Relevance and Therapeutic Implication of Macroprolactinemia Detection Using PEG 6000 in Women of Childbearing-Age with Hyperprolactinemia: Experience of a Tertiary Hospital
Abstract
Introduction: Macroprolactin (MacroPRL), a variant of human prolactin, may interfere with hormonal assay and falsely increase serum prolactin levels. Therefore, failure to identify macroprolactinemia can lead to inappropriate investigations and treatment in women already susceptible to anxiety and stress. We aimed to identify macroprolactinemia among women of childbearing age with hyperprolactinemia.
Materials and methods: We conducted a cross-sectional study in a tertiary care setting at the endocrine unit. Study participants were recruited from both endocrine and gynecological outpatient consultation services. They were women of childbearing age (18 to 49 years) consulting for signs and symptoms of gonadal dysfunction or hyperprolactinemia (PRL > 25 ng/ml). Total prolactin was measured using a Human direct ELISA method. Polyethylene glycol 6000 (PEG 6000) precipitation was used to detect macroprolactin.
Results: We enrolled 33 women with a mean age of 31 ± 7 years (range 21-48). Twenty-seven (81.8%) participants were symptomatic, and the majority, 23/27 (69.7%) reported having galactorrhea, and 21 (63.4%) women reported having an irregular menstrual cycle. The median pre-precipitation prolactinemia reduced significantly after PEG precipitation from 61.2 (IQR: 33.2-115.9) ng/ml to 33.8 (IQR: 17.9-70.5) ng/ml, p < 0.001. After PEG precipitation 5 participants had a serum prolactin recovery rate below 60%, and therefore a prevalence of macroprolactinemia at 15.2%. Four out of five (80%) women with macroprolactinemia presented with symptoms which were (Amenorrhea, oligomenorrhea and galactorrhea).
Conclusion: PEG 6000 permitted the detection of macroprolactinemia in women of childbearing age with hyperprolactinemia who otherwise would have been subjected to unnecessary medical investigations and treatment. Also, most of those with macroprolactin were symptomatic contrary to previous postulates.
Keywords
Prolactin, Macroprolactin, PEG, Prolactin recovery rate, Hyperprolactinemia
Abbreviation
MacroPRL: Macroprolactin; PEG 6000: Polyethylene glycol 6000; PRL: Prolactin
Introduction
Prolactin (PRL) is a single-chain protein synthesized and released by lactotroph cells of the anterior pituitary gland [1]. Its secretion is regulated by dopamine which has an inhibitory effect on lactotroph cells [1]. When prolactin secretion increases in the absence of pregnancy, clinical symptoms such as galactorrhoea and irregular menstrual cycles may occur. These menstrual abnormalities include spaniomenorrhoea and amenorrhoea which may contribute to infertility. Hyperprolactinemia is a well-recognized hormonal etiology of infertility amongst women of childbearing age. It affects 30-40% of infertile women and 15-20% of women with menstrual disorders [2]. Impairment of gonadal function and ultimately, infertility result from suppression of the pulsatile secretion of gonadotrophins [3]. The majority of prolactin molecules present as monomers which are biologically active but these may also exist as macromolecules (macro PRL) known as big and big-big prolactin which may interfere with laboratory measurements of the protein [4]. According to Vilaret, et al. in 2019, two Brazilian series reported macro PRL as being the third cause of non-physiological hyperprolactinemia after drugs and pituitary adenomas [5]. All three forms of prolactin are indistinguishable by routine laboratory assays.
Prolactin is measured in the biochemistry laboratory using immunometric methods on patient serum. The gold standard method to distinguish macroprolactin (big and big big forms) from the monomeric form is gel-filtration chromatography which is an expensive and time-consuming process [6]. Polyethylene glycol (PEG) has been used as a rapid and less costly method to screen for macroprolactinemia during routine analysis in clinical laboratories and research settings [7]. The presence of macro PRL may interfere with hormonal assay and falsely increase prolactin values [1]. As a result, misdiagnosis, unnecessary investigation and inappropriate treatments can be prescribed to these women who are already susceptible to anxiety and stress related to infertility.
Considering the psychological and financial impact these diagnostic errors may have on patient management and prognosis, we sought to determine the prevalence and effect of macroprolactinemia among Cameroonian women of childbearing age who present with signs of gonadal dysfunction using the PEG precipitation method which is an affordable and easily reproducible method used in clinical biology laboratories.
Materials and Methods
Study design, setting and population
We undertook a cross-sectional study at the endocrine unit of the Yaoundé Central Hospital, a tertiary hospital in Cameroon over 12 months (June 2020 to June 2021). Our study participants were drawn from outpatient consultations in the endocrinology unit of the hospital above. This included all consecutive women of childbearing age (18 to 49 years) consulting for gonadal dysfunction (galactorrhoea, spaniomenorrhoea and/or amenorrhoea) or hyperprolactinemia (PRL > 25 ng/ml) [8]). Pregnant or nursing mothers were not included in the study.
Clinical and biological sample collection
We provided all the information related to decision making as well as additional concerns on a consent and information forms. After this, we obtained, written informed consent from each individual who accepted to participate in the study. The participants were interviewed on the basis of a pre-established questionnaire. The clinical evaluation included: anthropometric parameters, vital parameters, gynecologic and pharmacologic history, medical history and breast examination. After clinical examination, we collected five milliliters of venous blood sample in a dry tube after ensuring asepsis. The patient sample was left to clot at room temperature and then centrifuged at 1500 revolutions/minute for 10 minutes and the resulting serum was collected and stored at -20 ℃ for prolactin and macroprolactin assay.
Prolactin assay
Prolactin was measured from serum samples through the direct ELISA method using reagents supplied by Human GERMANY (HUMAN ELISA test for quantitative determination of prolactin KIT® REF 53030 pack 21002) according to manufacturer's procedure. Reagents for the assay are ready- to- use and pre-dispensed in the sealed reagent strips. All of the assay steps were performed manually by the investigator.
Macroprolactin measurement
Macroprolactin was measured following precipitation with 25% polyethylene glycol 6000 [9]) (Polyethylenglykol 6000, ROTIPURAN® Ph.Eur. Carl Roth GmbH+ Co. KG- Schoemperlenstr. 3-5-D-76185 Kans).
Procedure of PEG precipitation
Four hundred microliters (400 µl) of 25% PEG solution was added to 400 µl of patient serum at room temperature. After mixing and stabilizing at room temperature for 30 minutes, this tube was centrifuged at 1800 revolutions per minute for 30 minutes, at 20 ℃ and then the supernatant isolated for PRL analysis as previously described. After correction for dilution, the results were compared with those obtained from unprecipitated serum. The PRL recovery rate (RR) was calculated using the formula:
Prolactin recovery rate less than 60% was considered as a significant level macroprolactin while recovery rate equal to or above 60% indicated the non-significant level of macroprolactin.
Laboratory analyses were performed at the CIAB EXACT laboratory which is a private laboratory located in the same town.
Statistical analysis
Data were analyzed using SPSS software version 21.0. Quantitative variables were reported as means and standard deviations or median and interquartile range depending on the distribution of data. Whereas, qualitative variables were expressed in terms of frequencies and proportions. The results of prolactin analysis were expressed as percentage of PRL recovery after precipitation.
Ethical consideration
The research protocol was submitted to the Regional Ethics Committee of human research of the Center region (clearance number 1591/CRERSHC/2020). Our study was carried out in strict accordance with the ethical principles of the Declaration of Helsinki. Results were disclosed to participants by the investigators at the end of the study.
Results
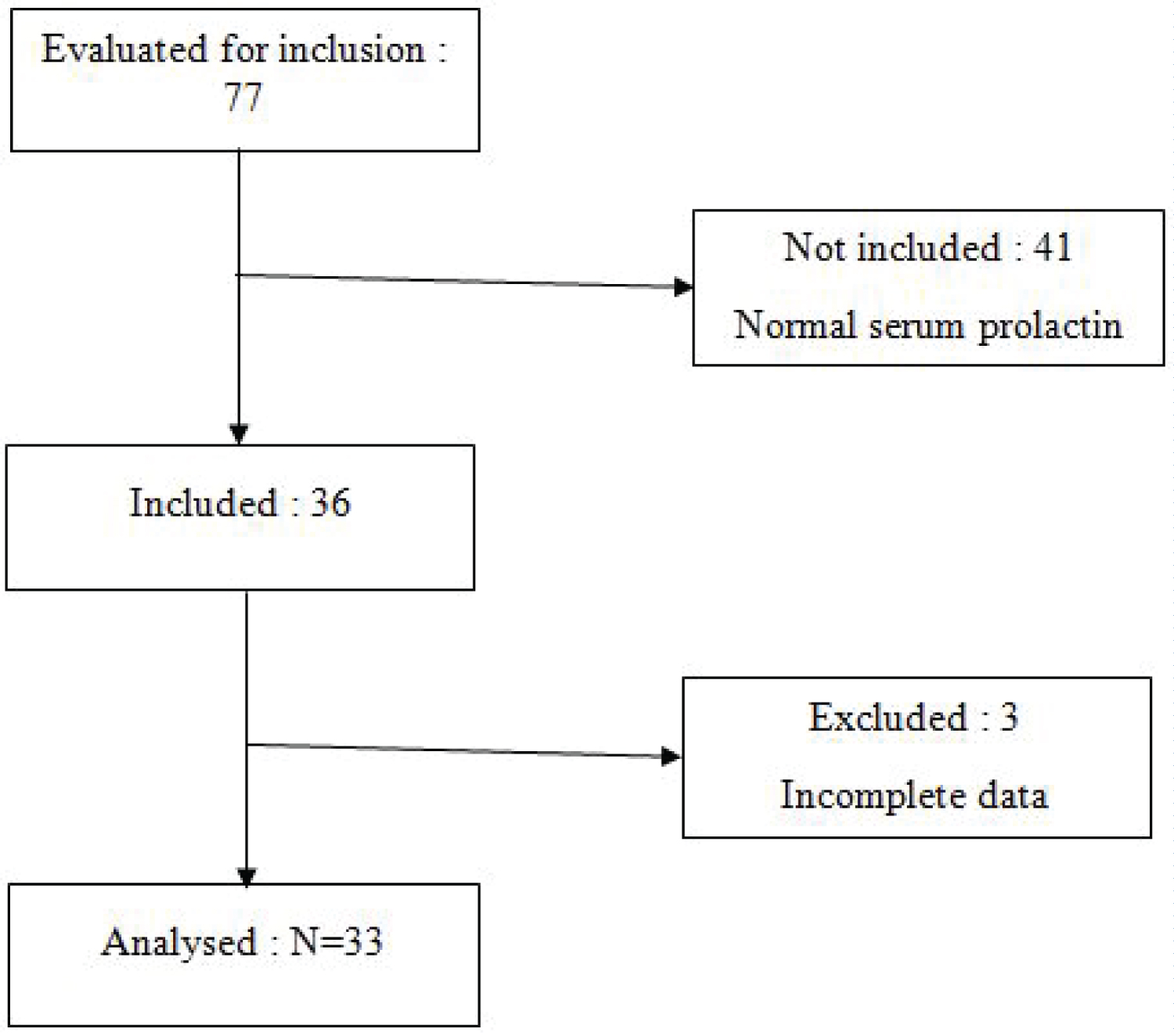
During the study period, 77 women were eligible for participation in our study. We did not include 41 due to normal serum prolactin and failure to give their informed consent. We later excluded 3 participants due to incomplete data giving a final participant number of 33 participants. The results that will follow are those of the 33 participants who completed the study (Figure 1).
Clinical characteristics of the study population
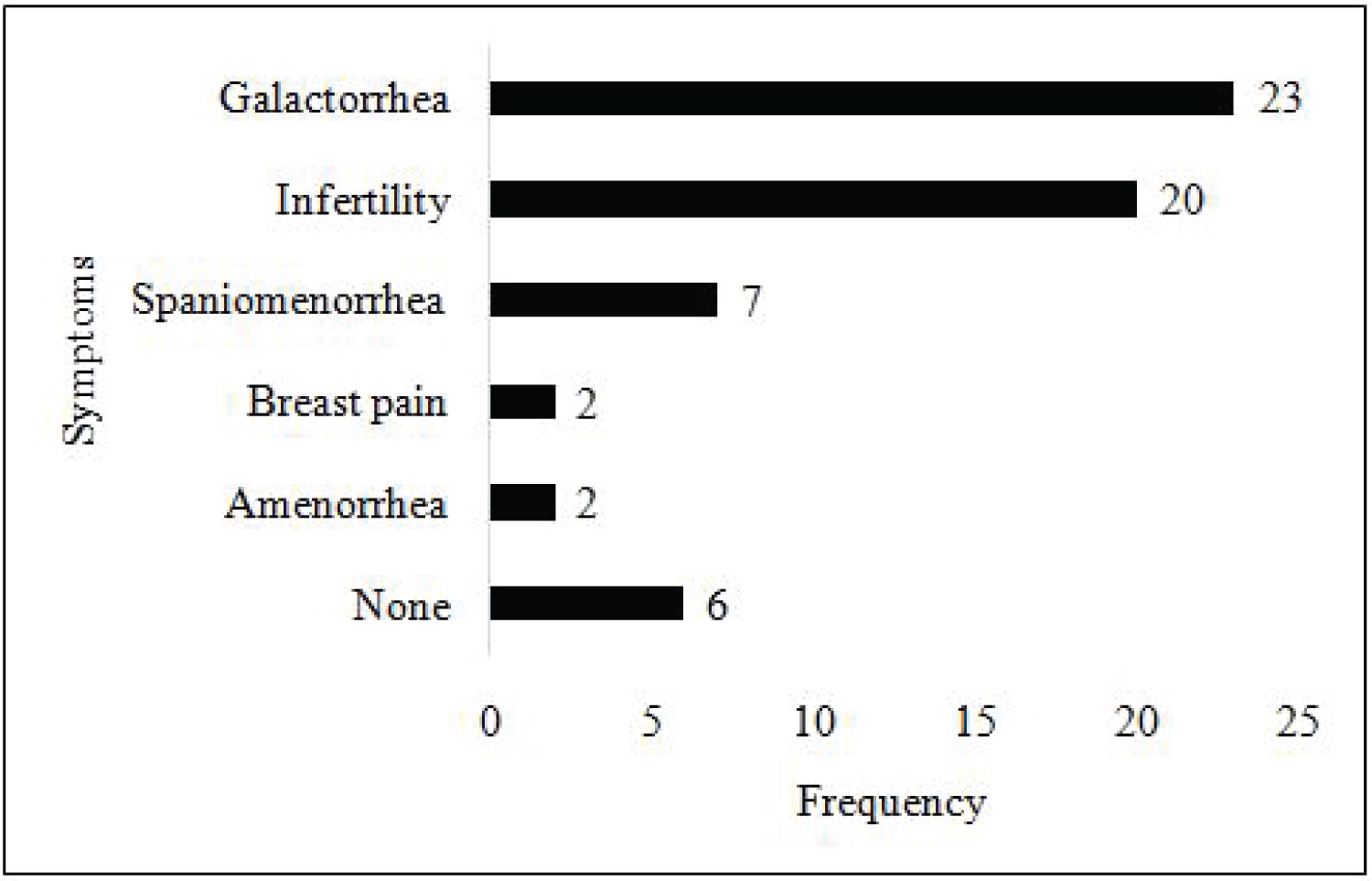
Overall, the women of our study population had a mean age of 31.7 ± 6.9 years (range 21-48). The majority of the participants reported symptoms of hyperprolactinemia. As regards symptoms the women reported, 23(69.7%) had galactorrhea while 6(18.2%) did not have any symptoms. Other symptoms reported were spaniomenorrhea, amenorrhea and infertility (Figure 2).
Basal prolactinemia, post-PEG prolactinemia and prolactin recovery rate
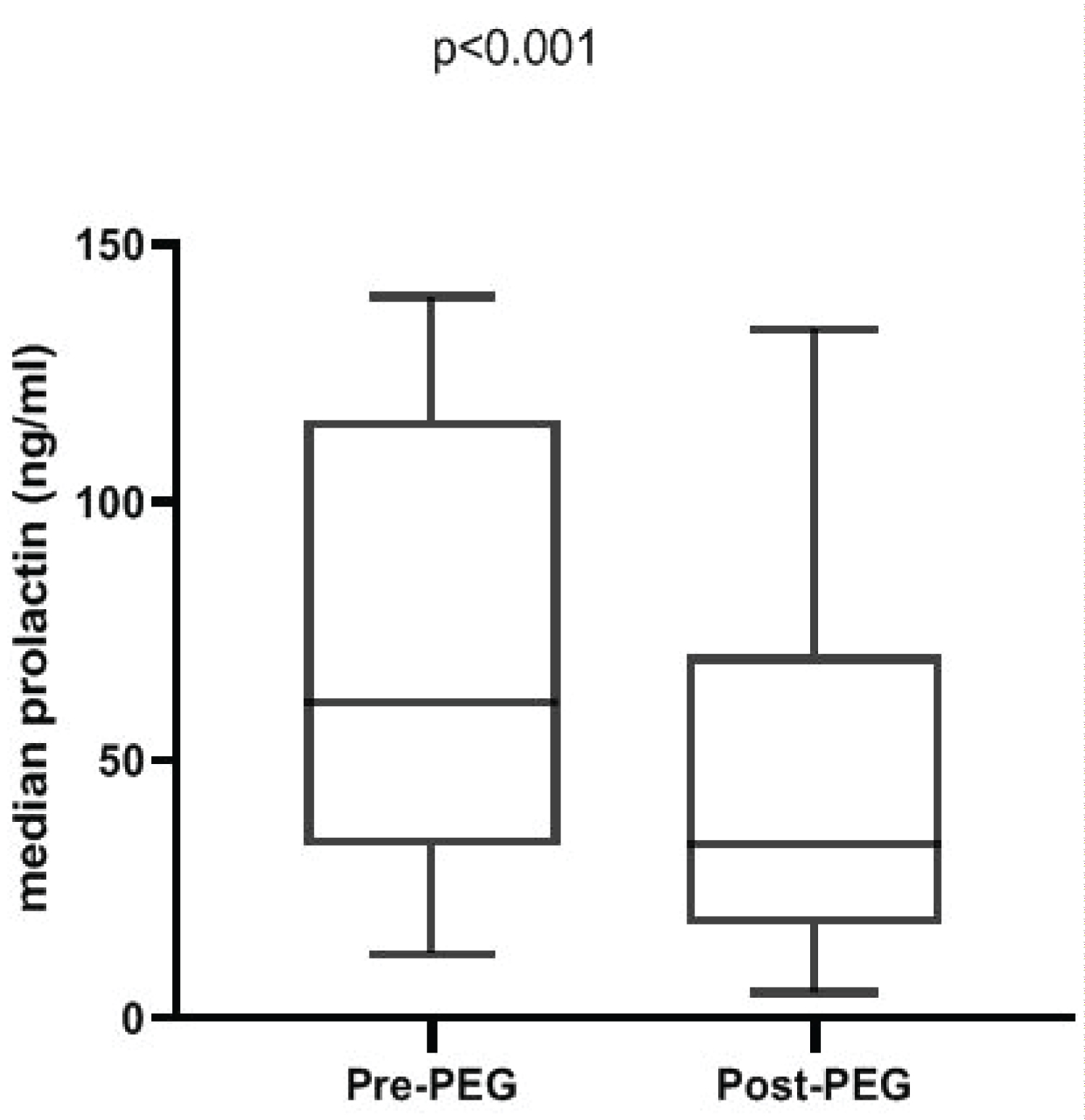
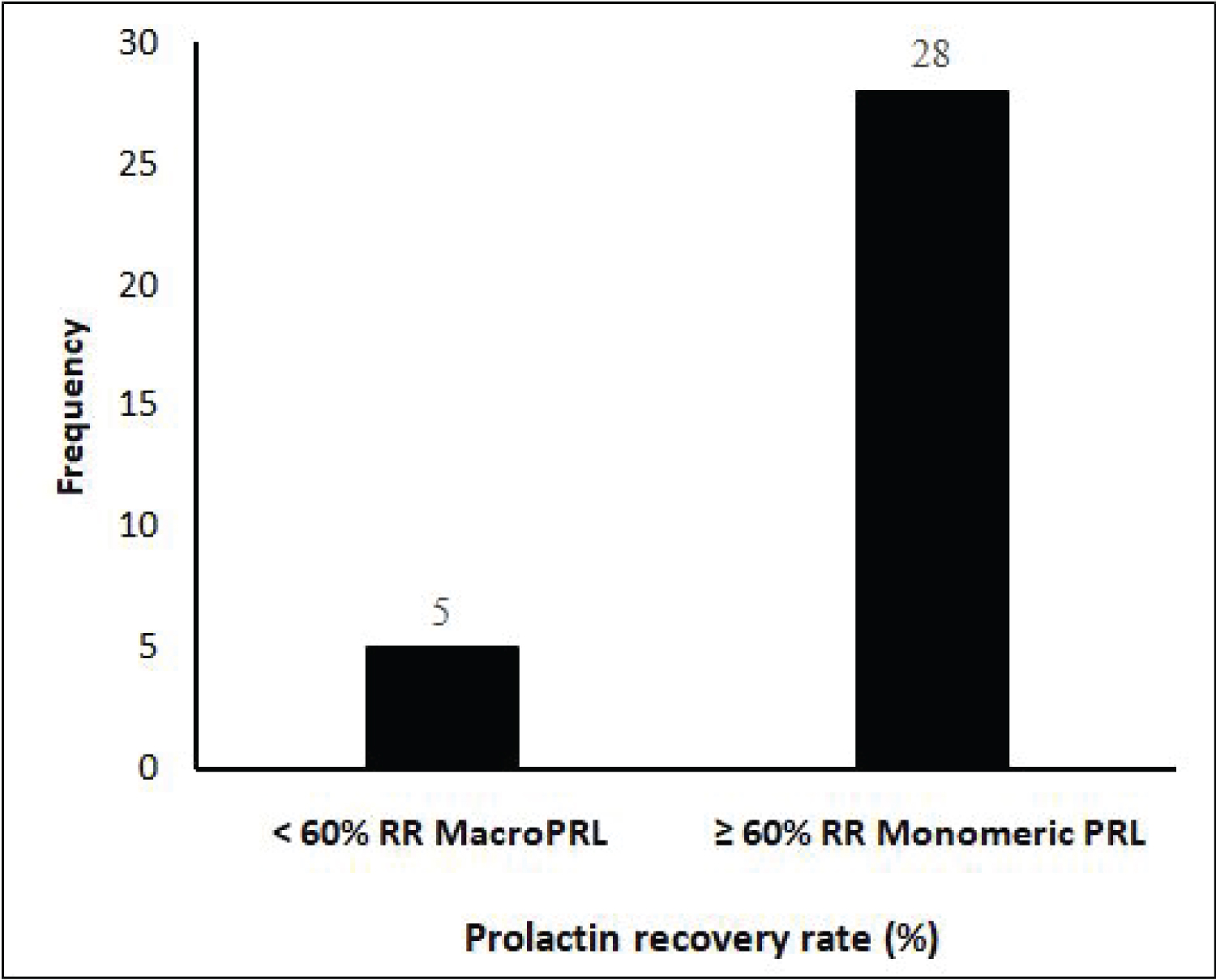
Median pre-PEG precipitation prolactinemia was 61.2 (IQR: 33.2-115.9) ng/ml. After PEG-precipitation, prolactin level decreased significantly (from 61.2 (IQR: 33.2-115.9) ng/ml to 33.8 (IQR: 17.9-70.5) ng/ml, p < 0.001) (Figure 3). After PEG precipitation, 5 participants had a prolactin recovery rate below 60%, representing those with significant macroprolactinemia (Figure 4). Thus, the prevalence of macroprolactinemia in our study population was 15.2%.
In order to identify factors which could be related to the presence of macroprolactin, we subdivided the study population into two groups according to the PRL RR: macroprolactinemia group (RR < 60%, n = 5) and true hyperprolactinemia group (RR > 60%, n = 28). As shown in Table 1, the different hyperprolactinemia-related symptoms as well as clinical parameters were comparable between the two groups. Moreover, 4 out of the 5 women with macroprolactinemia presented with symptoms of hyperprolactinemia.
Discussion
The aim of this study was to detect macroprolactinemia using PEG 6000, among a group of Cameroonian women of childbearing age with hyperprolactinemia. Several methods had been described in literature for the diagnosis of macroprolactinemia. These methods include polyethylene-glycol- (PEG-) precipitation method for the screening, and the confirmative and qualitative examinations include gel chromatography (GFC), protein A/G column, and 125I-PRL binding studies [10] despite the fact that PEG is not highly specific, comparison with other methods showed that PEG precipitation was superior and showed the best concordance with GFC. Moreover, PEG is widely used because it is simple and inexpensive. For these reasons PEG, was validated against gel filtration chromatography which is a gold standard for the diagnosis of macroprolactinemia, and it appears to be a cost-effective screening method which is affordable to use in our resource-limited setting. A valueless than 40% recovery after PEG precipitation of prolactin has been validated for the diagnosis of macroprolactinemia. With this cutoff, there is a 100% sensitivity in picking up macroprolactin [11].
We found that 5 out of 33 women included in this study had a prolactin recovery rate less than 60%, given a prevalence of macroprolactinemia of 15.2%.
Consistent with this finding, Soh and colleagues recently pooled data from 67 studies (n = 3770) and reported an overall prevalence of 18.9% for macroprolactinemia in patients with hyperprolactinemia [12]). However, the prevalence reported in this study is lower compared with the prevalence reported in other settings. Fahie-Wilson, et al. reported a prevalence of macroprolactin at 20% among England hyperprolactinemic patients [1]) while this prevalence ranged from 15 to 35% in Croatia hyperprolactinemic populations [13]) and from 8 to 42% in 9 European series [6]). All these showed that macroprolactin is not uncommon in studied populations and should be screened. Overall, the prevalence of macroprolactinemia in patients with hyperprolactinemia varies across the world regions and countries from 8-66%, with respect to the cut-off value for hyperprolactinemia used and also according to study designs [12]. Few studies are available in Africa and in the sub-Saharan region in particular. Thus, this study is among the first one that sought to determine the prevalence of macroprolactinemia in a cohort of hyperprolactinemic patients from sub-Saharan Africa.
It was argued that macroprolactin is confined to the vascular system and has limited access to the prolactin receptor of target organs resulting into limited bioactivity in vivo and asymptomatic hyperprolactinemia [13-15]. Consistent with this hypothesis, there was no association between macroprolactinemia and common symptoms of hyperprolactinemia in our study population. Macroprolactinemia is usually suspected when hyperprolactinemic patients do not present with symptoms of hyperprolactinemia. However, some patients present with symptoms such as menstrual disorders, galactorrhea or signs of hypogonadism which are thought to reveal concomitant pathologies such as polycystic ovarian disease (PCOS), monomeric prolactinemia or psychogenic erectile dysfunction in men [6]. Isik, et al. in 2012 among other studies have reported a prevalence of hyperprolactinemia symptoms in upto 45% of patients with macroprolactinemia [6,12,16,17]. In addition, Olukoga, et al. [18] suggested that the macroprolactin complex may dissociate in vivo in some cases, releasing bioactive, monomeric prolactin that causes the symptoms in these patients. This release of monomeric PRL, that may result from intermittent dissociation from the low affinity, high capacity IgG antibody to which it is bound in macroprolactin, may contribute to development of symptoms of hyperprolactinemia [11].
Conclusion
PEG 6000 has identified macroprolactinemia in our studied population with a prevalence of 15.2%. This allowed us to avoid extensive investigations and excessive treatment of hyperprolactinemia. Thus, screening for macroprolactin should be included in the routine investigation of all hyperprolactinemic patients when they are asymptomatic. PEG appears to be cost-effective for the detection of macroprolactin in a low resource-setting.
Availability of Data and Materials
Data are available as supplementary material.
Funding
No funding was received for this study.
Authors' Contributions
Conception and design: Ama Moor V, Sobngwi E, Ongmeb BA, Etoa EM. Acquisition of data: Ongmeb BA, Mendane MF, Dehayem M. Data analysis and interpretation: Falmata A, Ongmeb BA, Djahmeni E, Ndi Manga A. Drafting the manuscript or revising: Mbanya JC, Ongmeb BA, Feutseu C, Katte JC, Mbono Samba E. All authors approved the final manuscript.
Acknowledgements
Our gratitude and acknowledgements go to Djahmeni E and to all the women who gave their informed consent to participate and to all the health personnel of the various institutions in which the project took place; the Endocrinology unit of the Yaounde Central Hospital; PRIMA laboratory and CIAB EXACT laboratory.
Patient Consent for Publication
Not applicable.
Ethical Approval
Ethical clearance was obtained from the Regional Ethics Committee of human research of the Center region at the Ministry of Public Health, Cameroon (N° 1591/CRERSHC/2020). Before enrollment in the study, each participant provided a written informed consent.
References
- Fahie Wilson MN, John R, Ellis AR (2005) Macroprolactin; high molecular mass forms of circulating prolactin. Ann Clin Biochem 42: 175-192.
- Saleem MN, Martin H, Coates P (2018) Prolactin biology and laboratory measurement: An update on physiology and current analytical issues. Clin Biochem Rev 39: 3-16.
- Thirunavakkarasu K, Dutta P, Sridhar S, et al. (2003) Macroprolactinemia in hyperprolactinemic infertile women. Endocrine 44: 750-755.
- Kaiser UB (2012) Hyperprolactinemia and infertility: New insights. J Clin Invest 122: 3467-3468.
- Gibney J, Smith TP, McKenna TJ (2005) Clinical relevance of macroprolactin. Clin Endocrinol 62: 633-643.
- Vilar L, Vilar CF, Lyra R, et al. (2019) Pittfalls in the diagnostic evaluation of hyperprolactinemia. Neuroendocrinology 109: 7-19.
- Smith TP, Suliman AM, Fahie Wilson MN, et al. (2002) Gross variability in the detection of prolactin in sera containing big prolactin (macroprolactin) by commercial assays. J Clin Endocrinol Metabol 87: 5410-5415.
- Brue T, Delemer B (2007) Diagnosis and management of hyperprolactinemia: Expert consensus-French Society of Endocrinology. Ann Endocrinol 68: 58-64.
- Fahie Wilson MN, Soule SG (1997) Macroprolactinemia: Contribution to hyperprolactinemia in a district general hospital and evaluation of a screening test based on precipitation with polyethene glycol. Ann Clin Biochem 34: 252-258.
- Kavanagh L, McKenna TJ, Fahie Wilson MN, et al. (2006) Specificity and clinical utility of methods for the detection of macroprolactin. Clin Chem 52: 1366-1372.
- Olukoga AO, Kane JW (1999) Macroprolactinaemia: Validation and application of the polyethylene glycol precipitation test and clinical characterization of the condition. Clin Endocrinol 51: 119-126.
- Che Soh NAA, Yaacob NM, Omar J, et al. (2020) Global Prevalence of Macroprolactinemia among Patients with Hyperprolactinemia: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 17: 8199.
- Kasum M, Pavicic Baldani D, Stanic P, et al. (2014) Importance of macroprolactinemia in hyperprolactinemia. Eur J Obstet Gynecol Reprod Biol 183: 28-32.
- Kalsi AK, Halder A, Jain M, et al. (2019) Prevalence and reproductive manifestations of macroprolactinemia. Endocrine 63: 332-340.
- Hattori N, Ishihara T, Saiki Y, et al. (2010) Macroprolactinaemia in patients with hyperprolactinaemia: Composition of macroprolactin and stability during long-term follow-up. Clin Endocrinol 73: 792-797.
- Isik S, Berker D, Tutuncu YA, et al. (2012) Clinical and radiological findings in macroprolactinemia. Endocrine 41: 327-333.
- McCudden CR, Sharpless JL, Grenache DG (2010) Comparison of multiple methods for identification of hyperprolactinemia in the presence of macroprolactin. Clin Chim Acta 411: 155-160.
- Olukoga AO (2002) Paradoxical severe decrease in serum HDL-cholesterol after treatment with a fibrate. J Clin Pathol 55: 718.
Corresponding Author
Martine Claude Etoa Etoga, National Obesity Centre, Yaoundé Central Hospital, 87, Yaoundé, Cameroon, Tel: +237-67-481756.
Copyright
© 2023 Boli AO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.