Hepatocellular Carcinoma Encountered during Laparoscopic Bariatric Surgery: Two Case Reports and Discussion
Introduction
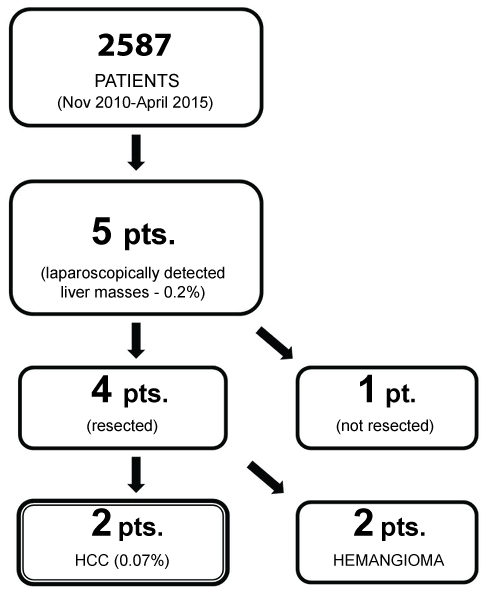
Recent epidemiological data demonstrate a rise in the prevalence of Hepatocellular Carcinoma (HCC) and Non-Alcoholic Fatty Liver Disease (NAFLD) in obesity and diabetes [1,2]. There is hence a greater chance of finding these liver pathologies during bariatric/metabolic surgery, as more patients undergo these surgeries. However, there have been no reports so far, of hepatocellular carcinoma encountered during bariatric surgery. Following a retrospective review of liver masses encountered during bariatric surgery (Figure 1), we report two patients with early HCC, discovered and managed successfully at the time of bariatric surgery in a bariatric and metabolic surgery center in Taiwan and discuss the relevance of the same.
Case Report 1
The first patient with HCC (Table 1, Case No.1) was a thirty-year-old lady admitted (in March 2015) for Duodenal-Jejunal Bypass with Sleeve Gastrectomy (DJB-SG). She had uncontrolled Type 2 Diabetes (T2DM) diagnosed for 10-years, on three oral hypoglycemic agents and insulin therapy. She also had fatty liver, hyperlipidemia, and hyperuricemia. She had no past history of jaundice and her hepatitis B and C viral statuses were both negative. Apart from hypertension (146/102 mmHg), her physical examination was normal. Her preoperative lab work up showed high HbA1C (10.4%), triglycerides (307 mg/dl), but liver chemistry was within normal limits. Ultrasound of her abdomen revealed marked fatty liver. However, no lesions or signs of portal hypertension were reported. Her chest x-ray was also normal.
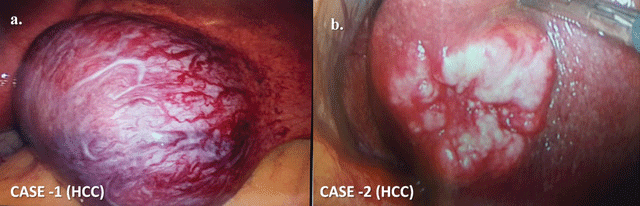
On laparoscopy during surgery, her liver showed a mass of size 6 × 5 × 4 cm, in the segment 3, involving the inferior border with prominent vessels on the surface (See Figure 2a). The right lobe was normal except for fatty changes. The stomach, duodenum, small and large bowel were all normal. There were no other intra-abdominal masses, regional lymph node involvement, peritoneal seeding or ascites. A laparoscopic wedge resection of the lesion with 5-10 mm margin all around was done. There was no vascular invasion. DJB-SG was carried out, as planned. The resected liver specimen was removed via an endobag at the end of the operation by enlarging the periumbilical port. The total operating time was 220 minutes. She had an uneventful postoperative recovery. Her postoperative CT and MRI scans did not reveal any residual lesion. Her AFP level was 1.37 ng/ml. The biopsy of the liver mass was reported as encapsulated hepatocellular carcinoma (histological grade I/II of Edmondson and Steiner) with non-involved margins. Surrounding liver tissue showing fatty changes and the portal areas, chronic inflammatory changes. She was discharged 72 hours following surgery, as per bariatric surgery protocol. The HCC stage being pT1 N0 M0, she was put on regular follow-up and did not receive any systemic chemotherapy. She was asymptomatic, had no recurrence and also achieved better glycemic control (HbA1C - 6.9%) on follow up, till two years after surgery.
Case Report 2
The second patient with HCC (Table 1, Case No. 2) was again a thirty-year-old female, non-diabetic, who was admitted for sleeve gastrectomy (in Nov 2010) after failed nonsurgical weight loss measures. Apart from obesity and hypertension (176/134 mmHg), her physical examination was normal. Her pre-operative lab work up showed hyperuricemia and her liver chemistry was normal. Chest x-ray did not reveal any abnormalities. Her pre-operative ultrasound of the abdomen reported fatty liver, but had no other significant findings of any lesion or portal hypertension.
On laparoscopy for sleeve gastrectomy, a 3 × 3 cm raised mass was seen on the inferior surface of segment 3 of the liver, with yellowish brown nodular areas and increased vascularity (See Figure 2b). Rest of the liver surfaces was normal. There was no ascites, lymph nodes, nor any other lesions in the abdominal or pelvic organs and peritoneal surfaces. After confirmation by a frozen section, laparoscopic wedge resection was done with a macroscopically clear margin of 5-10 mm all around. There was no vascular invasion. Laparoscopic sleeve gastrectomy was also performed, as planned. The resected liver specimen was removed via an endobag by enlarging the periumbilical port. The total operating time was 225 minutes. Her postop course was uneventful and she was discharged 48 hours after surgery. The biopsy of the tumor was reported as nodular growth of dysplastic hepatocytes with fibrotic bands, moderately differentiated hepatocellular carcinoma with involved margins. Her postoperative CT scan showed no other metastatic lesions in the liver. However, postoperatively, her alpha-fetoprotein levels were 74.0 ng/ml and were also found to be a hepatitis B virus carrier with HBV DNA levels > 250 IU/ml. The HBeAg was negative. There was no other hepatic or extrahepatic metastasis in this patient on postoperative CT scan report. As the patient had 'involved' margins and had refused angiography or further invasive treatments, the oncologist decided to give systemic chemotherapy and follow her up with serial ultrasound examination and monitoring of AFP levels. On follow-up, serial ultrasounds detected no abnormality in the liver, and her AFP levels had fallen to 1.73 ng/ml. At the time of the last follow-up, seven years post-procedure, her weight had reduced from 121.9 kg to 90 kgs. She had no complaints or symptoms except for a moderate weight regain of 5 kgs.
Discussion
During a laparoscopic bariatric procedure, detection of a small solitary liver mass, suspicious for malignancy, can pose a dilemma in its management. We report for the first time, the concurrent resection of early (T1, N0, M0) HCC during bariatric surgery, in two patients who had good outcomes as indicated above. Routine ultrasound examination (60% sensitivity) [3], can allow smaller liver masses escape detection. Larger masses detected during preoperative bariatric workup could be subjected to detail imaging studies and pre-operative planning, before proceeding with surgery. However, an unexpected and intraoperatively detected, suspicious, liver mass can pose a situation of a dilemma in management (including the abandonment of the planned procedure). The availability of a laparoscopic ultrasound would greatly improve the evaluation of such masses. Though laparoscopic distinction of HCC was studied by some [4], this will not amount to a definitive diagnosis, leaving the surgeon with the only other option, to proceed with resection of the suspicious liver mass. As the consent for surgery taken by the surgeon in this center, included 'any additional procedure that was found necessary' along with the planned procedure, the resections could be undertaken in these patients, who were primarily admitted for bariatric/metabolic surgery. We also add here, that in case of a major resection, the option of a less invasive procedure, or even suspension of the bariatric procedure, would be appropriate.
Laparoscopic liver resection for HCC has reduced hospital stay and complications with no differences in the recurrence rates compared to open liver resections [5]. In a laparoscopic setting, when discovered unexpectedly, we report the feasibility of resecting an early HCC lesion, safely. It should be clearly noted that in both these patients the tumors were small and in the (anterior) segment 3 of the liver with no vascular invasion. These were extracted using endobag, after resection, to avoid trochar site recurrences. There was no need for blood transfusions. However, the decision to proceed with the initial planned bariatric procedure should be weighed carefully in each setting, to avoid additional morbidity to the liver resection. We believe that during preoperative discussions with the patient, one should also include the scope for such unexpected intraoperative decisions in the informed consent.
Hepatocellular Carcinoma (HCC) is the 2nd leading cause of cancer deaths globally [6]. However, in Taiwan, it is the number one leading cause of death among cancers in men [7]. Obesity and diabetes, increases the risk of HCC, as reported by several population-based studies both in the Eastern and Western centers [8,9]. There is a 2-4 fold greater risk of HCC in diabetics as compared to non-diabetics, independent of other major HCC risk factors [10]. In a large healthcare database study in the US, NAFLD was the most common underlying HCC risk factor (59%), followed by diabetes (36%) and HCV infection (22%) [1]. NAFLD, which is present in up to 90% of all obese persons and up to 70% of T2DM patients [11], appears to play a key role in HCC development. Insulin resistance increased Tissue Necrosis Factor (TNF) activity, alterations in serum lipids associated with non-alcoholic fatty liver disease and non-alcoholic steatohepatitis may play important roles [12]. However, a comprehensive view of multiple pathways involved in NAFLD-related HCC pathogenesis, is currently lacking.
In our report, the diabetic patient (Case No.1) with HCC was negative for hepatitis B and C. However, as she had a fatty liver, high triglycerides, and a histopathology of the surrounding liver tissue (around the HCC) showing inflammatory changes around the portal areas, we suspect that NAFLD would have been the etiological factor for HCC in this case. However as the second (Case No.2) patient was found to be a hepatitis B carrier in addition to the fatty liver, the former (hepatitis B) could have been a possible cause of HCC. We however, could not retrieve any immunohistochemical staining for HBV from the records.
Whether bariatric surgery is protective against developing of HCC, is unclear. However, evidence from a large multicenter, retrospective study shows that admissions with a history of bariatric surgery had a 61% lower prevalence of liver cancer compared to those without a history of bariatric surgery [13]. There is also a rapid improvement in the liver histology of patients with NAFLD following bariatric surgery [14]. This could theoretically prevent the progression into steatohepatitis and subsequent hepatocellular carcinoma. However, there is currently, no unanimous consensus [15] for this and needs to be obtained from larger studies with long term follow-up of bariatric patients.
Conclusion
With the present increasing rate of global obesity, NAFLD-related HCC incidence is only expected to rise in the future. The role of bariatric surgery in the reducing the risk of HCC needs further study for confirmatory evidence. We report two patients with respectable, early HCC, encountered unexpectedly and managed successfully, during bariatric surgery. To our knowledge, there has been no publication of HCC managed in patients, while undergoing bariatric/metabolic surgery.
References
- Sanyal A, Poklepovic A, Moyneur E, et al. (2010) Population based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 26: 2183-2191.
- Mc Glynn KA, London WT (2011) The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 15: 223-243.
- Colli A, Fraquelli M, Casazza G, et al. (2006) Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha fetoprotein in diagnosing hepatocellular carcinoma: a systemic review. Am J Gastroenterol 101: 513-523.
- Kameda Y, Shinji Y (1992) Early detection of hepatocellular carcinoma by laparoscopy: yellow nodules as diagnostic indicators. Gastrointest Endosc 38: 554-559.
- Xiong JJ, Altaf K, Javed MA, et al. (2012) Meta-analysis of laparoscopic vs. open liver resection for hepatocellular carcinoma. World J Gastroenterol 18: 6657-6668.
- Jemal A, Bray F, Center MM, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90.
- (2008) Vital statistics 2007, Department of Health (DOH), The Executive Yuen, Taiwan.
- Koh WP, Wang R, Jin A, et al. (2013) Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer 108: 1182-1188.
- Polednak AP (2008) Estimating the number of U.S. incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detect Prev 32: 190-199.
- Hung CH, Wang JH, Hu TH, et al. (2010) Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol 16: 2265-2271.
- El-Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365: 1118-1127.
- Kew MC (2015) Obesity as a cause of hepatocellular carcinoma. Ann Hepatol 14: 299-303.
- Yang B, Yang H, Ward K, et al. (2016) Bariatric surgery and liver cancer in a consortium of academic medical centers. Obes Surgery 26: 696-700.
- Praveen Raj P, Gomes RM, Kumar S, et al. (2015) The effect of surgically induced weight loss on non-alcoholic fatty liver disease in morbidly obese Indians: "NASHOST" prospective observational trial. Surg Obes Relat Dis 11: 1315-1322.
- Koh JC, Loo WM, Goh KL, et al. (2016) Asian consensus on the relationship between obesity and gastrointestinal and liver diseases. J Gastroenterol Hepatol 31: 1405-1413.
Corresponding Author
Wei Jei Lee, Department of Surgery, Min-Sheng General Hospital, Touyuan, Taiwan.
Copyright
© 2017 Zachariah PJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.