Vero-Toxigenic Escherichia Coli (VTEC): To Sum up all we know
Verotoxigenic Escherichia coli renamed after detection of production ability of cytotoxin which kills Vero cells, a continuous cell line derived from the kidney of the African green monkey (Chlorocebus sp). These toxins are proved to cause severe diseases in humans. As an emerging food borne pathogen group, VTEC infection may lead to life threatening haemolytic-uremic syndrome (HUS). The occurrence of HUS is between 3-20% of sporadic and epidemic cases. The pathogens are found in different food matrices and may sometimes hide in plants. VTEC is an emerging topic for food microbiology. Many authors contribute this area with new studies. This review aims to summarize the VTEC.
Keywords
HUS, VTEC, TTP, Stx, Regulation
Subject Classification Codes
Microbiology, Food borne diseases, Emerging food borne pathogens
Introduction
Escherichia coli (E. coli) is a species of gram-negative facultative cylindrical rods, and usually motile. [1] Commensal E. coli bacteria are often found among the gut flora of warm-blooded animals [2]. E. coli are divided into groups according to serotypes based on the different surface antigens of the bacteria, which can be detected phenotypic or genotypic methods [3-5]. The O-antigen is the outer polysaccharide portion of lipopolysaccharide (LPS) found in the cell wall of gram-negative bacteria. The H-antigen shows flagellar proteins. E. coli may have K- (capsule) and F- (fimbrial) antigens [1]. Paragraph: use this for the first paragraph in a section, or to continue after an extract.
Although main proportion of E. coli strains is accepted as pathogen there is an exception of Nissle 1917 strain. This strain was isolated by Professor Alfred Nissle in 1917 during the World War I. Since, the strain was named after him as Escherichia coli Nissle 1917 and accepted as probiotic strain. It has been clinically studied and has been reviewed for over 80 years to prevent and treat an assortment of gastrointestinal disorders.
Pathotypes of Escherichia coli
Regulation of existing combined virulence factors gives E. coli the ability to trigger a specific disease in host. E. coli expressing these combined virulence factors are accepted as belonging to "pathotypes", characterized by the disease they cause [6]. Exceptional strains are widely observed but this grouping scheme makes the E. coli more comprehensive [7]. There are six major E. coli pathotypes causing diarrhoeal disease: enterohaemorrhagic (EHEC), enteropathogenic (EPEC), enterotoxigenic (ETEC), enteroaggregative (EAEC/EAggEC), enteroinvasive (EIEC) and diffusely adherent E. coli (DAEC). In addition, extraintestinal infections are caused by ExPEC, including uropathogenic E. coli (UPEC) and meningitis-associated E. coli (MNEC) [6].
Emerging Shiga-toxigenic Escherichia coli (STEC)/Vero-toxigenic Escherichia coli (VTEC)
EHEC pathotype is the most severe pathotype which is capable of leading fatal complications in healthy infected hosts. Subset Shiga-toxigenic Escherichia coli (STEC)/Vero-toxigenic Escherichia coli (VTEC) are characterized by the presence of genes encoding shigatoxins or verotoxins. The subset of VTEC causes haemorrhagic colitis in humans like the EHEC pathotype, all VTEC include a subset that are usually known as EHEC. In addition to the verotoxin itself, most EHEC carry the locus of enterocyte attachment and effacement (LEE), have some form of large virulence plasmid, and an additional repertoire of adhesion factors, toxins and secreted effector proteins. As there is no phylogenetic linearity between all VTEC only the acquired DNA is used as a marker for determination of the pathotype [8,9]. Most VTEC found in gut microflora of ruminants naturally, and genetic factors cause virulence in the human frequently play a role in colonization of cattle [10].
VTEC serotypes are different than each other with respect to occurrence and symptoms. Serotyping is mainly made using O and H antigens. The outer membrane of an E. coli cell contains lipopolysaccharide (LPS) molecules, that carry; O antigen, a polymer of immunogenic repeating oligosaccharides (1-40 units), Core region of phosphorylated nonrepeating oligosaccharides and Lipid A (endotoxin). The O antigen is used for serotyping E. coli and this O group designations start with O1 and end with O181. Some groups have been removed which are O31, O47, O67, O72, O93 (now K84), O94, and O122; and groups 174 to 181 are provisional (O174 = OX3 and O175 = OX7) or are under investigation (176 to 181 are STEC/VTEC). Moreover, many O groups have subtypes (e.g. O128ab and O128ac). The O antigen is encoded by the rfb gene cluster and rol (cld) gene encodes the regulator of lipopolysaccharide O-chain length. The H antigen is one of the major component of flagella, have role in E. coli movement. It is mainly encoded by the fliC gene. H antigens have 53 identified groups changing between H1 to H56 (H13 and H22 were not E. coli antigens but from Citrobacter freundii, and H50 was found to be the same as H10) [11,12] Serotypes causing severe disease in humans include O26:H11, O103:H2, O145:NM, O111:NM, O121:H19 and O104:H4 [13,14]. The serotype O157:H7, which is the most common source of EHEC outbreaks and cases in many countries [14].
Pathogenicity
Verotoxin or shiga-like toxin: Konowalchuk, et al. [15] are first to show that certain strains of E. coli produced a cytotoxin which kills vero cells, a continuous cell line derived from the kidney of the African green monkey (Chlorocebus sp). Since it kills vero cell lines, the toxin named as "verocytotoxin" or "verotoxin". Other alternative names are also called such as "shiga-like toxin" or simply "shiga toxin" due to high degree of similarity between verotoxin and the toxins produced by Shigella spp. this terminology lead a synonymous calling as VTEC or STEC. Thorpe, et al. [16] reported that stx is capable of interleukin-8 (IL-8) superinduction in a human colonic epithelial cell line. Despite a general blockade of mRNA translation, Stx treatment results in increased IL-8 mRNA as well as increased synthesis and secretion of IL-8 protein.
Verotoxin function: Verotoxins are classified under AB5 family, consisting of a single A subunit encoded by the vtx2A gene, and five non-covalent bounds associate with five B subunits encoded by vtx2B. AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria which cause human diseases like cholera, dysentery, and hemolytic-uremic syndrome. These toxins have a similar structure and same mechanism for entering targeted host cells. The cytotoxic effect of verotoxin is based on ribosomal inactivation. The B subunits have binding sites for the trisaccharide side chain of glycosphingolipid globotriaosylceramide (Gb3). Gb3 is present on the outer surface of several mammalian cell types. As toxin bound to Gb3, endocytosis occurs which leads the toxin entrance. The toxins route to cytoplasm completes by the help of Golgi apparatus and the endoplasmatic reticulum. As it activates by host cell proteases, the toxin is now able depurinate an adenine in the peptidyltransferase centre of the 28S rRNA, which is a critical component in the eukaryotic ribosome. This leads a blockage of elongation factor and tRNA binding, destroying the ribosome's protein synthesis ability [17]. Verotoxin is so potent that a single molecule can kill a cell and it not only inhibits the protein synthesis but also induces inflammatory pathways of apoptosis [18,19]. The higher Gb3 densities on cell surfaces, the more sensitivity to verotoxin expressed. Most cells in the human intestinal epithelium have little or no Gb3 receptors. On the contrary, human renal tubule cells, with their high Gb3 levels, prove the kidney damage associated with severe VTEC infection [18]. In a study, Exeni, et al. [20] reported that post-diarrheal hemolytic uremic syndrome (HUS) related to endothelial and epithelial cell damage induced by Shiga toxin (Stx), through an interaction with its globotriaosylceramide (Gb3) receptor. Although, Stx is the main pathogenic factor and necessary for HUS development, clinical and experimental evidence suggest that the inflammatory response is able to potentiate Stx toxicity. Lipopolysaccharides (LPS) and neutrophils (PMN) represent two central components of inflammation during a Gram negative infection. In this regard, patients with high peripheral PMN counts at presentation have a poor prognosis.
Verotoxin types: Two major types of verotoxin (VT) is identified as VT1 and VT2 which are encoded by vtx1 and vtx2 genes respectively. Each of these types includes several subtypes with assigned letters (e.g. VT1a, encoded by vtx1a).
Correlation between verotoxin types and severity of symptoms: It is shown that, the different verotoxin variants are found to be associated with lower or higher risk of severe symptoms for humans. To strengthen this evidence, several studies on O157 and non-O157 VTEC strains carrying VT2a alone or in combination with VT2c than other variants or combinations of variants, have shown in HUS patients more often [21-23]. On the contrary, detection of these verotoxin genes alone is not considered to be sufficient to determine the pathogenic potential of a VTEC strain [13]. It has been shown that verotoxin genes found in VTEC are encoded on lambdoid prophages inserted in the bacterial chromosome and vtx gene expression is regulated by phages borne genetic material [24]. In a study, it is shown that proinflammatory cytokine mRNAs, especially IL-8, were induced by Stx1 and Stx2 in Caco-2 cells. A non-toxic mutant of Stx1 which lacks N-glycosidase activity did not induce cytokine mRNAs. IL-8 production at the protein level was enhanced by Stx1 and Stx2, but not by the mutant Stx1. These results demonstrate that Shiga toxins induce expression and synthesis of cytokines in Caco-2 cells and their N-glycosidase activity is essential for the induction [25].
The locus of enterocyte attachment and effacement (LEE): The locus of enterocyte attachment and effacement (LEE) is a pathogenicity island found in most VTEC. The LEE helps VTEC to lead attaching-effacing (A/E) lesions in the host intestines. A/E lesions are typical by loss of enterocyte microvilli and tight attachment of VTEC bacteria to the enterocyte membranes on pedestals built by rearrangement of the host cell cytoskeleton [26]. A/E lesions result in disruption of the intestinal epithelium by the cytoskeletal structure changes, decreasing of tight junction integrity, and increase in apoptosis of epithelial cells [27]. This intimin-based mechanism of cell adhesion contributes to the diarrhoea in VTEC infections [28]. LEE encodes intimin protein which is an adhesion protein, a translocated intimin receptor (Tir), the components of type III secretion system (T3SS), a number of accessory genes and effector proteins transferred to the target cell via the secretion system [8]. VTEC infections caused by LEE-negative VTEC [13], especially the strain of VTEC/EAEC O104:H4, which led an outbreak in several European countries in 2011 with more than 4000 cases and 54 deaths [7] are significant for public health.
The O157:H7 large virulence plasmid (po157): Pathogenic E. coli frequently carry some of their genetic virulence determinants on plasmids [29]. Most VTEC serotypes are known to include one or more large plasmids encoding a set of virulence factors like haemolysin [8]. The O157:H7 large virulence plasmid pO157 plasmid carries genes encoding a kind of EHEC haemolysin that cause lysis of human microvascular endothelial cells and apoptosis, and it is likely to contribute to O157:H7 pathogenesis [30]. The plasmid is also in charge of putative virulence factors genes like, cytotoxin B (toxB), serine protease (espP), catalase/peroxidase (katP) [31], and some major components of a type II secretion system [32].
Other virulence factors: Cytolethal distending toxin (CDT) which is also found in Campylobacter jejuni [33], causes cell cycle arrest during G2 phase of mitosis and leads apoptosis in eukaryotic cells, and has been identified to exist in VTEC [34]. Several adhesion factors including fimbriae encoded in genes outside the LEE are identified in O157:H7 that provide initial attachment to the host cell before pedestal formation is initiated [35]. The type III secretion system (TTSS) of enteropathogenic Escherichia coli (EPEC) has been associated with the ability of these bacteria to induce secretion of proinflammatory cytokines, including interleukin-8 (IL-8), in cultured epithelial cells. However, the identity of the effector molecule directly involved in this event is unknown. In a study, we determined that the native flagellar filament and its flagellin monomer are activators of IL-8 release in T84 epithelial cells [36].
Characteristics of Infection
Minimal infectious dose of VTEC O157:H7 is as low as 100 cfu/g [37]. After ingested bacteria needs 3-4 days of incubation [38]. Infection patterns changes from undiagnosed to severe disease like O157:H7 [39]. If symptoms occur, patients will mostly experience watery diarrhoea which can resolve spontaneously in about a week, or progress to bloody diarrhoea, stomach cramps and more rarely fever [40]. Even this is an infection, antibiotic treatment is not recommended due to massive toxin release by the dead bacteria of concern. Generally, intravenous volume expansion and screening for complications is preferred [41,42].
Hemolytic Uremic Syndrome (HUS)
As an emerging food borne pathogen group, VTEC infection may lead life threatening haemolytic-uraemic syndrome (HUS). The occurrence of HUS is between 3-20% of sporadic and epidemic cases, respectively. Young especially, children up to 5 years, immunosuppressed, or elder patients are more likely to develop infection [43]. HUS is typical with a combination of microangiopathic haemolytic anaemia, thrombocytopenia, and acute kidney failure. Verotoxins not only induce cell death but also cause local inflammation in vascular epithelium, decrease in thromboresistance and complement activation. Ischemia is the result of restricted blood flow caused by thrombi formation in capillaries and arterioles. Erythrocytes fragment during their passage through the partially blocked vessels, resulting haemolytic anaemia. Verotoxin targets the Gb3 receptors which results damage in renal endothelium. Gb3 receptors are also found massively in brain and as verotoxins spread neurological symptoms like seizures, stroke or coma occur in around 25% of patients [44]. Neurologic deformation is the main cause of death [45]. Mortality is around 1-4% of HUS cases which has decreased from 30% with dialysis therapy [44,46]. Long term sequela develops in 20-40% of HUS patients.
Thrombotic thrombocytopenic purpura (TTP), mainly a disease of adults, a disorder with many overlapping clinical features with classic HUS, is known to occur due to either a congenital absence (Upshaw- Schulman-Syndrome) of von Willebrand factor (vWF) cleaving protease enzyme (ADAMTS 13) activity, or to an acquired IGg antibody directed against this enzyme. Recently, an additional cause has been discovered, namely novel mutations that either reduce vWF synthesis or result in abnormal mRNA that reduces vWF activity during severe episodes to less than 3% of normal [47].
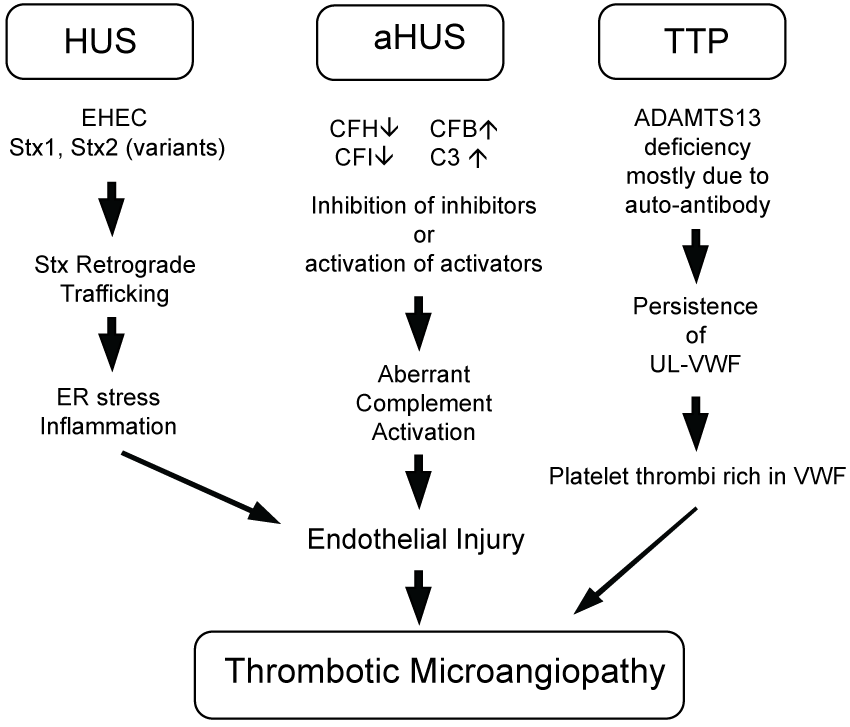
Microangiopathic haemolytic anaemia is one of the major determinant of HUS and characterized by negative Coombs' test. Haemoglobin values changes between 5.3 to 6.9 g/dL, peripheral blood smear shows up to 10% damaged red blood cells (schistocytes), mostly burr or helmet cells, which is result of mechanical trauma of the red blood cells in the vasculature. Increased lactate dehydrogenase is the most sensitive index of ongoing haemolysis. Additional findings include increased bilirubin level (mainly indirect), reticulocytosis and sharp decrease in haptoglobin levels [48,49]. Thrombocytopenia is another significant determinant. Platelet counts range between 25 × 109 to 55 × 109 cells/L.Verotoxin in E. coli O157:H7 decreases prostacyclin synthesis by endothelial cells and promotes platelet aggregation. Consequently, intravascular thrombi form, mainly in renal vessels, leads a reduction in platelet count [48,50]. Elevated transaminases as well as laboratory findings associated with renal failure are common. Serum levels of fibrin degradation product-E have been reported to be significantly increased in patients with bloody diarrhoea who ultimately developed HUS. Therefore, it is suggested that raised serum fibrin degradation product-E level may be a useful marker of HUS in the clinical setting [51]. Thrombotic Microangiopathy can result from different molecular pathways. Hemolytic uremic syndrome (HUS), atypical HUS (aHUS) and thrombotic thrombocytopenic purpura (TTP) have shared clinical manifestations, but differing molecular etiologies. EHEC-related HUS initiated by bacterial Stx injures endothelial cells by inducing endoplasmic reticulum (ER) stress responses and transcription events which include generation of inflammatory cytokines and chemokines. Endothelial injury and a pro-thrombotic environment in aHUS results from genetic mutations in complement pathway members and aberrant activation (complement factors H, IB, 3: CFH, CFI, CFB, C3). Coagulopathy during TTP results from inherited or immune-acquired deficiency in a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), needed to cleave von Willebrand Factor (VWF) released from endothelial cells to prevent accumulation of prothrombotic ultra-large VWF (UL-VWF) oligomers [52]. This is summarized in figure 1.
Zoonosis
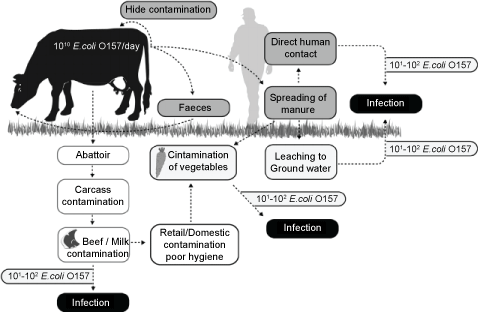
VTEC with its zoonotic character leads haemorrhagic colitis and HUS in humans. Many different animal species are identified as reservoirs for VTEC, but the most important animal source of human cases of infections with zoonotic VTEC is cattle [53,54]. Animals, particularly ruminants such as the cow, are usually colonized by organisms that are clearly pathogenic in humans and no infection symptoms are shown by these animals. In cows the carriage of O157 changes with age and feeding protocols. These organisms locate to mucosal lymphoid tissue in the rectum of the cow [55]. Reservoir animals excrete pathogens with feces. The level of excretion is important due to the risk of fecal contamination in all potential contamination routes. Thus, cattle persistently shedding VTEC, particularly at high numbers, give rise to a higher prevalence of VTEC in the farm environment, creating a higher risk of contaminated food products than cattle with occasional shedding [56,57]. The contamination of meat and milk products has been an emerging subject for food hygiene, noticed by well-publicised outbreaks resulting from undercooked ground beef, which is not the most common route for human exposure, as reported. VTEC shed on to pastureland survive over a wide range of temperatures and pHs and resist composting. They persist in soil; they can easily contaminate surface water. Fresh produces and domestic water supplies are contaminated. Additionally, farm visits is an important risk for visiting children [58]. After contamination of the soil, E. coli O157:H7 can become internalized within the root tissue of growing plants and subsequently can be transported via the vascular system to the aerial leaf tissue [59]. This internalization can limit the efficacy of postharvest wash regimes to combat pathogens of concern [60]. However, in some studies internalization of E. coli O157:H7 was restricted to seedlings and was less commonly associated with mature plants [61,62]. The routes of contamination are summarized in figure 2 [53].
Global Status of the VTEC Infections in Developed Countries
The incidence of zoonotic VTEC infections and shedding of VTEC from cattle have been clearly reported to be seasonal. Unsurprisingly, in industrialised countries, there is a seasonal pattern to the incidence of HUS, being greater in summer than in winter. This probably reflects increased exposure to countryside, animals and fresh uncooked foods. Bathing in contaminated water is an additional risk [57,62,63].
Two developed examples will be discussed to underline the emerging status of VTEC globally.
European union
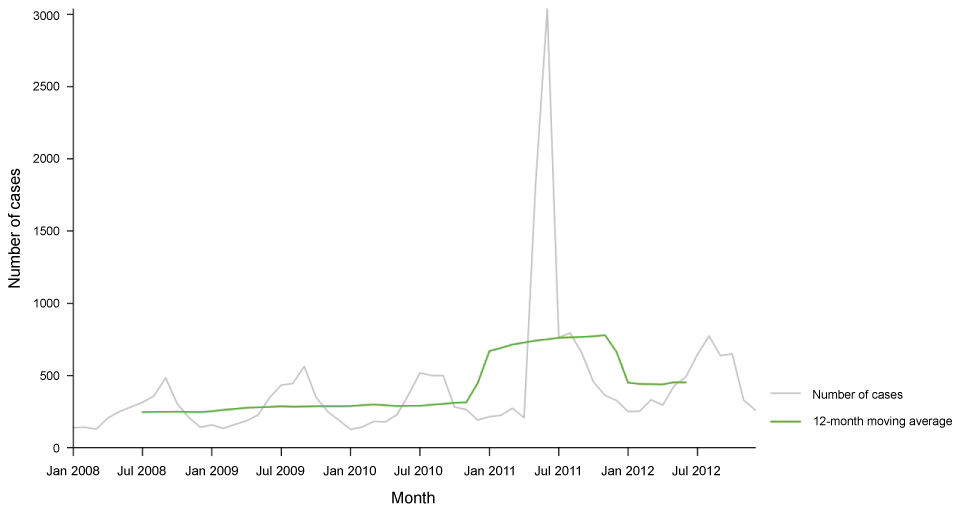
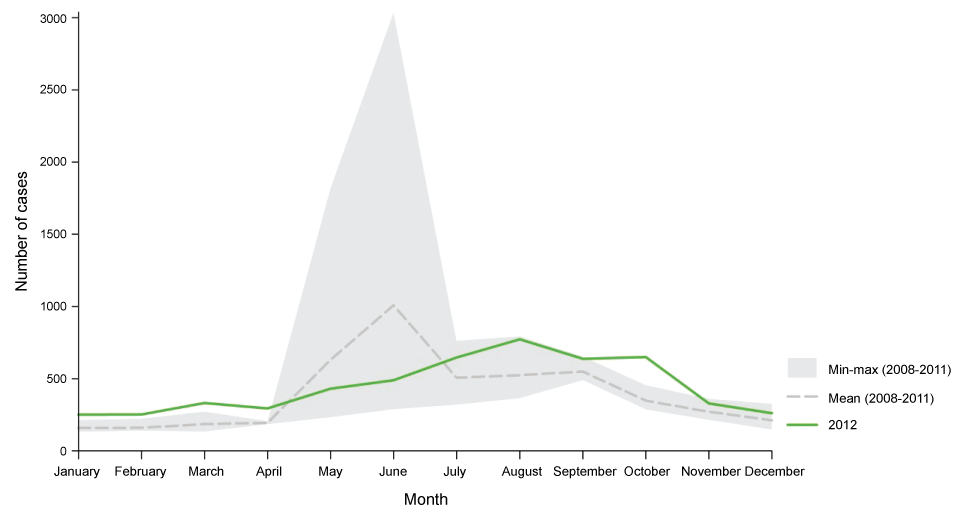
In a recent report published by ECDC (2014) it is declared that, VTEC infection increased in the EU/EEA in the five-year period 2008-2012. The number of confirmed VTEC cases was 5 748 and the overall notification rate was 1.5 cases per 100 000 population in the EU and EEA countries in 2012. The highest notification rate was in children aged 0-4 years, 7.6 cases per 100 000 population in both genders. The report includes a change in infection rate decreased by 66% with compared to 2011 which was the year of large VTEC O104:H4 occurs, but increased by 36% when comparing with years 2009 and 2010. The most commonly reported O-serogroups were O157 and O26 and 7% (382) of the confirmed VTEC cases developed haemolytic uremic syndrome (HUS). Figure 3 shows distribution of confirmed STEC/VTEC reported cases by month, EU/EEA, 2008-2012 and figure 4 shows distribution of confirmed STEC/VTEC reported cases by month in 2012 compared with 2008-2011 data, EU/EEA [62].
Additionally, In 2013, a total of 73 outbreaks caused by VTEC were reported, whereof 12 were supported by strong evidence. The main food vehicle was bovine meat and products thereof, followed by 'Vegetables and juices and other products thereof' and cheese [64].
United states
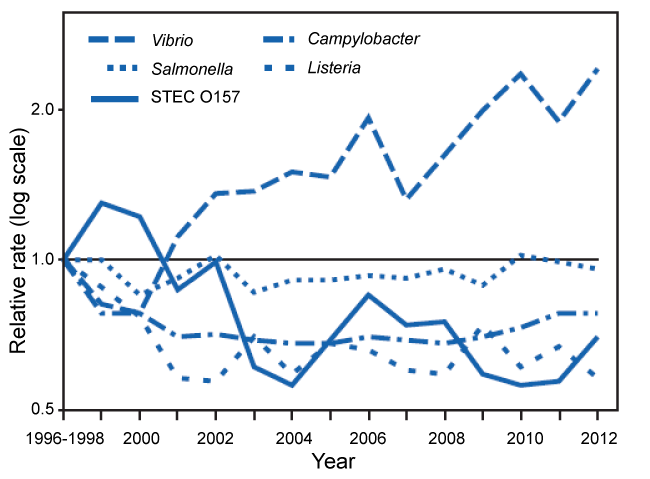
The summary incidence and trends of Foodborne Diseases between 1996 and 2010 reported by [65] indicates that contaminated food consumed in the United States leads an estimated 48 million illnesses, 128,000 hospitalizations, and 3,000 deaths annually [66,67]. The societal cost of a single fatal case of E. coli (STEC) O157 infection has been estimated at $7 million [68]. In 2009, FoodNet identified 66 children with HUS (0.59 cases per 100,000), of whom one died and 38 (58%) were aged < 5 years (1.2 case per 100,000). Compared with 2006-2008, the incidence was significantly lower for children aged < 5 years (36% decrease; CI = 7%-56%) but not significantly different for all children. In figure 5 it is clear that a decrease in VTEC O157 achieved. This decrease is reported to be achieved by a decrease in HUS. The major way to combat the pathogen of concern is reported as improved detection and investigation of STEC O157 outbreaks, resulting not only in contaminated products being removed before more persons became ill but also in enhanced knowledge about preventing contamination that was used to prevent future outbreaks and illnesses. Additionally, cleaner slaughter methods, microbial testing, and better inspections in ground beef processing plants [69]; regulatory agency prohibition of contamination of ground beef with STEC O157 (resulting in 234 beef recalls since STEC O157 was declared an adulterant in ground beef in 1994); improvements in the FDA model Food Code [70]; and increased awareness in food service establishments and consumers' homes of the risk of consumption of undercooked ground beef [65,71]. The figure 5 summarizes the report of Gillis, et al. [65].
Antibiotic Resistance of VTEC
Resistance patterns and geographical distribution of antimicrobial resistance of VTEC is summarized by Koluman and Dikici [72]. It is contra-indicated to apply antibiotics to VTEC infections [73]. Antimicrobial resistance starts from farm and it is common in VTEC serotypes, including multiple drug resistance to streptomycin, sulfisoxazole, and tetracycline [74,75], and there is some evidence that resistance may be increasing over time [74,76]. In a study held by Mora, et al. [75] a higher rate of antimicrobial resistance is recorded in E. coli O157 bovine strains compared to human strains. In a recent study, [77] it is indicated that the ketolide that has accumulated in epithelial cells may traffic back into the bacteria via the type III secretion system (T3SS). Considering that, nontoxic members of this class of antibiotics, such as solithromycin, should be considered for future testing and trials evaluating their use for treatment of EHEC infections.
Regulations Concerning the VTEC in Food
In European Union Commission Regulation EC 2073/2005 have the standards for the microbiological criteria of food. In this regulation it is replaced in the section 14 as: "The-Scientific Committee on Veterinary Measures relating to Public Health (SCVPH) issued an opinion on verotoxigenic E. coli (VTEC) in foodstuffs on 21 and 22 January 2003. In its opinion it concluded that applying an end-product microbiological standard for VTEC O157 is unlikely to deliver meaningful reductions in the associated risk for the consumers. However, microbiological guidelines aimed at reducing the faecal contamination along the food chain can contribute to a reduction in public health risks, including VTEC. The SCVPH identified the following food categories where VTEC represents a hazard to public health: raw or undercooked beef and possibly meat from other ruminants, minced meat and fermented beef and products thereof, raw milk and raw milk products, fresh produce, in particular sprouted seeds, and unpasteurised fruit and vegetable juices" [78]. This underlines the importance of indicator organism for faecal contamination. However, many member states continues to control food matrices for VTEC and isolates are send to Istituto Superiori di Sanita (ISS) which is European Union Reference Laboratory for VTEC for further analyzes. In 2012, European Union's the Directorate-General Health and Consumers of the European Commission decided to initiate the Union-wide collection of molecular typing data for isolates of Salmonella, Listeria and Verocytotoxin-producing E. coli from food and animals [3]. Also with the hard works of ISS a new established method is published for detection of VTEC [79]. This identification method is comprehensive and will be an additional value for future studies, however application of the method needs sophisticated laboratory experience and machinery.
Conclusion and Future Marks
VTEC is an emerging subject for food microbiology. We only know for several decades. We recently started to isolate and identify them. We hardly recognize the pathogenic mechanisms and changes of these mechanisms according to the serotype. There is a long path to discover and surely this path is not an easy one. To combat VTEC is important for additional value in public health and slow down the economic burden caused by VTEC infections. Regulations concerning VTEC should be based on risk assessment results. VTEC should be controlled with zero tolerance policy. An easier screening method should be developed and these methods should be applied during the processing. ISO 22000 standards should be applied widespread in all food producing environments.
Declaration of Interest
The authors have no conflict of interest to declare.
Acknowledgement
We started the journey of science with the powerful light of Rumi's saying "What word that belongs to yesterday, is gone with yesterday, and now is the time to say new things".
References
- Greenwood D, Slack RCB, Peutherer JF (2002) Medical microbiology. Elsevier Science.
- Eckburg PB, Bik EM, Bernstein CN, et al. (2005) Diversity of the human intestinal microbial flora. Science 308: 1635-1638.
- Caprioli A, Maugliani A, Michelacci V, et al. (2014) Molecular typing of Verocytotoxin producing E coli VTEC strains isolated from food, feed and animals: state of play and standard operating procedures for pulsed field gel electrophoresis PFGE typing, profiles interpretation and curation. EFSA supporting publication 2014: 704-755.
- Madic J, Peytavin de Garam C, Vingadassalon N, et al. (2010) Simplex and multiplex real-time PCR assays for the detection of flagellar (H-antigen) fliC alleles and intimin (eae) variants associated with enterohaemorrhagic Escherichia coli EHEC serotypes O26:H11 O103:H2 O111:H8 O145:H28 and O157:H7. J Appl Microbiol 109: 1696-1705.
- Gannon VP, D'Souza S, Graham T, et al. (1997) Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J Clin Microbiol 35: 656-662.
- Kaper JB, Nataro JP, Mobley HL (2004) Pathogenic Escherichia coli. Nat Rev Microbiol 2: 123-140.
- Karch H, Denamur E, Dobrindt U, et al. (2012) The enemy within us: lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO Mol Med 4: 841-818.
- Ogura Y, Ooka T, Iguchi A, et al. (2009) Comparative genomics reveal the mechanism of the parallel evolution of O157 and non-O157 enterohemorrhagic Escherichia coli. Proc Natl Acad Sci USA 106: 17939-17944.
- Ogura Y, Ooka T, Whale A, et al. (2007) TccP2 of O157:H7 and non-O157 enterohemorrhagic Escherichia coli EHEC: challenging the dogma of EHEC-induced actin polymerization. Infect Immun 75: 604-612.
- Sheng H, Lim JY, Knecht HJ, et al. (2006) Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect Immun 74: 4685-4693.
- Brenner DJ, Noel R Krieg, James T, et al. (2005) The Gammaproteobacteria. Bergey's Manual of Systematic Bacteriology. (2nd edn), Williams & Wilkins, New York.
- Wang L, Rothemund D, Curd H, et al. (2003) Species-Wide Variation in the Escherichia coli Flagellin (H-Antigen) Gene. J Bacteriol 185: 2396-2943.
- (2013) Scientific Opinion on VTEC-seropathotype and scientific criteria regarding pathogenicity assessment. EFSA Journal 11: 3138.
- Karmali MA, Mascarenhas M, Shen S, et al. (2003) Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J Clin Microbiol 41: 4930-4940.
- Konowalchuk J, Speirs JI, Stavric S (1977) Vero response to a cytotoxin of Escherichia coli. Infect Immun 18: 775-779.
- Thorpe CM, Hurley BP, Lincicome LL, et al. (1999) Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun 67: 5985-5993.
- Walsh MJ, Dodd JE, Hautbergue GM (2013) Ribosome-inactivating proteins: potent poisons and molecular tools. Virulence 4: 774-784.
- Tesh VL (2010) Induction of apoptosis by Shiga toxins. Future Microbiol 5: 431-453.
- Tam PJ, Lingwood CA (2007) Membrane cytosolic translocation of verotoxin A1 subunit in target cells. Microbiology 153: 2700-2710.
- Exeni RA, Fernandez GC, Palermo MS (2007) Role of polymorphonuclear leukocytes in the pathophysiology of typical hemolytic uremic syndrome. ScientificWorldJournal 7: 1155-1164.
- Brandal LT, Wester AL, Lange H, et al. (2015) Shiga toxin-producing Escherichia coli infections in Norway, 1992-2012: characterization of isolates and identification of risk factors for haemolytic uremic syndrome. BMC Infect Dis 15: 324.
- Persson S, Olsen KE, Ethelberg S, et al. (2007) Subtyping method for Escherichia coli shiga toxin verocytotoxin 2 variants and correlations to clinical manifestations. J Clin Microbiol 45: 2020-2024.
- Friedrich AW, Bielaszewska M, Zhang WL, et al. (2002) Escherichia coli harboring Shiga toxin 2 gene variants: frequency and association with clinical symptoms. J Infect Dis 185: 74-84.
- Herold S, Karch H, Schmidt H (2004) Shiga toxin-encoding bacteriophages-genomes in motion. Int J Med Microbiol 294: 115-121.
- Yamasaki C, Natori Y, Zeng XT, et al. (1999) Induction of cytokines in a human colon epithelial cell line by Shiga toxin 1 (Stx1) and Stx2 but not by non-toxic mutant Stx1 which lacks N-glycosidase activity. FEBS Lett 442: 231-234.
- Jores J, Rumer L, Wieler LH (2004) Impact of the locus of enterocyte effacement pathogenicity island on the evolution of pathogenic Escherichia coli. Int J Med Microbiol 294: 103-113.
- Serna AT, Boedeker EC (2008) Pathogenesis and treatment of Shiga toxin-producing Escherichia coli infections. Curr Opin Gastroenterol 24: 38-47.
- Schmidt MA (2010) LEEways: tales of EPEC ATEC and EHEC. Cell Microbiol 12: 1544-1552.
- Johnson TJ, Nolan LK (2009) Pathogenomics of the virulence plasmids of Escherichia coli. Microbiol Mol Biol Rev 73: 750-774.
- Bielaszewska M, Aldick T, Bauwens A, et al. (2014) Hemolysin of enterohemorrhagic Escherichia coli: structure, transport, biological activity and putative role in virulence. Int J Med Microbiol 3045: 521-529.
- Burland V, Shao Y, Perna NT, et al. (1998) The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Res 26: 4196-4204.
- Schmidt H, Henkel B, Karch H (1997) A gene cluster closely related to type II secretion pathway operons of gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol Lett 148: 265-272.
- Koluman A (2010) Detection of Campylobacter jejuni Contamination in Poultry Houses and Slaughterhouses. Turk Hij Den Biyol Derg 67: 57-64.
- Heywood W, Henderson B, Nair SP (2005) Cytolethal distending toxin: creating a gap in the cell cycle. J Med Microbiol 54: 207-216.
- Farfan MJ, Torres AG (2012) Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect Immun 80: 903-913.
- Zhou X, Giron JA, Torres AG, et al. (2003) Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect Immun 71: 2120-2129.
- Tilden J Jr, Young W, McNamara AM, et al. (1996) A new route of transmission for Escherichia coli: infection from dry fermented salami. Am J Public Health 86: 1142-1145.
- Bell BP, Goldoft M, Griffin PM, et al. (1994) A multistate outbreak of Escherichia coli O157:H7-associated bloody diarrhea and hemolytic uremic syndrome from hamburgers The Washington experience. JAMA 272: 1349-1353.
- Sartz L ,De Jong B, Hjertqvist M, et al. (2008) An outbreak of Escherichia coli O157:H7 infection in southern Sweden associated with consumption of fermented sausage aspects of sausage production that increase the risk of contamination. Epidemiol Infect 136: 370-380.
- Yoon JW, Hovde CJ (2008) All blood no stool: enterohemorrhagic Escherichia coli O157:H7 infection. J Vet Sci 9: 219-231.
- Goldwater PN, Bettelheim KA (2012) Treatment of enterohemorrhagicEscherichia coli EHEC infection and hemolytic uremic syndrome HUS. BMC Medicine 10: 12.
- Karch H, Tarr PI, Bielaszewska M (2005) Enterohaemorrhagic Escherichia coli in human medicine. Int J Med Microbiol 295: 405-418.
- Mead PS, Griffin PM (1998) Escherichia coli O157:H7. Lancet 352: 1207-1212.
- Mele C, Remuzzi G, Noris M (2014) Hemolytic uremic syndrome. Semin Immunopathol 36: 399-420.
- Trachtman H, Austin C, Lewinski M, et al. (2012) Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol 8: 658-669.
- Spinale JM, Ruebner RL, Copelovitch L, et al. (2013) Long-term outcomes of Shiga toxin hemolytic uremic syndrome. Pediatr Nephrol 28: 2097-2105.
- Siegler R, Oakes R (2005) Hemolytic uremic syndrome pathogenesis treatment and outcome. Curr Opin Pediatr 17: 200-204.
- Amirlak I, Amirlak B (2006) Haemolyticuraemic syndrome: An overview. Nephrology (Carlton) 11: 213-218.
- Ruggenenti P, Remuzzi G (1998) Pathophysiology and management of thrombotic microangiopathies. J Nephrol 11: 300-310.
- Karch H, Bitzan M, Pietsch R, et al. (1988) Purified verotoxins of Escherichia coli O157:H7 decrease prostacyclin synthesis by endothelial cells. Microb Pathog 5: 215-221.
- Aihara Y, Nakamura T, Unayama T, et al. (2000) Usefulness of serum fibrinogen degradation product-E in sporadic cases of classical hemolytic uremic syndrome. Pediatr Int 42: 523-527.
- Mayer CL, Leibowitz CS, Kurosawa S, et al. (2012) Shiga Toxins and the Pathophysiology of Hemolytic Uremic Syndrome in Humans and Animals. Toxins 4: 1261-1287.
- Larsen MH, Dalmasso M, Ingmer H, et al. (2014) Persistence of foodborne pathogens and their control in primary and secondary food production chains. Food Control 44: 92-109.
- Nataro J P, Kaper J B (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11: 142-201.
- Naylor SW, Low JC, Besser TE, et al. (2003) Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect Immun 71: 1505-1512.
- Ogden I D, Hepburn N F, MacRae M, et al. (2002) Long-term survival of Escherichia coli O157 on pasture following an outbreak associated with sheep at a scout camp. Lett Appl Microbiol 34: 100-104.
- Chapman PA (2000) Sources of Escherichia coli O157 and experiences over the past 15 years in Sheffield, UK. Journal of Applied Microbiology 88: 51S-60S.
- Werber D, Behnke SC, Fruth A, et al. (2007) Shiga toxin-producing Escherichia coli infection in Germany-different risk factors for different age groups. Am J Epidemiol 165: 425-434.
- Warriner MJ, Ibrahim K F, Dickinson M, et al. (2003) Interaction of Escherichia coli with growing spinach plants. J Food Prot 66: 1790-1797.
- WarrinerMJ, Ibrahim K F, Dickinson M, et al. (2003) Internalization of human pathogens within growing salad vegetables. Biotechnology and Genetic Engineering Reviews 20: 117-134.
- Hora R, Warriner K, Shelp BJ, et al. (2005) Internalization of Escherichia coli O157:H7 following Biological and Mechanical Disruption of Growing Spinach Plants. J Food Protec 68: 2506-2509.
- ECDC (2014) Annual epidemiological report, food- and waterborne diseases and zoonoses, Stockholm.
- Edrington TS, Callaway TR, Ives SE, et al. (2006) Seasonal shedding of Escherichia coli O157:H7 in ruminants: a new hypothesis. Foodborne Pathog Dis 3: 413-421.
- EFSA and ECDC (2015) The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2013. EFSA Journal 13: 3991.
- Gilliss D, Cronquist A, Cartter M, et al. (2011) Vital Signs: Incidence and Trends of Infection with Pathogens Transmitted Commonly Through Food - Foodborne Diseases Active Surveillance Network, 10 US Sites, 1996-2010. MMWR 60: 749-755.
- Scallan E, Griffin PM, Angulo FJ, et al. (2011) Foodborne illness acquired in the United States-unspecified agents. Emerging Infectious Diseases 17.
- Scallan E, Hoekstra RM, Angulo FJ, et al. (2011) Foodborne illness acquired in the United States-major pathogens. Emerg Infect Dis 17: 7-15.
- Frenzen PD, Drake A, Angulo FJ, et al. (2005) Economic cost of illness due to Escherichia coli O157 infections in the United States. J Food Prot 68: 2623-2630.
- (2011) Food Safety and Inspection Service Microbiological testing program for Escherichia coli O157:H7. US Department of Agriculture, Washington, DC.
- (2011) Food and Drug Administration Food Code 2009. US Department of Health and Human Services, Food and Drug Administration, Washington, DC.
- Patil SR, Cates S, Morales R (2005) Consumer food safety knowledge practices and demographic differences: findings from a meta-analysis. J Food Prot 68: 1884-1894.
- Koluman A, Dikici A (2013) Antimicrobial resistance of emerging foodborne pathogens: status quo and global trends. Crit Rev Microbiol 39: 57-69.
- Karmali MA, Gannon V, Sargeant JM (2010) Verocytotoxin-producing Escherichia coli VTEC. Vet Microbiol 140: 360-370.
- Kim HH, Samadpour M, Grimm L, et al. (1994) Characteristics of antibiotic-resistant Escherichia coli O157:H7 in Washington State 1984-1991. J Infect Dis 170: 1606-1609.
- Mora A, Blanco JE, Blanco M, et al. (2005) Antimicrobial resistance of Shiga toxin verotoxin-producing Escherichia coli O157:H7 and non-O157 strains isolated from humans cattle sheep and food in Spain. Res Microbiol 156: 793-806.
- White DG, Zhao S, Simjee S, et al. (2002) Antimicrobial resistance of foodborne pathogens. Microbes Infect 4: 405-412.
- Fernandez-Brando RJ, Yamaguchi N, Tahoun A, et al. (2015) Type III Secretion-Dependent Sensitivity of Escherichia coli O157 to Specific Ketolides. Antimicrob Agents Chemother. 60: 459-470.
- Official Journal of the European Union (2005) Commission Regulation EC No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs.
- (2012) ISO /TS 13136:2012 Horizontal method for the detection of STEC and the determination of O157, O111, O26, O103, O145 serogroups.
Corresponding Author
Dr. Ahmet Koluman, Associate Professor, Ministry of Food Agriculture and Livestock, Food Control Laboratory, Turkish Republic, Adana, Turkey.
Copyright
© 2017 Koluman BU, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.