Comparisons In floristic Composition and Plant Species Diversity amongst Granite Outcrops of the Mamabolo Mountain Bushveld, South Africa
Abstract
The geographical landscapes together with environmental conditions are well recognised drivers in determining a suitable habitat for plants and animals on rocky as well as granite outcrops. Granite outcrops of the Mamabolo Mountain Bushveld (Limpopo Province, South Africa) are ideal in investigating aspects of landscape geography and specific abiotic factors in order to understand floristic compositions amongst outcrops themselves and that of their surrounding vegetation matrix. A total of 13 outcrops and 24 matrix plots were sampled to compare plant species composition, diversity and richness. The effect landscape geography was incorporated as a management regime effect, thus communal (disturbed) versus protected (undisturbed) of sampled areas. At a landscape level, outcrops in protected areas within the same geographical location were found to have similar floral composition to those in communal areas, but with poorer species richness and diversity than those in communal areas. Matrices were similar in floristic composition irrespective of geographical area, with no difference in species richness and diversity between protected and communal areas. Abiotic factors are important in determining outcrop plant structures and this extends to respective slopes within the outcrop. Upon outcrops themselves, southern slopes are more species rich compared to the north-facing slopes. Our findings confirm the importance of landscape geography and abiotic factors as key possible drivers in determining outcrop plant structures. Furthermore, outcrops act as refugia in those areas prone to disturbance (communal areas) in maintaining plant species diversity and richness.
Keywords
Floristic composition, Disturbance, Granite outcrops, Mamabolo Mountain Bushveld, Species richness, Species diversity
Introduction
Over recent decades, granite outcrops have become increasingly recognized as important habitats for plants, owing to their distinct physical and biotic environments [1]. Outcrops in areas such as the Piedmont Plateau in Georgia, USA [2,3], eastern and western Australia [1,4-6], Brazil [7,8] and several African countries [9-11], have been found to be characterised by harsher environmental conditions relative to their surrounds, thereby creating unique microhabitats for several distinct plant communities [12,13].
Within landscapes, outcrops vary tremendously in size, shape, and position [14], and are mainly dominated by large granite boulders and rock. They are found within forests, grasslands and savanna, and are recognized as 'islands' within these habitats, with distinct vegetation composition and a variety of microclimates [2,10]. Given that outcrops do to a certain extent have plant communities that are different to their surrounding vegetation, they do however share several species and thus tend not to be unique but somehow "special habitats" [15]. For this reason, Burke [16] argued that outcrops cannot be labelled as 'islands', but agrees that they do offer special microhabitats (dependant on variable factors) associated with distinct communities. Traditionally, islands have played a crucial role in ecological studies designed to achieve a better understanding of factors influencing species richness and diversity [17].
Outcrops, however, offer excellent subjects for comparative phytogeographical studies, as well as for research on the factors determining species richness in isolated plant communities [18]. This is because they occur in spatial isolation over large distances [15], with those in different geographical regions having different floristic compositions, whereas floristic differences are usually low at a local scale [10]. The same concept can thus be resembled to that of islands [17]. However, Burke [16] and Parmentier, et al. [19] further added that outcrops variations depends solemnly on outcrop positioning within the landscape, climatic conditions (responsible for niche differentiation), dispersal capabilities/limitations and other factors (i.e. history of vegetation changes) of the matrix.
A study by Parmentier [19] on outcrops in the forests of Equatorial Guinea found outcrop vegetation to differ from that of the surrounds, and that even over short distances (100-500 m), the floristic composition of plant communities on different neighbouring outcrops was distinct. Parmentier [19] hypothesised that the nature of the matrix in which the outcrops are found (e.g. forest, savanna) might influence similarities in plant species composition between outcrops. Burke [16] is of the idea that various activities (grazing, resource use, anthropogenic influences and clearing of corridors) in the surrounding matrix are a key determent of the outcrop vegetation composition. As such, management regime (disturbed versus non disturbed areas) can also be considered to be a crucial factor when comparison in floral composition between outcrop and their surround vegetation matrix is made.
Plant species and community variations on outcrops and their surrounding habitats are also primary driven by the abiotic component. Environmental conditions on granite outcrops are harsh [2], and are characterized by high solar irradiance [20]. Furthermore, water availability is relatively limited because of rapid run-off from the rock surface and low water-holding capacity of shallow sandy soils [21]. With limited soil [22], severe soil nutrient limitation [23], water scarcity [15] and high daily temperatures [24], granite outcrops are a challenging environment for any inhabitant [5]. In spite of these harsh conditions, outcrops have also been shown to serve as refuge areas for species (for example, bats, euros and rock wallabies) during periods of environmental stress in the surrounding landscapes [6].
Upon outcrops themselves, abiotic factors vary with aspect, with, in the southern hemisphere, a non-linear moisture gradient in which the northern slope is the most xeric [25]. The number of plant species has been found to vary greatly between north and south aspects of outcrops in South Africa [26] and South America [24]. Mares [24] hypothesised that the southern slopes of outcrops in the southern hemisphere have higher plant diversity compared to the northern slopes due to the abiotically favourable (cooler and moister) habitat on southern aspects of outcrops. However, for the South African study, the investigated outcrops were limited in scale (< 4) and in one confined area (campus of the University of Limpopo) and therefore did not fully represent the occurrence of outcrop with the Mamabolo Mountain Bushveld. In particular, those in communal lands and exclusively protected areas (e.g. Nature Reserves). This made it imperative to look at outcrops in different localities within this vegetation types taking into account factors reviewed from the literature above and addressing these based on outcrop plant communities' differences to that of their surrounding matrix - prioritising the effect of management regime and geographic location. A few selected abiotic factors on a limited number of outcrops are also assessed owing to the characteristic nature of environmental conditions on outcrops.
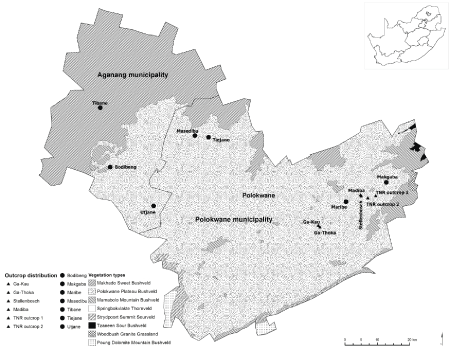
The selected study site in this paper, the Mamabolo Mountain Bushveld, occurs within a savanna matrix that comprises of seven other vegetation types (Figure 1). The granite outcrops (unique to this vegetation type) are distributed mostly on the east and west side of the region, while communal (disturbed) and protected (non-disturbed) areas co-occur thought out the landscape. The savanna matrix within communal areas is used and subject to free roaming and grazing of domestic livestock, cultivation and development (mainly for housing), while the matrix within protected areas is browsed and grazed by indigenous wildlife such as kudu (Tragelaphus strepsiceros Pallas, 1766), wildebeest (Connochaetes gnou Zimmermann, 1780) and giraffe (Giraffacamelo pardalis Linnaeus, 1758); farm stocks (Nguni cattle). Outcrops in both communal and protected areas are generally observed to be grazed by small mammals, primarily rock dassies (Procavia capensis Pallas, 1766) and rodents (e.g. Saccostomus campestris Peters, 1846 and Tatera leucogaster Peters, 1852).
The setting dynamics of this area is ideal for investigating and assessing the effect of degree of disturbance (i.e. management) relative to geographical location of various outcrops as these are some of the aspects that remain unclear when substrate differences are discussed between outcrops themselves and also their surrounding vegetation.
In this paper, we selected outcrops of similar size to enable us to examine the effects of management regime and outcrop plant species composition, richness and diversity, whilst keeping outcrop size comparable. The following questions were addressed: (1) Are plant communities found on outcrops different to those found in the matrix, due to management regime and geographic location?; (2) Upon outcrops themselves, are there differences in species richness according to aspect (i.e., North and South facing slopes)?, and (3) How variable are factors such as temperature, humidity and soil moisture of the respective slopes? In this instance, plant communities on outcrops are expected to differ from those of the matrix [2,10] , with geographic location [10,15] management [16,19] , abiotic conditions [2,10,19] being the major drivers. The south slopes on outcrops are expected to yield more species compared to the north slopes and to further have favourable abiotic conditions than the latter [24, 26].
Methods
Study area
The Mamabolo Mountain Bushveld vegetation type extends along the mountain habitats of the Strydpoort, Wolkberg and Drakensberg ranges, as far west as the Mogoshi Mountain Ranges and De Loskop to the north. Annual rainfall across the study sites ranges from 450 to 750 mm. Average daily maximum summer (November-January) and winter (May-July) temperatures are 33.4 ℃ and 24.9 ℃, respectively [27]. The geology is variable, including basement granite and gneiss clastic sediments of the Pretoria group (Vaalian) and ultra mafic and mafic metal volcanics of the Pietersburg group (Swazian) [28]. Soil is mostly coarse, sandy and shallow [27]. Generally, the Mamabolo Mountain Bushveld vegetation is a mixture of dense grass layer (mainly Themeda triandra (Poaceae) Forssk., Panicum maximum (Poaceae) Jacq. and Eragrostis curvula (Schrad.Nees), dense shrubby thickets, and both small Acacia and broad-leaved trees, with Euphorbia clivicola (Euphorbiaceae) R.A. Dyerand Khadia media (Mesembryanthemaceae) P.J.D. Winter & N. Hahnbeing the only two documented endemics [27].
Granite outcrops, which give this habitat its uniqueness, are characterised by granite boulders that vary tremendously in shape and size (~10-230 m in width, while their height extends up to 180 m). These outcrops support a great variety of plant life including Euphorbia cooperi (Euphorbiaceae) N.E.Br. ex A. Berger, and various Acacia, Combretum, Cussonia, Euclea, and Ficus species. Bird species are associated with these outcrops include several species amongst others, the Southern Bald Ibis (Geronticus calvus Boddaert, 1783), Rock Kestrel (Falco tinnunculusLinnaeus, 1758), Freckled Nightjar (Caprimulgus tristigma Rüppell, 1840), Rock Martin (Ptyonoprogne fuligula Lichtenstein, 1842) and Lazy Cisticola (Cisticola aberrans Smith, 1843) (Birdlife South Africa, 2009).
Selection criteria of study sites
Sites were selected on the East and West regions of the Mamabolo Mountain Bushveld as theseareas had outcrops that were more equal in size and had at least 60% vegetation cover as opposed to those outcrops mostly dominated by granite rocks. Therefore, those on the East and West regions were suitable for the desired sampling criteria for this study. A total of 13 outcrops (seven in communal areas and six in protected/privately-owned areas, and 24 matrix plots (eight in communal areas and 16 in protected/privately-owned areas) were selected representing vegetation in the direct vicinity of the six outcrops (Figure 1).
In this paper, outcrops in non-protected/communal areas shall be referred to as 'OC', with their matrix being 'MC', while outcrops in will be referred to as 'OR' and the matrix as 'MR'. We take it that outcrops and matrices in protected/privately-owned areas are indeed under some form of protection, we therefore consider and refer to these areas as "Reserves", thus 'OR' for outcrop and 'MR' for the matrix.
Outcrops had to be more than 50 m in length and higher than 5 m (measurements accounted for using Google Earth), fairly erect on all four outcrop sides, with a mixture of medium-large boulders and not dominated by bare rock. In addition, we chose outcrops with an even distribution of vegetation cover (to enable adequate vegetation sampling/avoid rock- and boulder-dominated outcrops with less vegetation cover) in all four major compass directions. Outcrops from communal areas were a minimum of 8 km apart (to account for matrix vegetation sampling), although this minimum distance was not possible for outcrops in protected areas/private game farms, as these areas were too small to accommodate this criterion. For these three sets of paired outcrops the minimum distance was kept at 150-450 m.
Sampling
Sampling was carried out during the summer (Nov 2009-Jan 2010), with revisits in the autumn (Feb-Apr) and spring (Aug-Oct) of 2010, to ensure maximum representivity of the plant assemblages in the data set, and to facilitate identification of plant species. This blooming period has a good correlation with the rainfall in the area as it is a typical savanna region with summer rainfall and dry winters. Annual rainfall ranges from 450-750 mm [27]. Two 20 m wide sampling transects were used for each outcrop, one from the northern and one from the southern side. These transects ran from the base towards the apex of the outcrop. Within each 20 m transect, five meter division areas were demarcated throughout the transect to enable easy species documentation. This was done by walking in a zigzag pattern within the five meter division to count species individually - to account for abundance. In places where the terrain was flat, the step-point method, adopted from Werger [29], was used to document species abundance. A Braun-Blanquet field form was used to survey vegetation on both outcrops and in the surrounding matrix vegetation.
Matrix vegetation was sampled using a single quadrat of 15 × 15 m (= 225 m2) in each of the four major compass directions (therefore, 4 quadrats per outcrop), approximately 60-100 m from the outcrop. This was to avoid having the plots just at the foot of the outcrops or being a continuation of outcrop vegetation as it would have not accounted substantially for similarities/differences in vegetation structure. Matrix plots for the other seven outcrops could not be surveyed as the matrix was transformed to ploughed fields and residential areas (three sides in most instances). The selection of four quadrats around the outcrop was to enable a thorough documentation of the surrounding vegetation matrix.
In both outcrop and matrix plots, all plant species encountered were recorded and grouped into various categories (trees, shrubs, herbs, succulents and grasses). Plants were identified on site, while those that could not be identified were collected and sent to the Larry Leach Herbarium located at the University of Limpopo for identification. The abundance of all species and differences in presence/absence of species in north versus south transects were also recorded. This was done by individual plant counts in the step-point method. Field guides consulted for plant identification included van Wyk and Malan [30], Fabian and Germishuizen [31], van Wyk and van Wyk [32], and van Oudtshoorn [33]. Plant nomenclature followed that of Germishuizenand Meyer [34].
Temperature and humidity readings on four outcrops (Madiba, Stellenbosch, TNR1 and TNR2) occurring in protected/privately-owned areas were documented. Readings we recorded throughout June; the onset of the winter season, and October for the summer period. All readings on the four outcrops were measured on the seventh day of the week throughout the month. These climatic factors were monitored and recorded on transitional, middle and apex zones of the outcrop's north and south slopes. The transitional area represents the boundary between the outcrop and matrix vegetation. A hygrometer (model: Ama-ditit ad 90 h) was used to measure both humidity and temperature between 13h00 and 14h00. Soil moisture was measured using the ThetaProbe Soil Moisture Sensor, Type ML1. The ThetaProbe measures volumetric moisture content and expresses the ratio of water to soil volume, which was then converted to a percentage by multiplying by 100.
Data analysis
An Analysis of Similarities (ANOSIM) was carried out using PRIMER v.5.1.2 [35] for similarity analysis on plant assemblages between outcrops and matrix plots. A similarity matrix was constructed using the Bray-Curtis similarity coefficient on square-root-transformed data. A square-root transformation was used to reduce the influence of common species [35]. A hierarchical cluster analysis was performed using group averages and a dendrogram was plotted. Groupings for similarity index accounted for management (protected/privately-owned and communal areas) and locality (East and West region).
Shannon-Weiner diversity (H') was used to determine species richness [36] and diversity of outcrops and matrix plots. An unpaired t-test was then run to compare species richness between OR and OC, then MR and MC. Species richness comparison between outcrops and matrix plots was not legible since plots sampled on the two respective habitats were not equal in size. Diversity (H') values were calculated using PRIMER v.5.1.2 [35]. Comparison of species richness on the northern and southern sides of slopes was carried out using a paired t-test. All statistical tests were carried out in R.
Results
Floristic composition outcrop and matrix vegetation
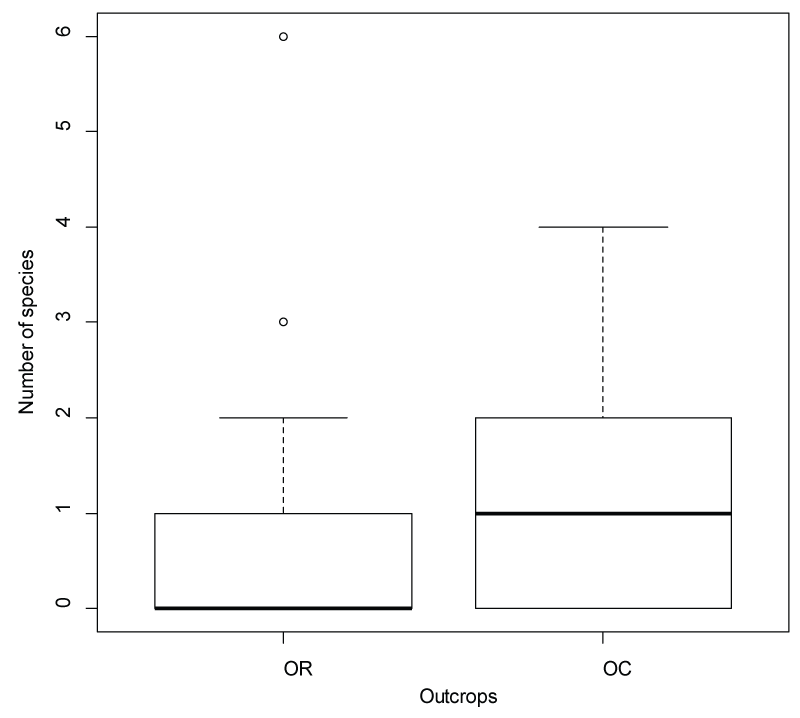
A total of 174 species from 57 families were recorded on the sampled outcrops and matrix plots. Outcrops had a higher number of families and percentage of plant species (17 families and 49.4% of species) unique to them relative to the matrix (3 families and 6.8% of species). When comparing the number of species restricted to OR versus OC (not found in the matrix), OC had a significantly higher (t = -2.9, df = 114, P = 0.004) number of species in contrast to OR (Figure 2) Plants mostly restricted to outcrops included amongst others (i) Hypoestes forskaolii (Vahl) R.Br, (ii) Ficus glumosa (Miq.) Del. and (iii) Melhania acuminate K. Schum. Plants present on both outcrops and matrix were the likes of (i) Grewia bicolour Juss; (ii) Acacia karroo Hayne and (iii) Indigofera tinctoria Linn. For the matrix vegetation, Agelanthus natalitius (Meisn.) Polhill & Wiens; Eulophia streptopetala Lindl. And Hermannia boraginiflora Hook. Were some of the plants restricted to the plots. Families well-represented on outcrops included Asteraceae, Anacardiaceae, Crassulaceae, Euphorbiaceae and Poaceae. A full plant list indicating the plants species occurrence on either outcrops or matrix is provided as Appendix A (Supplementary Table 1) and the species diversity of each sampled site in Appendix B.
For species richness and diversity comparison, OC was found to be significantly more species rich (t = -2.60, df = 24, P = 0.02) and diverse (t = -2.49, df = 24, P = 0.02, = 5.29) than OR, while matrix plots did not differ significantly in species richness (t = 1.19, df = 22, P = 0.23) or diversity (t = 0.49, df = 22, P = 0.62,) according to management.
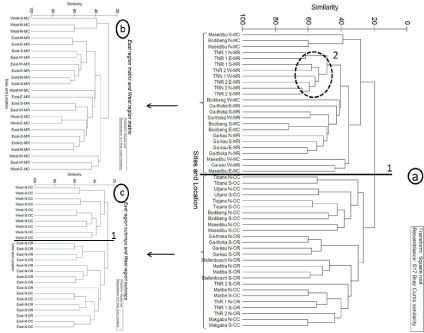
Matrix and outcrop sites had plant communities that were distinct from each other (Figure 3). In contrast, Matrix TNR 1 and TNR 2 had a very similar species composition. There was no clear distinction in terms of floristic composition on outcrops according to management, but the eastern region outcrops were separately grouped from those in the west. Matrices (MR and MC) did not show any distinct groupings according to management or geographical location (Figure 3).
North versus south slopes
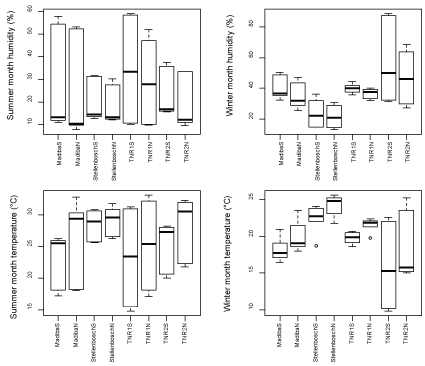
Summer and winter month's temperature recordings revealed the northern slope to be slightly warmer than the southern slope across the four outcrops (Figure 4). The mean winter month temperature ranges for the south and north slopes were 15.9-22.5 ℃ and 18.4-24.2 ℃, respectively, while the summer month temperatures ranged from 23.1-28.5 ℃ and 25.3-29.1 ℃ for the south slopes and north slopes, respectively. TNR1N was the hottest in summer (33.1 ℃), compared to the coolest, which was TNR1S (14.8 ℃), while Stellenbosch N was much warmer (25.6 ℃) in winter as opposed to the cooler TNR2N (15 ℃).
For humidity, the south slopes were generally more humid than the north slopes, variation were however still much distinct for respective outcrops (Figure 4).
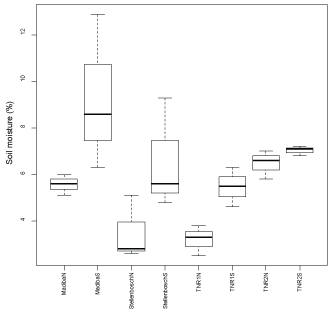
Across the four outcrops, soil moisture recordings were always higher on the south slopes compared to the north slopes (Figure 5). Mean soil moisture content ranged from 5.5-9.3% for the south slopes, while it was 3.2-6.5% for the north slopes. Madiba's southern slope had the highest maximum of 12.9%, whereas TNR2's northern slope had the maximum at 7%. Again, variation in soil moisture differed vastly between outcrops (e.g. TNR1S had very low variation in soil moisture, but TNR2S had very high variation).
For species richness comparison amongst slopes, the mean species richness for southern slopes (34.4) was higher than for northern slopes (30.9). That is 34.4 and 30.9, respectively. The difference was, however, not significant (paired t-test: t = -1.76, df = 12, P = 0.10). A number of plant species were also restricted to respective slopes. On the north it was species such as Aloe camperi Masson and Carissa macrocarpa (Eckl.) A.DC.) and on the south (Senecio affinis DC. and Urochloa mosambicensis (Hack.) Dandy.
Discussion
Floristic composition outcrop and matrix vegetation
Clear differentiation in species composition between outcrop and matrix plots was found. These findings are consistent with previous studies [10,12,13,19] . These differences have been attributed to disparities in abiotic conditions [2,10,19], which have been recognized as the primary determinants of floristic and vegetation structural pattern in most, if not all, ecosystems [37].
Plant assemblages on outcrops were more similar to those on outcrops in closer proximity, consistent with biogeographic theory [17]. Other studies [10,15] have also concluded that outcrops in different geographical regions differ in floristic composition. Parmentier [19] did not find similarities on outcrops in close proximity to each other, and attributed this to environmental factors on outcrops and the nature of the surrounding vegetation making dispersal less likely, although these findings were based on a small sample size (n = 3).
There was no clear distinction in floristic composition between MR and MC plots. This suggests that management has little influence on plant species composition. This is unexpected given that a number of studies [38-40] have observed changes in plant species composition with different grazing intensities. It is possible that historically heavy grazing has resulted in a certain species composition [41,42].
As a rule, habitats in communal lands tend to be heavily impacted, owing to high intensity grazing, firewood collection and other human activities, which leads to both habitat and biodiversity loss [43]. This was not the case for outcrops in communal areas, however, which were found to be significantly more species rich and diverse compared to OR. Again when it came plant species of restricted outcrops, OC outnumbered OR. Since protected areas are less prone to disturbance compared to non-protect/communal areas [44], OR was expected to be more species rich and diverse than OC. We had also expected this to extend to the compared number of restricted. In both instances OC exceeded expectations owing to the understanding that perhaps outcrop ecosystem are much more complex that we make of them. That is environmental conditions that result in varied microclimates which creates suitable niches for various specialised life forms.
Outcrops are also generally known not to attract much agriculture owing to their harshness, terrain and topography [15], but it is likely that some livestock (e.g. goats) do browse and graze on outcrops, perhaps creating a level of disturbance beneficial to species diversity. Fire regimes may also differ between communal and protected areas, and this might also influence levels of species diversity, and would need further investigation. However, protected (nature reserves) and lower-impacted areas (privately-owned areas) likely remain important for maintaining biodiversity, particularly when surrounded by highly-impacted landscapes (communal areas), as they may act as refugia for some species.
North versus south slopes
Mares [24] and Masehela [26] noted that the southern slopes have better abiotic conditions for plant life, and so had more species relative to the northern slope. The study findings was consistent with those stated above, as the southern slopes were much cooler, more humid and had slightly higher soil moisture content than the north slope across the four sampled outcrops. Although the study findings did not show wide variation in species richness between the two slopes, southern slopes were still slightly more species rich than northern slopes. Such trends are strongly driven by abiotic variation [24], associated with the non-linear moisture gradient making the northern slope more xeric [25]. Given that this study found some species to be limited to certain slopes, these species might be restricted by their niche requirements, further studies would however be essential to further substantiate or add clarity to this phenomenon.
In conclusion, the study findings support the idea that outcrops are "special habitats", their inaccessibility and harsh conditions, making them refugia for some species. Geographical separation of outcrops is critical in species composition as the study clearly depicted to resemble similar plant assemblages with their neighbouring outcrops as opposed to those in a different geographic location. Many other factors drive the resultant plant communities on outcrops, including fire, seed dispersal mechanisms, bird, insect and small-mammal life and these factors should also be considered in understanding outcrop plant community composition. Since outcrops vary in floristic composition over geographic gradients, occur randomly and widely within their surrounding vegetation matrix (in both communal and protected areas), future effective conservation planning would be best achieved by incorporating them with those of surrounding habitats or by an area wide network.
Acknowledgements
We thank Mr. Tsamaelo Malebu, Biodiversity Planner-Landuse and Environmental management division at SANBI, for his assistance with GIS. Mrs. Bronwyn Egan, curator at the Larry Leach Herbarium (University of Limpopo), her assistance in plant identification is highly appreciated.
References
- SD Hopper, AP Brown, NG Marchant (1997) Plants of western Australian granite outcrops. Journal of the Royal Society of Western Australia 80: 141-158.
- Madeline P Burbanck, Robert B Platt (1964) Granite outcrop communities of the Piedmont Plateau in Georgia. Ecology 45: 292-306.
- DL Phillips (1981) Succession in granite outcrop shrub-tree communities. American Midland Naturalist 106: 313-317.
- JT Hunter (2003) Factors affecting range size differences for plant species on rock outcrops in eastern Australia. Diversity and Distributions 9: 211-220.
- PC Withers (2000) Overview of granite outcrops in Western Australia. Journal of the Royal Society of Western Australia 83: 103-108.
- B York Main (1997) Granite Outcrops: A collective ecosystem. Journal of the Royal Society of Western Australia 80: 113-122.
- CM Jacobi, do Carmo FF, Vincent RC, et al. (2007) Plant communities on ironstone outcrops: A diverse and endangered ecosystem. Biodiversity and Conservation 16: 2185-2200.
- S Porembski, G Martinelli, R Ohlemüller, et al. (1998) Diversity and ecology of saxicolous vegetation mats on inselbergs in the Brazilian Atlantic rainforest. Diversity and Distributions 4: 107-119.
- MF Poelchau, S Mistry (2006) Forb diversity and community similarity of kopjes in the Serengeti National Park, Tanzania. African Journal of Ecology 44: 38-46.
- S Porembski, R Seine, W Barthlott (1997) Inselberg vegetation and the biodiversity of granite outcrops. Journal of the Royal Society of Western Australia 80: 193-199.
- MF Thomas (1994) Geomorphology of the Tropics. Wiley, Chichester.
- JV Lencher (1990) Kopjes: Islands in a sea of grass. The Science Teacher 57: 5.
- C Sarthou, J Villers (1998) Epilithic Plant communities on Inselbergs in French Guiana. Journal of Vegetation Science 9: 847-860.
- S Porembski (2000) The invasibility of tropical granite outcrops ('inselbergs') by exotic weeds. Journal of the Royal Society of Western Australia 83: 131-134.
- S Porembski (2007) Tropical Inselbergs: Habitat types, adaptive strategies and diversity patterns. Rev Bras Bot 30: 579-586.
- A Burke (2003) Inselbergs in a changing world - global trends. Diversity and Distributions 9: 375-383.
- RH MacArthur, EO Wilson (2001) The Theory of Island Biogeography. Princeton University Press, Oxfordshire, UK, 3-19.
- S Porembski, G Brown, W Barthlott (1995) An inverted latitudinal gradient of plant diversity in shallow depressions on Ivorian Inselbergs. Vegetatio 117: 151-163.
- I Parmentier (2003) Study of the vegetation composition in three inselbergs from Continental Equatorial Guinea: Effects of site, soil factor and positive relative to forest fringe. Belgian Journal of Botany 136: 63-72.
- DJ Cotter, RB Platt (1959) Studies on the ecological life history of Portulacasmallii. Ecology 40: 651-668.
- DN Wiggs, RB Platt (1962) Ecology of Diamorpha cymosa. Ecology 43: 654-670.
- H Bremer, H Sander (2000) Inselbergs: Geomorphology and Geoecology. In: S Porembski, W Barthlott, Inselbergs - biotic diversity of isolated rock outcrops in tropical and temperate regions. Ecological Studies, Springer-Verlag, Berlin.
- S Dörrstock, S Porembski, W Barthlott (1996) Ephemeral flush vegetation on inselbergs in the Ivory Coast (West Africa). Candollea 51: 407-419.
- MA Mares (1997) The geobiological interface: Granitic outcrops as a selective force in mammalian evolution. Journal of the Royal Society of Western Australia 80: 131-139.
- Juan J Armesto, Jose A Martínez (1978) Relations between vegetation structure and slope aspect in the Mediterranean region of Chile. Journal of Ecology 66: 881-889.
- TS Masehela (2008) An investigation into the biodiversity of the Madiba and Ramahela granite hills on the campus grounds of the University of Limpopo, Turfloop Campus. Unpublished Honours Mini-dissertation. University of Limpopo, Sovenga.
- L Mucina, MC Rutherford (2006) The Vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria, 478-479.
- AB Low, AG Rebelo (1996) Vegetation of South Africa, Lesotho and Swaziland. A companion to the vegetation of South Africa, Lesotho and Swaziland. Department of Environmental Affairs and Tourism, Pretoria, 478.
- MJA Werger (1974) On concepts and techniques applied in the Zurich-Montpellier method of vegetation survey. Bothalia 11: 309-323.
- B vanWyk, S Malan (1988) Field Guide to the Wild Flowers of the Witwatersrand and Pretoria, including the Magaliesberg and Suikerbosrand. Struik Publishers, Cape Town.
- A Fabian, G Germishuizen (1997) Wild Flowers of Northern South Africa. Tien Wah Press (Pty) Ltd., Singapore.
- B van Wyk, P van Wyk (1997) Field Guide to Trees of Southern Africa. Struik Publishers, Cape Town, South Africa.
- F van Oudtshoorn (1999) Guide to Grasses of Southern Africa. Briza Publications, Arcadia, Pretoria.
- G Germishuizen, NL Meyer (2003) Plants of southern Africa: An annotated checklist. Strelitzia 14. National Biodiversity Institute, Pretoria, South Africa.
- KR Clarke, RN Gorley (2001) Plymouth Routines in Multivariate Ecological Research (PRIMER). Primer-E. Ltd, Plymouth.
- NJ Gotelli, RK Colwell (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379-391.
- KK Caylor, HH Shugart (2006) Pattern and process in savanna ecosystems. In: P D'Odorico, A Porporato, Dryland Ecohydrology. Springer, Dordrecht, 259-281.
- BW Allred, SD Fuhlendorf, FE Smeins, et al. (2012) Herbivore species and grazing intensity regulate community composition and an encroaching woody plant in semi-arid rangeland. Basic and Applied Ecology 13: 149-158.
- G Martindale (2007) Influence of livestock grazing on plant diversity of Highland Sourveld grassland in KwaZulu-Natal. Unpublished MSc, Dissertation, University of Witwatersrand, South Africa.
- DG Milchunas, OE Sala, WK Lauenroth (1988) A generalized model of the effects of grazing by large herbivores on grassland community structure. The American Naturalist 132: 87-106.
- MM Hlaka (2010) The effect of organic and inorganic fertilizer in terms of dry matter production and nutritional value on the rejuvenation of an old sward of Blue Buffalo grass under dry land conditions. Unpublished MSc. Dissertation. University of Limpopo, Sovenga.
- CL Seymour, SJ Milton, SJ Joseph, et al. (2010) Twenty years of rest returns grazing potential, but not palatable plant diversity, to Karoo rangeland, South Africa. Journal of Applied Ecology 47: 859-867.
- S Lin (2007) The distribution and role of an invasive plant species, Lantana camara, in disturbed roadside habitats in Moorea, French Polynesia. Student Research Papers, Fall 2007, UCB Moorea Class: Biology and Geomorphology of Tropical Islands, University of California, Berkeley.
- ASL Rodrigues, SJ Andelman, SJ Bakarr, et al. (2004) Effectiveness of the global protected area network in representing species diversity. Nature 428: 640-643.
Corresponding Author
Tlou S. Masehela, Applied Biodiversity Research, Kirstenbosch Research Centre, South African National Biodiversity Institute, Private Bag X7, Claremont 7735, South Africa, Tel: +27-78-285-2553.
Copyright
© 2017 Masehela TS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.