The Effect of Root and Shoot Extracts of Seriphium Plumosum as Allelopathic Agents
Abstract
Seriphium plumosum is a declared indicator of bush encroachment and poses a serious threat to the management of sustainable utilization in all grasslands. The successful invasiveness of Seriphium plumosum is attributed to its competitive ability and an apparent highly allelopathic potential. A study was conducted at the University of Limpopo (South Africa) to investigate the effect of S. plumosum on the germination and radicle length of Lactuca sativa. Roots and shoots material of S. plumosum were collected and used to make an infusion. The materials were chopped and finely grinded and each soaked in distilled water as solvent (150 g of shoots in 2000 ml of distilled water, and 150 g of roots in 2000 ml of distilled water). The soaking process was done at room temperature for 24 hours to produce aqueous extracts of the different plant parts. Fifty L. sativa seeds were placed in petri dishes lined with Whatmann number one filter paper and treated with 3 ml of the roots and shoots extracts. Distilled water was used as a control. The petri dishes were sealed with cling wrap and placed in a germination chamber. The chamber was set to 90% humidity with all lights on for 12 hours during the day and off for 12 hours during the night, limited to a 25 Ω dew point temperature, for a period of seven to 21 days. Lactuca sativa was sensitive to roots and shoots infusions, but the effect of shoots infusion differed highly significantly (P ≤ 0.01) from those of roots infusion. Lactuca sativa was sensitive to both summer and winter collected materials, but plant material collected in winter had a bigger effect than plant material collected in summer.
Keywords
Allelochemicals, Germination, Radicle length, Receiver species
Introduction
The term allelopathy is from the Greek-derived compounds allele and pathy (meaning "mutual harm" or "suffering"), and was first used in 1937 by Austrian scientist Hans Molisch in the book "Der Einflusseiner Pflanze auf dieandere - Allelopathie" (The Effect of Plants on Each Other) [1]. In his definition it refers to both detrimental and beneficial biochemical interactions among all classes of plants, including microorganisms. This has led to allelopathy being defined as: "any direct or indirect harmful or beneficial effect by one plant (including microorganisms) on another, through the production of chemical compounds that escape into the environment" [2]. Snyman [3] further defined allelopathy as a process which involves the production of secondary metabolites by plants and microorganisms, which influence growth and development of biological systems.
It is believed that certain plants might have inhibitory effects on neighbouring plants by releasing allelopathic substances into the soil, either as exudates from the living tissues or as decomposing plant residues [4]. These substances are known as allelochemicals, and can have beneficial (positive allelopathy) or detrimental (negative allelopathy) effects on the target organisms [5]. They can be present in several plant parts [6,7], including roots, rhizomes, leaves, stems, pollen, seeds and flowers [2]. It is believed that allelochemicals, are released into the environment by root exudation, leaching from aboveground parts, volatilisation and/or by decomposition of plant materials. Wind-pollinated plants, of which Seriphium plumosum is a good example, usually have this allelopathic characteristic [8].
The genus Seriphium consists of 36 species, with two species indigenous to Madagascar and 34 in South Africa, of which Seriphium plumosum is recognised as the most aggressive-growing species. Lately, this species is viewed as an encroacher in grasslands in South Africa [3]. In South Africa, Seriphium plumosum is mainly found in the Limpopo Province, North West, Free State, Eastern Cape, Mpumalanga, Gauteng and certain parts of KwaZulu-Natal [9]. Seriphium plumosum encroachment in South Africa has converted extensive areas of grassland into less productive shrubland-grassland [10], and considerable economic inputs are made annually in South Africa on its chemical control [9].
Seriphium plumosum encroachment has a devastating effect on the grazing capacity of veld. It causes land degradation which, in turn, leads to financial losses, since farmers are forced to obtain supplementary forage for livestock. The species is known for being very difficult to control, and of being extremely unpalatable for livestock and game animals. It is also highly flammable and aggravates the spread of uncontrolled veld fires, which makes it a problem plant in areas where it occurs [11,12]. The invasion of veld by S. plumosum is accompanied by competition among plants for resources such as light, water and nutrients [13]. Once an area is invaded by S. plumosum, it reduces its biodiversity, the function of the ecosystem is reduced and severe veld deterioration occurs [5].
According to Naderi and Bijanzadeh [14], rice leaves had a strong growth inhibitory activity on barnyard grass, followed by roots and stems. This was supported by Fateh, et al. ; Grisi, et al. and Pirzad, et al. [6,7,15], who reported that different plant parts could have different effects on the growth of receiver plant species. In the case of S. plumosum, Snyman [10] found that high-concentration of S. plumosum extracts derived from fresh roots and shoots materials had a greater effect on the germination of dicot species such as L. sativa. All aspects relating to the phenology and physiology of this problem species thus need investigation to develop a means of preventing its successful establishment and growth. Since little has been published on the physiological, phenological and ecological aspects of this plant species under South African circumstances, the aim of this study was to confirm if this species is allelopathic and to establish which part of the plant is the main source of allelopathy.
Methodology
Roots and shoots of S. plumosum were collected at the Mabula Private Game Reserve (Mabula), which is situated approximately 45 km north west of the town Bela-Bela in the Limpopo Province (24°42'S and 24°50'S and 27°50'E and 27°58'E). The altitude ranges between 1140 and 1432 m above sea level. The reserve occupies an area of 8500 ha and is situated in the savanna biome [16]. Soils at Mabula can be classified into two main types. Soils originating from igneous rock (granites) occur in the southeast of the reserve, and soils originating from sedimentary rocks (arinitic rocks) in the north-west. Soils from igneous rocks generally have a higher pH, and are less leached than soils from a sedimentary origin. Red soils at Mabula indicate better drainage and aeration with red oxidation layering around the grains. The majority of soils at Mabula are of a sandy texture with less than 15% clay. The clay content increases in low-lying areas, due to the natural process of accumulation of finer material in areas with smaller gradients [17].
Mabula has a unimodal, subtropical savanna climate [16]. The mean annual rainfall is 611.3 mm. The rainfall is seasonal, with the majority of precipitation occurring during the warmer months (September to April). The coolest month is June, with a mean monthly maximum temperature of 12.7 ℃. The warmest month is January, with a mean monthly temperature of 23.3 ℃ [18].
Plant material (shoots and root material) of S. plumosum were collected from 30 randomly chosen plants in an area, situated in the south-eastern part of the Mabula Private Game Reserve that was severely encroached by S. plumosum, during summer and winter month of 2014. At the collection site, the vegetation is classified as Sour and Mixed Bushveld [19]. Dominant grasses are Hyperthelia dissoluta, Heteropogon contortus, Digitaria eriantha, Eragrostis species and Melenis repens, while a woody component such as Vachillia and Senegalia species (Acacia species), Dichrostachys cinera and Terminalia sericea occur [20].
This study was conducted at the Plant Production at the University of Limpopo (23°53′10″S, 29°44′15″E). The influence of the possible allelopathic effect of S. plumosum was tested on Lactuca sativa.
The experimental layout was a 2 × 2 × 2 factorial, in a randomized block design, replicated four times. Plant materials of S. plumosum were collected during the summer and winter of 2014 and separated into root and shoot material. The fresh materials were chopped and finely grinded, and each soaked in distilled water as solvent (150 g of shoots in 2000 ml of distilled water, and 150 g of roots in 2000 ml of distilled water). The soaking process was done at room temperature for 24 hours to produce aqueous extracts of the different plant parts. The experiment thus consisted of the following treatments: an infusion of roots collected in summer, an infusion of roots collected in winter, an infusion of shoots collected in summer, an infusion of shoots collected in winter and a control treatment, consisting of distilled water only, as an infusion.
For the purpose of this study, the allelopathic potential of S. plumosum was investigated, using only the effects of the infusions at an unknown concentration of the allelopathic agent.
Fresh certified seeds of L. sativa were bought from a private seed company. Fifty seeds were placed in petri dishes lined with Whatmann number one filter paper, and treated with 3 ml of the roots and shoot extracts and distilled water. The petri dishes were sealed with cling wrap and placed in a germination chamber. The chamber was set to 90% humidity with all lights on for 12 hours during the day and off for 12 hours during the night, limited to a 25 ℃ dewpoint temperature, for a period of seven to 21 days.
Seedling counts of were done on a daily basis from the day of soaking for a period of 21 days, the aim being to determine the number of days to first and maximum germination, and to determine the germination percentage. The radicle length of four randomly selected seedlings in each petri dish was measured, using a ruler.
Generalized Linear Model (GLM) analysis was applied to the number of days to first germination and to the number of days to maximum germination with the Poisson distribution (for counts) and logarithmic link function, testing for differences between the effects of two plant parts, two seasons and two concentrations of infusions, as well as all their interactions. Germination percentage data was analysed with GLM, but with the Binomial distribution (for proportions) and the log it link function, testing for differences between the effects of two plant parts, two seasons and two concentration of infusions, as well as all their interactions. The radicle lengths were positively skewed, and therefore analysed with GLM and the Gamma distribution, testing for differences between the effects of two plant parts, two seasons and two concentrations of infusions, as well as all their interactions. All predictions were compared with Fisher's protected least significant test at the 5% level (P ≤ 0.05). Data were analysed, using the statistical program GenStat® [21].
Results and Discussion
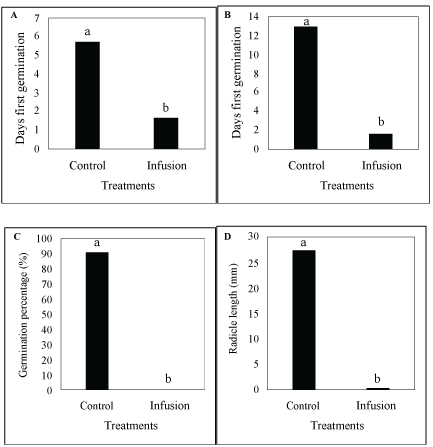
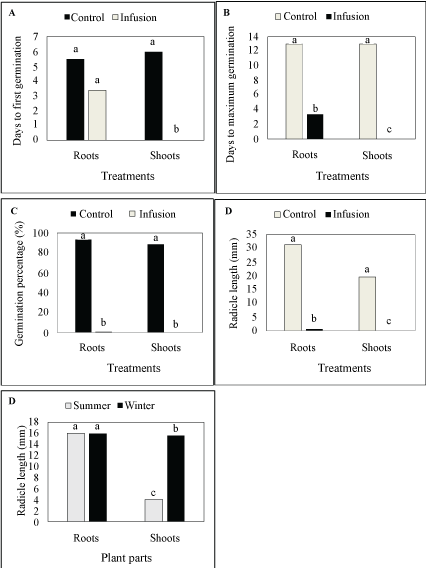
Differences in days to first germination were highly significant (P ≤ 0.01) (Table 1) between infusions and the interaction between infusions and plant parts (Table 1). All other factors, including plant parts and season as main factors, had no influence. Infusions [(37.522/150.387) × 100 = 24.95%] and the interaction between plant parts and infusions [(33.128/150.387) × 100 = 22.03%] comprised 46.98% of the total deviance. Significant differences occurred between the infusions (Figure 1A), but where interaction between infusions and plant parts were concerned, only the shoots infusion was significantly different from others (Figure 2A). In the control treatment, L. sativa started germinating five to six days after treatments started and after three days where the roots infusion collected in summer was applied, but did not germinate where the shoots infusion collected in summer was applied (Table 2). Similarly, L. sativa started germinating four days after the infusion of roots collected in winter was applied, but did not germinate where the infusion of shoots collected in winter was applied (Table 2).
Differences in days to maximum germination were highly significant (P ≤ 0.01) (Table 1) between infusions (Figure 1B) and the interaction between infusions and plant parts. All other factors, including plant parts and season as main factors, were not significant (P ≥ 0.05). Infusions (58.21%) and the interaction between plant parts and infusions (12.63%) comprised 70.84% of the total deviance. No significant differences occurred between the plant parts and days to maximum germination (P ≤ 0.05) (Table 1), but where interaction between infusions and plant parts were concerned, the shoots infusion was significantly different from the root infusion and both the root and shoot infusions were significantly different to the control treatments (Figure 2B). In the control treatments, L. sativa reached maximum germination between 12 and 14 days after treatments started. It did not germinate where the shoots infusion of material collected in summer was applied (Table 2), but reached maximum germination four days after the infusion of roots collected in summer was applied. Similarly, L. sativa reached maximum germination four days after the infusion in roots collected in winter was applied, and did not germinate where the infusion of shoots collected in winter was applied.
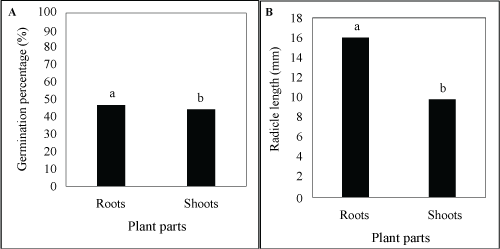
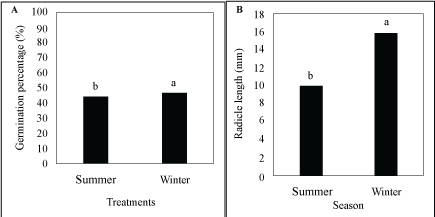
Differences in germination percentage were highly significant (P ≤ 0.01) between infusions, plant parts and seasons (Table 1). All other factors were insignificant. Infusions (97.96%), plant parts (0.39%) and season (0.32%) comprised 98.67% of the total deviance. Significant differences occurred between the infusions (Figure 1C) and between plant parts (Figure 3A) as well as between seasons (Figure 4A), as well as the interaction between roots and shoots (Figure 2C). Only 1% of the seeds germinated where the infusion in roots collected in summer, while none germinated with the infusion of shoots collected in summer was applied (Table 2). In the control treatment, 94% of the seeds germinated.
Differences in radicle length were highly significant (P ≤ 0.01) between infusions, the interaction between infusions and plant parts and the interaction between plant parts and seasons (Table 1). Plant parts and seasons as the main factors were significant (P ≤ 0.05). All other factors were not significant (P ≥ 0.05). Infusions (85.10%), plant parts (1.43%), season (1.20%), the interaction between plant parts and infusions (1.80%) and the interaction between plant parts and season (3.64%) comprised 93.17% of the total deviance. Significant differences occurred between the infusions (Figure 1D), between plant parts (Figure 3B) as well as between seasons (Figure 4B). However, where the interaction between plant parts and infusions were concerned, the roots and shoots infusions were significantly different from the control treatments and they were also significantly different from each other (Figure 2D and Figure 2E). In the control treatment, L. Sativa had reached a radicle length of 31 mm when the germination study ended after 21 days (in both treatments that represented infusions from roots and shoots, collected in both summer and winter) (Table 2). With the root infusions (summer and winter collected), the average radicle length was 1 mm, while with shoot infusions, no radicle development occurred.
Overall, the infusion had major depressing effects on all traits that were studied. Where plant parts were concerned, both the shoots and roots infusion had similar inhibitory effects on days to first and maximum germination, but the shoots infusion had a bigger effect on the germination percentage and radicle length, compared to the roots infusions. This was in agreement with Snyman [3], who found that different plant parts differed significantly in allelochemical potential and realized that it is important to evaluate the allelopathic potential of different plant parts of S. plumosum. Snyman [12] also stated the fact that not only germination, but also early seedling development, are inhibited by S. plumosum.
Where seasons were concerned, infusions from material collected in summer and winter had similar inhibitory effects on days to first and maximum germination, but material collected in summer had a bigger inhibitory effect on the germination percentage and radicle length. The shoots infusion resulted in a short radicle, while the roots infusion had no effect. The results supported the findings of Hansen-Quartey, et al. [22] which stated that leaf extracts had a more pronounced adverse effect on the seed germination of selected test species than stem and root extracts.
Conclusions
Infusions of S. plumosum shoots and roots severely depressed the germination and radicle length of L. sativa, while distilled water alone no effect, which proved that different plant parts had an allelopathic effect on the germination of the receiver species. The shoots infusion had a bigger effect on the germination and radicle length, while the roots infusion had a little effect. This showed that different plant parts have an allelopathic effect on the germination of the receiver species.
Infusions made from plant material collected in winter had a bigger effect on the germination and radicle length of L. Sativa than infusions made from plant material collected in summer, which indicated a seasonal effect of allelopathy on the germination of the receiver plant species. The reason for the allelopathic effect being less effective in winter might be attributed to the absence of rain during winter. Less leaching of allelopathic agents out of above-ground plant parts thus occur, which possibly increases the allelopathic effect of S. plumosum [23]. This also points towards increased allelopathy of S. plumosum during droughts, when receiver plants might be more susceptible to allelopathy.
In practice, it is recommended, especially in the case of mechanical control (cutting or mowing), that excess plant material be removed from the treated area as soon as possible to minimize the effect of allelopathy. This applies to both winter and summer clearing treatments. Since roots also have an influence, total removal of S. plumosum plants might be better, but it is more important to remove the above-ground material from the infested site, since leaves and stems appear to be the main source of allolopathy.
References
- Willis RJ (2010) The History of Allelopathy. Springer, Dordrecht, Netherlands.
- Kruse M, Strandberg M, Strandberg B (2000) Ecological Effects of Allelopathic Plants-a Review. NERI Technical Report No. 315. National Environmental Research Institute, Silkeborg, Denmark, 12: 66-68.
- Snyman HA (2010) Allelopathic potential, seed ecology and germination of the encroacher shrub Seriphium plumosum. African Journal of Range and Forage Science 27: 29-37.
- Batlang U, Shushu DD (2007) Allelopathic activity of sunflower (Helianthus annuus L.) on growth and nodulation of Bambara Groundnut (Vigna subterranean L.). Journal of Agronomy 6: 541-547.
- Singh HP, Batish DR, Kohli RK (2003) Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. Critical Reviews in Plant Sciences 22: 239-311.
- Fateh E, Sohrabi SS, Gerami F (2012) Evaluation of allelopathic effect of bindweed (Convolvulus arvensis L.) on germination and seedling growth of millet and basil. Advances in Environmental Biology 6: 940-950.
- Grisi PU, Gualtieri SCJ, Ranal MA, et al. (2012) Allelopathic interference of Sapindus saponaris root and mature leaf aqueous extracts on diaspore germination and seedling growth of Lactuca sativa and Allium cepa. Brazilian Journal of Botany 35: 1-9.
- Van Wyk AE (2004) Biomes of South Africa: Grasslands. In: Conserving threatened species and ecosystems. Endangered Wildlife Trust, Johannesburg, South Africa.
- Jordaan D (2009) Bankruptbush (Slangbos) - A silent threat to grasslands? Grassroots: Newsletter of the Grassland Society of Southern Africa 9: 40-42.
- Snyman HA (2010) Knowledge of seed ecology essential for Seriphium plumosum control. Grassroots 10: 9-14.
- Snyman HA (2009) A philosophical approach to the distribution and spread of Seriphium plumosum. Grassroots 9: 29-37.
- Snyman HA (2009) Germination potential of Seriphium plumosum (bankrupt bush, slangbos or vaalbos). Grassroots 9: 43-48.
- Vyvyan JR (2002) Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 58: 1631-1646.
- Naderi R, Bijanzadeh E (2012) Allelopathic potential of leaf, stem and root extracts of some Iranian rice (Oryza sativa L.) cultivars on barnyard grass (Echinochloa crus-galli) growth. Plant Knowledge Journal 1: 37-40.
- Pirzad A, Jamali M, Zareh MA, et al. (2012) Effect of water extract originated from different parts of Russian knapweed (Acroptilon repens L.) on growth of Amaranthus retroflexus L. International Journal of Agriculture: Research and Review 2: 589-594.
- Low AB, Rebelo AG (1996) Vegetation of South Africa, Lesotho and Swaziland. Department of Environmental Affairs and Tourism, Pretoria, South Africa.
- Kriel A (2000) Die Geologie van Mabula Wildreservaat. BSc. Honours Mini-Dissertation, University of Pretoria, South Africa.
- ISCW (2007) Climate annual and monthly totals and averages. ISCW Agromet, Pretoria, South Africa.
- Acocks JPH (1988) Veld types of South Africa. (2nd edn), Memoirs of the Botanical Survey of South Africa 57, Government Printer, Pretoria.
- Smallwood D (2007) Vegetation monitoring system for the Mabula Game Reserve. M.Sc. Dissertation, University of Pretoria, South Africa.
- Roger Payne, Darren Murray, Simon Harding, et al. (2014) Introduction to GenStat® for Windows™. (15th edn), VSN International, Hemel Hempstead, Hertfordshire, UK.
- Hansen-quartey JA, Nyamapfene K, Materechera SA (1998) Effects of aqueous extracts from Artemisia afra parts and soil on seed germination and early seedling development in selected plant species. South African Journal of Plant and Soil 15: 1-5.
- Snyman HA (2008) Slangbos - bedreiging in weiveld. SA Co-op 26: 8-10.
Corresponding Author
Jorrie J Jordaan, School of Agriculture & Environmental Science, University of Limpopo, Private Bag X1106, Sovenga, 0727, South Africa.
Copyright
© 2017 Mokou BM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.