Plant Histaminase as Bioactive Agent to Lower the Histamine Level: A Mini-Review
Abstract
Histamine is a biogenic amine originating endogenously or exogenously from Histidine by enzymatic decarboxylation. Endogenous histamine is mostly generated by basophils and mast cells that, when activated, will release histamine that is involved in various regulatory processes but also will induce multiple allergic effects (hypotension, tachycardia, vascular risks) including anaphylactic shock and possible death. For some histamine related symptoms (itching, asthma, hyperacidity), there are antihistaminic drugs (e.g. Desloratadine-Aerius®, Loratadine-Claritin®, Ranitidine-Zantac®) whereas for anaphylaxis, epinephrine (EpiPen®) is required. Exogenous histamine, frequently associated to fermented food and beverages, some fruits, fish, may induce a food histaminosis and trigger pseudo-allergic phenomena for which there is no current treatment available. Histamine may also exert some pro-inflammatory effects, particularly damaging for subjects with Inflammatory Bowel Diseases (IBD) as Crohn's Disease (CD) and Ulcerative Colitis (UC), mostly treated with anti-inflammatory drugs. Histamine deleterious effects and those produced as side-effects of antihistaminic drugs (allergic phenomena) or of anti-inflammatory drugs are inconvenient (nausea, diarheea, dizziness).
For this reason, novel enzymatic strategies with Diamine Oxidase (DAO, histaminase) to decrease the histamine levels in allergic reactions to food and in ulcerative colitis were recently proposed. This may alleviate the symptoms associated with IBD and food allergens. The therapeutic concept is that histaminase will decrease the level of histamine in the intestine by oxidation, involving the dissolved oxygen. Since the by-product of the DAO reaction is hydrogen peroxide (H2O2), a pro-oxidant agent generating undesirable oxidative damaging effects, a combination of catalase and vegetal histaminase in tablet formulations for oral administration was proposed. Catalase will decompose H2O2 generating in situ more oxygen, promoting thus the decomposition of histamine under histaminase action. Thus, the dual enzymatic histaminase + catalase tablets, could contribute to a healthier intestinal mucosa.This DAO approach seems a non-toxic way to improve the treatment IBD and related inflammatory pathologies.
Keywords
Histamine, Diamine oxidase (DAO, Histaminase), Catalase, Inflammatory Bowel Diseases (IBD), Food histaminosis, Lower intestine and Colon delivery
Introduction
Histamine is a biologically active molecule: a biogenic amine generated by the alpha-decarboxylation of the amino acid histidine by histidine decarboxylase. This biogenic amine is widely distributed and is involved in various important biological processes (regulation of gastric acidity, activity of smooth muscles as well as inflammatory and immunological reactions) through the activation of one or more of the four specific histamine receptors H1, H2, H3, H4 on target cells [1,2]. In general, H1 and H2 receptors are present in the vascular smooth muscle cells (SMCs) and endothelial cells, H3 and H4 receptors are mostly expressed in central nervous system and in the enteric nervous system and H4 is expressed in bone marrow, liver, lung, spleen, small intestine and colon.
Histamine: friend & foe and its signalling issues
Histamine can act as vasodilator and as vasoconstrictor of smooth muscle such as uterine and gut smooth muscle [3-5]. As a vasoactive biogenic amine, histamine is able to act on H1-H4 receptors activating the G protein and trigger signals modulating vasomotricity. Histamine may strongly increase vascular permeability and regulate the blood pressure. Histamine may also induce tachycardia and arrhythmias depending on the activated receptor and location of target cells [6]. Furthermore, histamine also mediates neurotransmission in the central nervous system [7], immunomodulation [8], hematopoiesis [9], cell proliferation [10], angiogenesis in tumour models i.e., adenocarcinoma of stomach or large bowel [11-13]. It also promotes mucosal and gastric acid secretion. As a constrictor of smooth muscle, histamine activates H1 receptors located on SMCs (smooth muscle cells) inducing peristalsis [2,14].
Origin of histamine
Endogenous origin
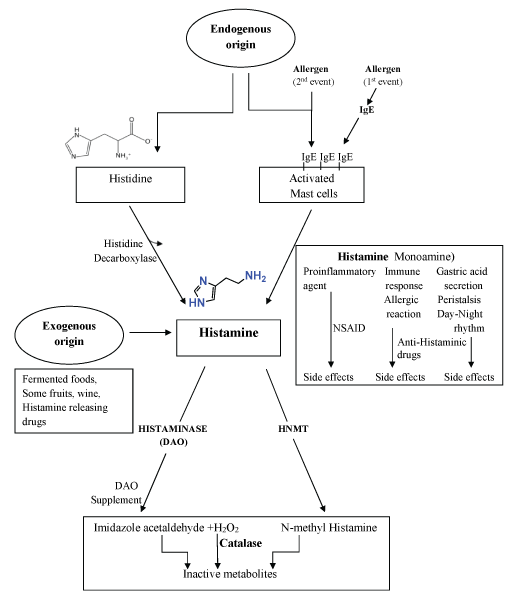
Histamine is produced by the organism itself and is present in many tissues. Histamine is mostly generated in gastric enterochromaffin-like (ECL) cells, histaminergic neurons, basophiles and mast cells [15,16] which store it in intracellular vesicles (Figure 1). Histamine is released as result of allergens and other exogenous factors. For instance allergens, at a first allergic event, will trigger specific antibodies (IgE) that will rapidly act on basophiles and on mast cells. At a subsequent allergic event, the allergen will be recognised by the IgE antibodies retained by the basophil and mast cells inducing degranulation of histamine vesicles with histamine release, which will induce drastic allergic phenomena (Figure 1). Besides allergens, mast cells degranulation may also be mediated by non-immuno stimuli such as neuropeptides [17], hyperosmolarity [18], lipoproteins and platelet activating factor [19], adenosine [20]. In addition to these mechanisms, various medications and many agents such as opiates [21,22], muscle relaxants [23] plasma expanders and radiocontrast materials [24] as well as physical factors (i.e. extreme temperature, vibrations) [25,26] can be responsible of histamine release. All these mentioned allergens, chemical and physical factors are responsible of deleterious histamine able to induce allergic phenomena including anaphylactic shock and possibly death.
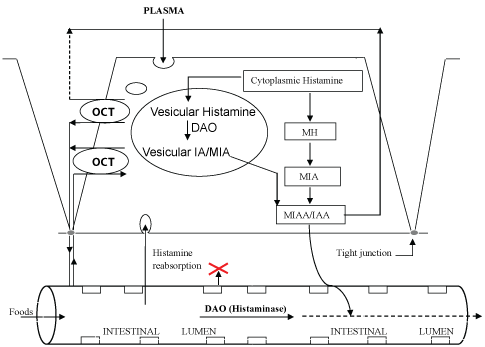
Most recent knowledge on histamine functions was acquired in the last decade. Histamine is normally catabolized by copper diamine oxidase (DAO) and FAD (Flavin-Adenine-Dinucleotide) monoamine oxidase (MAO) and also by histamine N-methyltransferase (HNMT). These enzymes are localized at cellular level but histamine is unable to easily enter the intracellular space except when the transport is mediated by organic cationic transporters (OCT) as described by [27] Ogasawara, et al. The OCT facilitate in and out processes from extracellular space into the cells and from cells to plasma or other external fluids (Figure 2). For instance the access of biogenic amines (as cationic agents) from plasma into the cells can be mediated by OCT. Thus histamine may be converted in products that will be eliminated (Figure 1 and Figure 2). Histamine not metabolised can be eliminated via OCT into the intestinal lumen. Cannot be excluded the access of exogenous histamine via OCTs disposed on Enterocyte's tight junctions. In this case, an excess of histamine may be a risk for pseudoallergic phenomena.
Exogenous origin
Histamine may often be present in certain foods, particularly fish, cheese, dairy products, some fruits and fermented food items (some wines, beers, sauerkraut [6,28,29]. It is almost impossible to remove histamine present in food through freezing or cooking [15]. This ingested exogenous histamine can generate a condition called food histaminosis and the symptoms are similar to those induced by allergenic factors; this is why food-histaminosis is considered as a pseudo-allergy with duration of 3-4 days and for which there is no current treatment.
Amine oxidases and physiological catabolism of histamine
The diamine oxidase (DAO, also known as Histaminase), is a copper-enzyme (EC 1.4.3.22) present in different tissues (kidney, placenta) and In intestinal mucosa where is abundant [30]. It regulates cells proliferation via degradation (oxidative deamination) of polyamines (known to be involved in control of protein synthesis, growth, differentiation and cells proliferating mechanism [31,32]. DAO also inactivates the endogenous and exogenous excess of dietary histamine preventing "pseudo-allergic" reactions such as food histaminosis (Figure 2).
Histamine can be metabolised principally by two processes, via oxidative deamination by DAO copper-enzyme or by monoamine oxidase (MAO, a FAD-enzyme) and via histamine N-methyl transferase (HNMT) by histamine methylation [6,33,34]. These mechanisms depend on the localisation of histamine and generate different end products. Stored in secretory granules structures associated to plasma membrane of cells, DAO protein is released in circulation via heparin and by immunitary stimulation inducing degranulation. DAO activity is elevated in intestinal mucosa and villosities, kidney and placenta [6,35]. HNMT is a cytosolic enzyme which inactivates intracellular histamine [15].
Intoxication with histamine
It is a food histaminosis caused by ingestion of a high content of histamine [15,36]. Common symptoms of histaminosis include headache, arrhythmia, tachycardia, nausea, anxiety, abdominal cramps, diarrhea and nasal congestion. Other conditions such as reduction of diamine oxidase activity or the presence of factors inducing degranulation of mast cells can also increase histamine toxicity. For instance, in the case of scombroid fish poisoning, in addition to the high level of histamine, the decomposition process of fish may also release other biogenic amines (cadaverine, putrescine) which can further potentiate toxicity through inhibition of intestinal diamine oxidase [15,37]. In addition to histamine-rich foods, many other foods may increase histamine release via allergenic factors by mast cells degranulation (Figure 1) and/or inhibition of DAO activity. A fragility of duodenal mast cells with elevated degranulation and histamine release was shown in subjects with pseudo allergic history [6].
Role of histamine in enteric dysfunctions
In gastrointestinal tract, histamine is involved in various physiological processes such as inflammatory responses, regulation of intestinal motility and gastric acid secretion [38,39]. It is also implicated in gastrointestinal ailments such as gastric ulcers, Inflammatory Bowel Diseases, ulcerative colitis and food allergies [2].
Histamine and gastric acidity - Due to stimulation by mediators (including histamine and gastrin), parietal cells of stomach secrete gastric acid (hydrochloric acid, pH 1.2-3.8), with the role to solubilize ingested foods and to induce the release of pepsin [40,41]. Histamine released from ECL cells, stimulates gastric acid secretion through activation of histaminic receptors H2 on parietal cells [2,42,43]. Histamine may contribute to the formation of duodenal ulcers by increasing gastric acid release [44,45]. Receptors H2-antagonists are able to inhibit gastric acidity and are used in treatment of peptic ulcers [46-48].
Histamine as mediator of acute inflammatory response-On the endothelium, the histamine-activation of H1-receptors stimulates PLC (Phospholipase C), the key enzyme responsible for the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to 1,2-diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) intracellular messengers. The DAG stimulates protein kinase C (PKC) implicated in vasoconstriction, and IP3 triggers the release of calcium (Ca2+) ions from reticulum endoplasmic to cytosol [49] forming calcium/calmodulin complexes and activating endothelial nitric oxide synthase (eNOS) [50]. The nitric oxide (NO) binds to the sGC (sodium Guanylyl cyclase) increasing the level of cGMP and vasorelaxation. The NO is also oxidized to peroxynitrite anions (ONOO-), which stimulates nuclear factor kappa B (NFKB) to (pro) inflammatory mediators and enhances cyclooxygenase-2 (COX-2) prostaglandins and thromboxane A2 (TX2).
Histamine intolerance
Histamine released from granulocytes and transported in blood stream is usually rapidly inactivated. This mechanism is effective in healthy body but can be disrupted by: i) a strong release of histamine (histamine intoxication) and ii) a low activity of the DAO due to some food contents (biogenic amines) as well as enteric dysfunctions (i.e. intestinal inflammatory conditions and damaged enterocytes, known as related to a low level of DAO). Low DAO activity can also be due to genetic predisposition [15].
Drugs-induced histaminosis
Some drugs have the capacity to inhibit the DAO activity or favour the release of histamine and enhance histamine intolerance [6,51-53].
Inflammatory bowel diseases and current treatment
IBD is a group of disorders (Crohn's disease, Ulcerative colitis) characterised by severe inflammation and potential ulceration of sections of the gastrointestinal tract. In Crohn's disease the inflammation commonly affects the small intestine and/or colon but may attain any area of gastrointestinal tract from mouth to anus. In ulcerative colitis the inflammation is mostly limited to the colon. Both can increase the risk of colorectal cancer. Degranulation and number of mast cells is strongly increased in mucosa of the ileum and of the colon of patients with IBD [54,55].
Histamine released from mast cells and that eliminated from plasma via OCT [27,56] as well as that from food intake may enhance the inflammation level and markedly contribute to severity of IBD [55]. Anti-inflammatory drugs as 5-aminosalicylic acid (5-ASA, mesalazine), corticosteroids, NSAID (non-steroidal anti-inflammatory drugs), immune-suppressor drugs (infliximab, cyclosporine, azathioprine) and antibiotics are currently used for IBD treatment [57]. Furthermore, certain drugs have side's effects such as loss of sense of smell, drowsiness, nausea, vomiting, dizziness and urinary disorders. Some vegetal supplements as curcumin [58,59], Cissampelos glaberrima [60] and recently cannabis [61,62] were found to produce significant clinical benefits to patients with IBD, without side effects. These treatments reduce symptoms, may provide remission for various durations, but still not afford complete recovery (cissaglaberrimine and trilobinine-two alkaloids) are plant antihistaminic.
It is worth to note that in gastrointestinal diseases including Crohn's disease and ulcerative colitis, the level and activity of Diamine oxidase is reduced, leading to a lower degradation and accumulation of histamine (pro-inflammatory factor) [63-65].
Novel Approach of Histaminase: Catalase in Treatment of IBD
The premises of the novel approach are: a) In IBD, the level and activity of Diamine oxidase is reduced, leading to a lower degradation and accumulation of histamine as pro-inflammatory agent [63,65] b) Current treatments target histaminic receptors to reduce allergic effects but cannot reduce the level of histamine in the intestinal lumen; c) An oral Pig-Kidney DAO supplement is commercially available as Histame® or DAOSIN® capsules recommended for food histaminosis and histamine-related intestinal dysfunctions [66].
Recently, a novel approach [67,68] was proposed to treat histamine-related dysfunctions with a vegetal DAO extract from White Pea (Lathyrus sativus). Another recent study on vegetal DAO alone or in combination with catalase, showed that H2O2 produced by DAO and histamine at concentrations higher than 1 mM is toxic to the Caco-2 cells and that in the presence of catalase, the DAO-induced increase of histamine toxicity was abolished [69]. Theseresults support the hypothesis that adding catalase to formulation of DAO will protect against H2O2 produced by DAO (H2O2 may act as pro-oxidant). Catalase is a homo-tetrameric enzyme (EC 1.11.1.6) able to rapidly decompose H2O2 [70].
The novel approach of oral administration of combined DAO and catalase (Figure 3) appears of interest for the treatment of food histaminosis and of histamine-related enteric dysfunctions and also to ameliorate the treatment of IBD: Crohn's disease (particularly ileocolitis, jejunoileitis) and ulcerative colitis [67,71]. Concerning the ammonia by-product of DAO generated during the oxidative desamination it is known that it may play various deleterious effects as Neurotoxic [72] and as an agent causing colon mucosal cell damage [73]. Further studies are needed to evaluate the amounts of NH3 released following the administration of DAO pills related to the amount generated by intestinal bacteria and that eliminated (intestinal absorption and by feces). This approach based on a vegetal DAO associated to Catalase is now in development and the bioactive enzymes will be formulated for oral administration as monolithic tablets conceived for lower intestine and colon delivery [66,67,74].
Acknowledgements
Financial support from Fondation Courtois (Canada) is gratefully acknowledged. Thanks are due to Dr. Pompilia Ispas-Szabo for helpful discussions.
References
- Jutel M, Akdis M, Akdis CA (2009) Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy 39: 1786-1800.
- Benly P (2015) Role of Histamine in Acute Inflammation. J Pharm Sci & Res 6: 373-376.
- Manera M, Giammarino A, Peruqini M, et al. (2008) In vitro evaluation of gut contractile response to histamine in rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Res Vet Sci 84: 126-131.
- Rangachari PK (1992) Histamine: mercurial messenger in the gut. Am J Physiol 262: 1-13.
- Szelag A, Merwid-Lad A, Trocha M (2002) Histamine receptors in the female reproductive system. Part I. Role of the mast cells and histamine in female reproductive system. Ginekol Pol 73: 627-635.
- Maintz L, Novak N (2007) Histamine and Histamine intolerance. Am J Clin Nutr 85: 1185-1196.
- Schwartz JC (1975) Histamine as a transmitter in the brain. Life Sciences 17: 503-517
- Bäumer W, Rossbach K (2010) Histamine as an immunomodulator. J Dtsch Dermatol Ges 8: 495-504.
- Schneider E, Bertron AF, Dy M (2011) Modulation of hematopoiesis through histamine receptor signaling. Front Biosci (Schol Ed) 3: 467-473.
- Molina-Hernández A, Velasco I (2008) Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J Neurochem 106: 706-717.
- Kusche J, Biegański T, Hesterberg R, et al. (1980) The influence of carcinoma growth on diamine oxidase activity in human gastrointestinal tract. Agents Actions 10: 110-113.
- Norrby K (1995) Evidence of a dual role of endogenous histamine in angiogenesis. Int J Exp Pathol 76: 87-92.
- Raithel M, Ulrich P, Hochberger J, et al. (1998) Measurement of gut diamine oxidase activity. Diamine oxidase as a new biologic marker of colorectal proliferation? Ann N Y Acad Sci 859: 262-266.
- Leurs R, Brozius MM, Smit MJ, et al. (1991) Effects of histamine H1-, H2- and H3-receptor selective drugs on the mechanical activity of guinea-pig small and large intestine. Br J Pharmacol 102: 170-185.
- Kovacova-Hanuscova E, Buday T, Gavliakova S, et al. (2015) Histamine, histamine intoxication and intolerance. Allergol Immunopathol (Madr) 43: 498-506.
- Smolinska S, Jutel M, Crameri R, et al. (2014) Histamine and gut mucosal immune regulation. Allergy 69: 273-281.
- Gaudenzio N, Sibilano R, Marichal T, et al. (2016) Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest 126: 3981-3998.
- Proud D, Bailey GS, Naclerio ML, et al. (1992) Tryptase and histamine as markers to evaluate mast cell activation during the responses to nasal challenge with allergen, cold, dry air, and hyperosmolar solutions. J Allergy Clin Immunol 89: 1098-1110.
- Nilsson G, Metcalfe DD, Taub DD (2000) Demonstration that platelet-activating factor is capable of activating mast cells and inducing a chemotactic response. Immunology 99: 314-319.
- Marquardt DL, Parker CW, Sullivan TJ (1978) Potentiation of Mast Cell Mediator Release by Adenosine. J Immunol 120: 871-878.
- Casale TB, Bowman S, Kaliner M (1984) Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: Evidence for opiate and nonopiate receptor participation. J Allergy Clin Immunol 73: 775-781.
- Blunk JA, Schmelz M, Zeck S, et al. (2004) Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo micro dialysis study in human skin. Anesth Analg 98: 364-370.
- Koppert W, Blunk JA, Petersen RJ, et al. (2001) Different patterns of mast cell activation by muscle relaxants in human skin. Anesthesiology 95: 659-667.
- Genovese A, Stellato C, Marsella AC, et al. (1996) Role of Mast Cells, Basophils and Their Mediators in Adverse Reactions to General Anesthetics and Radiocontrast Media. Int Arch Allergy Immunol 110: 13-22.
- Casale TB, Keahey MT, Kaliner M (1986) Exercise- induced anaphylactic syndromes. Insights into diagnostic and pathophysiologic features. JAMA 255: 2049-2053.
- Huston DP, Bressler RB, Kaliner M, et al. (1986) Prevention of mast-cell degranulation by ketotifen in patients with physical urticarias. Ann Intern Med 104: 507-510.
- Ogasawara M, Yamauchi K, Satoh Y, et al. (2006) Recent advances in molecular pharmacology of the histamine systems: organic cation transporters as a histamine transporter and histamine metabolism. J Pharmacol Sci 101: 24-30.
- Bodmer S, Imark C, Kneubühl M (1999) Biogenic amines in foods: Histamine and food processing. Inflamm Res 48: 296-300.
- Silla Santos MH (1996) Biogenic amines: their importance in foods. Int J Food Microbiol 29: 213-231.
- Wolvekamp MC, de Bruin RW (1994) Diamine oxidase: an overview of historical, biochemical and functional aspects. Dig Dis 12: 2-14.
- Heby O (1981) Role of polyamines in the control of cell proliferation and differentiation. Differentiation 19: 1-20.
- Fukudome I, Kobayashi M, Dabanaka K, et al. (2014) Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol 47: 100-107.
- Valen G, Kaszaki J, Szabo I, et al. (1996) Activity of histamine metabolizing and catabolizing enzymes during reperfusion of isolated, globally ischemic rat hearts. Inflamm Res 45: 145-149.
- Klocker J, Mätzler SA, Huetz GN, et al. (2005) Expression of histamine degrading enzymes in porcine tissues. Inflamm Res 54: S54-S57.
- Schwelberger HG, Stalzer B, Maier H, et al. (1998) Expression and cellular localisation of diamine oxidase in the gastrointestinal tract of pigs. Inflamm Res 47: S62-S63.
- Visciano P, Schirone M, Tofalo R, et al. (2014) Histamine poisoning and control measures in fish and fishery products. Front Microbiol 5: 500.
- Preston L (2011) Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 28: 1547-1560.
- Fargeas MJ, Fioramonti J, Bueno L (1989) Involvement of Different Receptors in the Central and Peripheral Effects of Histamine on Intestinal Motility in the Rat. J Pharm Pharmacol 41: 534-540.
- Poli E, Pozzoli C, Corruzi G (2001) Role of histamine H(3) receptors in the control of gastrointestinal motility. An overview. J Physiol Paris 95: 67-74.
- Smith JL (2003) The Role of Gastric Acid in Preventing Food borne Disease and How Bacteria Overcome Acid Conditions. J Food Prot 66: 1292-1303.
- Serfaty-Lacrosnière C, Wood RJ, Voytko D, et al. (1995) Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr 14: 364-368.
- Schubert ML (1999) Regulation of gastric acid secretion. Curr Opinion in Gastroenterology 15: 457.
- Barcocelli E, Ballabeni V (2003) Histamine in the control of gastric acid secretion: a topic review. Pharmacol Res 47: 299-304.
- Troidl H, Lorenz W, Rohde H, et al. (1976) Histamine and peptic ulcer: a prospective study of mucosal histamine concentration in duodenal ulcer patients and in control subjects suffering from various gastrointestinal diseases. Klin Wochenschr 54: 947-956.
- Man WK, Boesby S, Michalowski A, et al. (1990) Histamine, histamine formation capacity and gastrin in cysteamine-induced peptic ulcer. J Exp Pathol 71: 95-104.
- Gledhill T, Howard OM, Buck M, et al. (1983) Single nocturnal dose of an H2 receptor antagonist for the treatment of duodenal ulcer. Gut 24: 904-908.
- Parsons ME (1985) Histamine and the pathogenesis of duodenal ulcer disease. Gut 26: 1159-1164.
- Deakin M, Williams JG (1992) Histamine H2-receptor antagonists in peptic ulcer disease. Efficacy in healing peptic ulcers. Drugs 44: 709-719.
- Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281-312.
- Lantoine F, Iouzalen L, Devynck MA, et al. (1998) Nitric oxide production in human endothelial cells stimulated by histamine requires Ca2+ influx. Biochem J 330: 695-699.
- Sattler J, Hesterberg R, Lorenz W, et al. (1985) Inhibition of human and canine diamine oxidase by drugs used in an intensive care unit: relevance for clinical side effects? Agents Actions 16: 91-94.
- Sattler J, Hesterberg R, Schmidt U, et al. (1987) Inhibition of intestinal diamine oxidase by detergents: a problem for drug formulations with water insoluble agents applied by the intravenous route? Agents Actions 20: 270-273.
- Sattler J, Häfner D, Klotter J, et al. (1988) Food-induced histaminosis as an epidemiological problem: Plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO). Agents Actions 23: 361-365.
- He SH (2004) Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol 10: 309-318.
- Nolte H, Spjeldnaes N, Kruse A, et al. (1990) Histamine release from gut mast cells in patients with Inflammatory Bowel Disease. Gut 31: 791-794.
- Aschenbach JR, Honscha KU, von Vietinghoff V, et al. (2009) Bioelimination of histamine in epithelia of the porcine proximal colon of pigs. Inflamm Res 58: 269-276.
- Triantafillidis JK, Merikas E, Georgopoullos F (2011) Current and emerging drugs for the treatment of inflammatory bowel disease. Drugs Des Devel Ther 5: 185-210.
- Hanai H, Sugimoto K (2009) Curcumin has bright prospects for the treatment of inflammatory bowel disease, Curr Pharm Des 15: 2087-2094.
- Deguchi Y, Andoh A, Inatomi O, et al. (2007) Curcumin prevents the development of dextran sulfate Sodium (DSS)-induced experimental colitis. Dig Dis Sci 52: 2993-2998.
- Alves MF, Scotti MT, Scotti L, et al. (2016) Secondary metabolites from cissampelos, A possible source for new leads with anti-inflammatory activity. PMID: 28029072
- Ahmed W, Katz S (2016) Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol Hepatol 12: 668-679.
- Naftali T, Bar-Lev Schleider L, Dotan I, et al. (2013) Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol 11: 1276-1280.
- D'Agostino L, Daniele B, Pignata S, et al. (1988) Postheparin plasma diamine oxidase in subjects with small bowel disease: Diagnostic efficiency of a simplified test. Digestion 41: 46-54.
- Kuefner MA, Schwelberger HG, Weidenhiller M, et al. (2004) Both catabolic pathways of histamine via histamine-N-methyltransferase and diamine oxidase are diminished in the colonic mucosa of patients with food allergy. Inflamm Res 53: S31-S32.
- Schmidt WU, Sattler J, Hesterberg R, et al. (1990) Human intestianal diamine oxidase (DAO) activity in Crohn's disease: a new marker of disease assessment? Agents Action 30: 267-270.
- Missbichler A, Mayer I, Pongracz C, et al. (2010) 19 Supplementation of enteric coated diamine oxidase improves intestinal degradation of food-borne biogenic amines in case of histamine intolerance. Clinical Nutr Suppl 5: 11.
- Mateescu MA, Calinescu C, Ispas-Szabo P, et al. (2011) Oral enzyme compositions for intestinal delivery. Canadian patent 2831535
- Mondovi B, Fogel WA, Federico R, et al. (2013) Effects of Amine Oxidases in Allergic and Histamine-Mediated Conditions. Recent Pat Inflamm Allergy Drug Discov 7: 20-34.
- Jumarie C, Séïde M, Marcocci L, et al. (2017) Diamine Oxidase from White Pea (Lathyrus sativus) Combined with Catalase Protects the Human Intestinal Caco-2 Cell Line from Histamine Damage. Appl Biochem Biotechnol doi: 10.1007/s12010-016-2390-2393
- Scibior D, Czeczot H (2006) [Catalase: structure, properties, functions]. Postepy Hig Med Dosw (Online) 60: 170-180.
- Calinescu C, Mondovi B, Federico R, et al. (2012) Carboxymethyl starch: Chitosan monolithic matrices containing diamine oxidase and catalase for intestinal delivery. Int J Pharm 428: 48-56.
- Albrecht J (1998) Roles of neuroactive amino acids in ammonia neurotoxicity. J Neurosci Res 51: 133-138.
- Lin HC, Visek WJ (1991) Colon mucosal cell damage by ammonia in rats. The Journal of nutrition 121: 887-893.
- Blemur L, Le Tien C, Marcocci L, et al. (2016) Carboxymethyl starch/alginate microspheres containing diamine oxidase for intestinal targetging. Biotechnol Appl Biochem 63: 344-353.
Corresponding Author
Mircea Alexandru Mateescu, Department of Chemistry, Université du Québec à Montreal, CP 8888, Branch A, Montreal, Québec, H3C 3P8, Canada.
Copyright
© 2017 Mateescu MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.