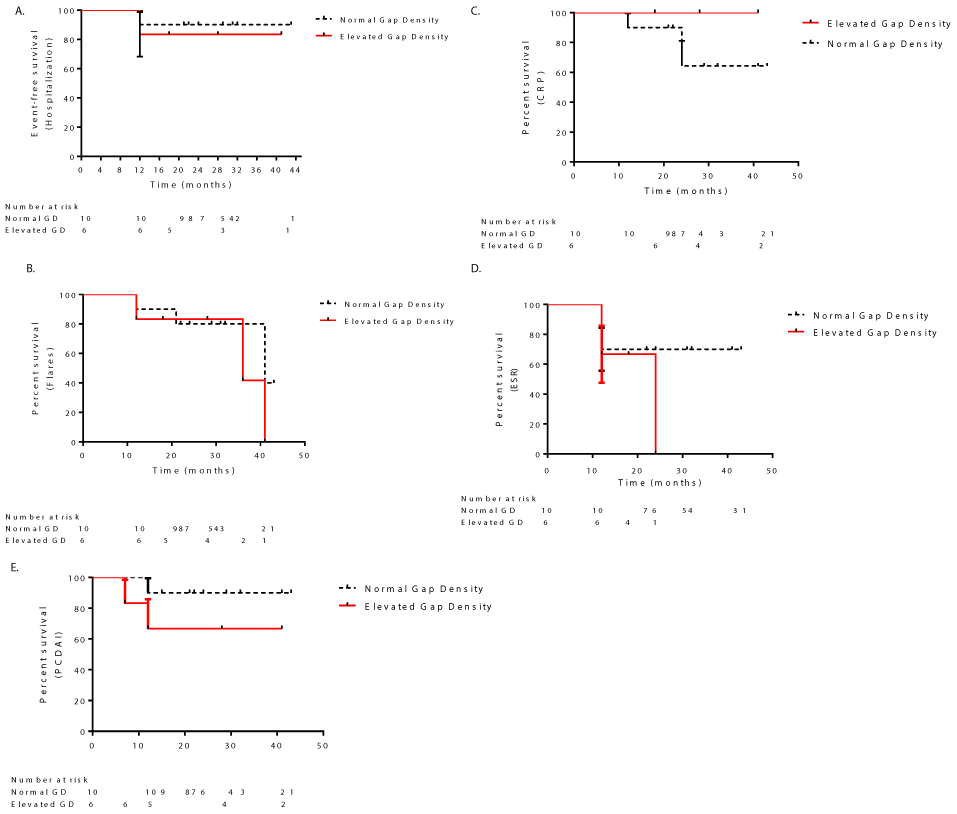
Event-free survival probabilities and inflammatory parameters did not differ significantly amongst CD patients with normal and high epithelial gap density who were newly diagnosed and had active disease
Analysis of event-free survival probabilities was done with the Kaplan-Meier method and comparison was made using log-rank tests. None of the patients in either group required surgery. Although patients in the normal and high epithelial gap groups had different trends of clinical events/inflammatory markers, there was no significant difference in any inflammatory parameter or events between the groups, and cox proportional hazard models did not identify gap density to be a predictor of clinical events or inflammatory markers (Figure 2: log-rank and Gehan-Breslow-Wilcoxin tests P > 0.05).
In CD patients with either normal or high epithelial gaps who were newly diagnosed and had active disease, there was only one incidence of hospitalization in each group at the 12-month point (Figure 2A). Three patients in the CD group with normal epithelial gaps had flares at 12, 21, and 41 months after pCLE; whereas flares occurred at 36 and 41 months in two patients in the high epithelial gap group (Figure 2B). Of the two patients in the high epithelial gap group, neither patient had elevated CRP levels, whereas, of the 6 patients with normal epithelial gaps, 2 had elevated CRP at 12 and 24 months each (Figure 2C). At 12 months, 1 patient with high and 3 patients with normal epithelial gaps in the CD group had increased ESR; 1 patient with high epithelial gaps had elevated ESR at 24 months (Figure 2D). One patient each in the normal and high epithelial gap group had a high PCDAI score at 12 months (Figure 2E). Statistical analysis conducted on the CD group with chronic disease treated with either infliximab or azathioprine did not show any significant differences amongst the clinical events or clinical parameters between the normal and high epithelial gap groups (Supplemental Digital Content 1 and Supplemental Digital Content 2). However, in both the CD groups with normal and high epithelial gaps, 3 patients were treated with infliximab after endoscopy. CD patients with higher gap densities who were treated with infliximab had a significant decrease in ESR levels from baseline to 12 months, (Supplemental Digital Content 3A, Student's t-test, P < 0.05). In contrast, patients with normal gap density treated with infliximab did not show a significant reduction in ESR, despite having a higher baseline level (Supplemental Digital Content 3B, Student's t-test, P > 0.05). Thus, in CD patients with elevated epithelial gap density, high ESR levels are more responsive to treatment with infliximab than in those with normal gaps.
Disease location at diagnosis and use of other medications were not correlated with response to infliximab or presence of gaps; however, given the small number of patients in each group, we cannot exclude such a correlation. Of the patients with normal gaps who were treated with infliximab 1 patient with high ESR levels also had high CRP levels, the other 2 patients had high ESR with normal CRP levels at 12 months follow-up. The patients with high gaps who were treated with infliximab had normal CRP levels in addition to lowered ESR levels at 12 months and were in remission.
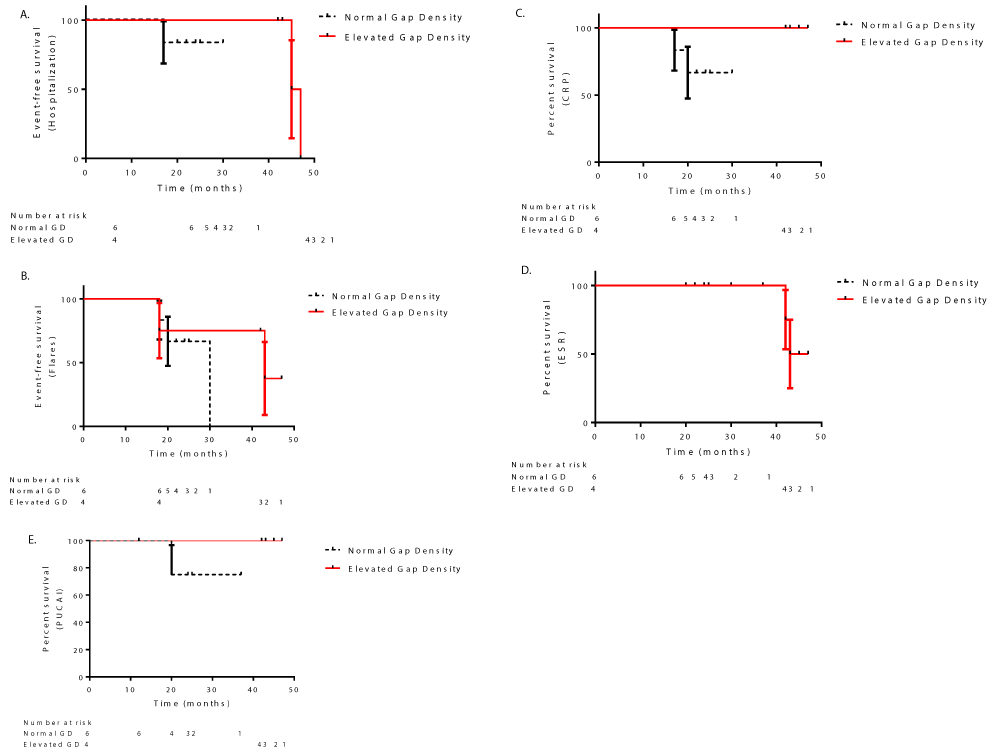
Event-free survival probabilities and inflammatory parameters did not differ significantly between UC patients with normal and high epithelial gap density
In the UC group there were no significant differences amongst the clinical events or clinical parameters between the normal and high epithelial gap groups. One patient (of 6) in the normal epithelial gap group was hospitalized at 17 months, and 2 patients (of 4) in the high epithelial gap group were hospitalized at 45 and 47 months, respectively (Figure 3A). Three patients of the normal epithelial gap group had flares at 18, 20, and 30 months, whereas in the high epithelial gap group, two patients had flares at 18 and 43 months, respectively (Figure 3B). In the normal epithelial gap group, 2 patients had elevated CRP levels at 17 and 20 months (Figure 3C). Two patients in the high epithelial gap group had elevated ESR levels at 42 and 43 months, (Figure 3D). One patient who had normal epithelial gaps had a high PUCAI score at 20 months (Figure 3E).
Analysis of event-free survival probabilities and inflammatory parameters did not show a significant difference between chronic UC patients with normal and high epithelial gap density treated with either infliximab or azathioprine (Supplemental Digital Content 4 and Supplemental Digital Content 5).
UC vascular flow follow up
Event-free survival probabilities and inflammatory parameters did not differ significantly between patients with normal and high capillary flow rates (data not shown).
Discussion
Given our recent findings, demonstrating changes in the form of increased epithelial gaps in an uninvolved bowel section (duodenum) of pediatric IBD patients, we conducted this follow-up study to determine if the clinical course of IBD patients with increased epithelial gap density is different from that of IBD patients with normal gap density, and if epithelial gaps are predictive of clinical events.
The gut barrier is greatly affected in IBD. Increased gut permeability is not only present in IBD patients [13,14], but also in unaffected relatives of CD patients, as assessed by lactulose/mannitol ratios which could possibly be linked with mutations in the NOD2 gene, or could be due to subclinical mucosal inflammation [15].
Epithelial gap density of the gut barrier is of profound importance in IBD, is increased in adult IBD patients [16], and is predictive of disease course [5]. pCLE determined that gap density was related to hospitalization in adult CD patients [16]. Epithelial gaps analyzed with pCLE were also found to be higher in the duodenum of adult IBD patients than in healthy controls [7]. In vitro cell line models using T-84 cells demonstrated that epithelial shedding was induced by caspase-1, which also caused increased cell shedding in mice and altered epithelial permeability in T84 cells. Analysis of human ileum biopsies from IBD patients reported caspase-1-mediated apoptosis to be a possible mechanism involved in epithelial cell shedding [17].
In the epithelial gap study cohort, we observed some variation in patients who had maintained normal inflammatory parameters (PCDAI/PUCAI < 10, normal lab values of CRP and ESR) within the follow up period, but it was not statistically significant.
Analysis of individual clinical events and inflammatory parameters in the follow up period showed that there was neither a specific trend, nor a significant difference between patients with normal and high epithelial gap density whether they were newly diagnosed or had chronic disease. We cannot exclude that a lack of association is mainly due to the very low rate of flares/complications/active disease seen in our cohorts during the follow-up period and the small number of patients included in the study in the first place, which makes a type 2 error possible. In contrast to Sharvov, et al. [18], who showed that high epithelial gap density in the terminal ileum (TI) of pediatric patients was predictive of relapse, we did not find duodenal epithelial gaps to be predictive. This could be because the TI is a commonly involved site in IBD, whereas, duodenum is typically unaffected in IBD. It is possible that as our findings are in the duodenum, they could be very early changes in our patients and perhaps over time, these changes do become predictive of relapse.
Disease activity and treatments are variables that could impact the outcomes of our study. We subdivided the patients having active and inactive disease and per treatment groups and did not find an effect, and it also further reduced the numbers of patients in each group, increasing the risk of a type 2 error. Interestingly, our results show that at the 12 month follow-up point, there was a significant decrease in serum ESR levels in CD patients treated with infliximab, present in those with higher epithelial gap density but not those with normal gaps. Infliximab is successfully used to achieve remission in many IBD patients [19]. The inability of PCDAI/PUCAI to predict response is not surprising (given the limited correlation between disease outcomes and symptom-based scores) but the fact that CRP was not a predictor does require attention. We hypothesize that this might be due to the fact that some patients do not mount a CRP response, therefore diluting the predictive capacity of CRP.
Small sample size is a major limitation of our study. It is possible that a larger cohort could have indicated an ability to predict outcomes. With a larger cohort, it may also be possible to determine the predictive value of epithelial gap density in the duodenum for surgeries, hospitalizations and flares in IBD patients, although our study was not able to support this statement.
In the vascular study cohort, the percent of patients with normal clinical parameters was similar between the groups. Similar to the results of the epithelial gap follow up, there was no difference in the clinical events and inflammatory parameters between patients with normal and high capillary flow rates.
Nevertheless, since high epithelial gaps are a significant feature present in pediatric IBD patients, their clinical importance needs to be explored more. Thus, pCLE has a potential for being a marker of biological processes that could help correlate the presence of high epithelial gaps with molecular pathogenic changes in pediatric IBD patients, and better define the basic biological defects leading to disease pathogenesis.
Author Contribution
DZ and EW designed the study and collected the data. The data were interpreted and analyzed by all authors. The manuscript was prepared by DZ and EW, and the final version was approved by all authors.
Acknowledgements
We want to specially thank our patients and the hospital staff, specially Cheryl Kluthe, RN and Pavel Medvedev, EPIC research coordinator.
Funding Sources
Conflicts of interest and source of funding
Dr. Eytan Wine, Dr. Hien Huynh and Dr. Matthew Carroll are members of the advisory boards of Janssen and AbbVie. This study was funded by Women and Children's Health Research Institute (WCHRI), for graduate studentship for DZ, Alberta Innovates Health Solutions (AIHS), The Crohn's and Colitis Foundation of America (CCFA), WCHRI, and Crohn's and Colitis Canada (CCC) for the Wine lab.
References
- Benchimol EI, Manuel DG, Guttmann A, et al. (2014) Changing age demographics of inflammatory bowel disease in Ontario, Canada: A population-based cohort study of epidemiology trends. Inflamm Bowel Dis 20: 1761-1769.
- Atreya I, Atreya R, Neurath MF (2008) NF-kappaB in inflammatory bowel disease. J Intern Med 263: 591-596.
- Liu JJ, Madsen KL, Boulanger P, et al. (2011) Mind the gaps: Confocal endomicroscopy showed increased density of small bowel epithelial gaps in inflammatory bowel disease. J Clin Gastroenterol 45: 240-245.
- Watson AJ, Chu S, Sieck L, et al. (2005) Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology 129: 902-912.
- Turcotte JF, Wong K, Mah SJ, et al. (2012) Increased epithelial gaps in the small intestine are predictive of hospitalization and surgery in patients with inflammatory bowel disease. Clin Transl Gastroenterol 3: e19.
- Kiesslich R, Duckworth CA, Moussata D, et al. (2012) Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut 61: 1146-1153.
- Lim LG, Neumann J, Hansen T, et al. (2014) Confocal endomicroscopy identifies loss of local barrier function in the duodenum of patients with Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 20: 892-900.
- Zaidi D, Jorgensen M, Huynh HQ, et al. (2016) Increased epithelial gap density in the non-inflamed duodenum of children with inflammatory bowel diseases. J Pediatr Gastroenterol Nutr 63: 644-650.
- Zaidi D, Churchill L, Huynh HQ, et al. (2017) Capillary flow rates in the duodenum of pediatric ulcerative colitis patients are increased and unrelated to inflammation. J Pediatr Gastroenterol Nutr 65: 306-310.
- Solem CA, Loftus EV Jr, Tremaine WJ, et al. (2005) Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 11: 707-712.
- Turner D, Griffiths AM, Walters TD, et al. (2012) Mathematical weighting of the pediatric Crohn's disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis 18: 55-62.
- Turner D, Otley AR, Mack D, et al. (2007) Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology 133: 423-432.
- Bruewer M, Samarin S, Nusrat A (2006) Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci 1072: 242-252.
- Weber CR, Turner JR (2007) Inflammatory bowel disease: Is it really just another break in the wall? Gut 56: 6-8.
- Teshima CW, Dieleman LA, Meddings JB (2012) Abnormal intestinal permeability in Crohn's disease pathogenesis. Ann N Y Acad Sci 1258: 159-165.
- Liu JJ, Wong K, Thiesen AL, et al. (2011) Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: Density matters. Gastrointest Endosc 73: 1174-1180.
- Liu JJ, Davis EM, Wine E, et al. (2013) Epithelial cell extrusion leads to breaches in the intestinal epithelium. Inflamm Bowel Dis 19: 912-921.
- Shavrov A, Kharitonova AY, Davis EM, et al. (2016) A pilot study of confocal laser endomicroscopy to predict barrier dysfunction and relapse in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 62: 873-878.
- Guo Y, Lu N, Bai A (2013) Clinical use and mechanisms of infliximab treatment on inflammatory bowel disease: A recent update. Biomed Res Int 2013: 581631.
Corresponding Author
Eytan Wine, MD, PhD, Division of Pediatric Gastroenterology and Nutrition, Department of Pediatrics, University of Alberta, Edmonton Clinic Health Academy, Room 4-577, 11405 87th Avenue, Edmonton AB, T6G 1C9, Canada, Tel: 780-248-5420, Fax: 1-888-353-1157.
Copyright
© 2018 Zaidi D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.