Baseline Serum Total Cortisol during the Primary Coronavirus Infection in the Beginning of the COVID-19 Pandemic in Cameroon
Abstract
Background: Improvement of critically ill patients Covid-19 positive placed on glucocorticoids may suggest impairment of the adrenal function. Our objective was to evaluate baseline serum cortisol in covid+ patients.
Methods: This was a descriptive and analytical cross-sectional study conducted in a population of patients infected with SARS-CoV-2 in Cameroon. Patients receiving corticosteroids irrespective of the route of administration within 3 months prior to recruitment and those with any hypothalamic-pituitary-adrenal axis disease were excluded. We reviewed the medical records of patients to collect socio-demographic and clinical data. Patients were then sampled at 8 a.m. for serum cortisol assay by the competitive ELISA method. Statistical analyses were performed using the Student's test to compare means. The significance level was set at p < 0.05.
Results: We included 80 covid+ patients, predominantly males (45, 56.3%) with a mean age of 43 ± 13 years. The co-morbidities found were hypertension (9, 11.3%), diabetes mellitus (7, 8.8%), cardiovascular disease (8, 10%) and obesity (4, 5%). The most common symptoms were asthenia (44, 55%), fever (36, 45%) and respiratory symptoms (75, 93.7%). The non-severe form was the most common (70, 87.5%). Only 12.5% (10) of patients received oxygen therapy within our study. The mean baseline serum cortisol in the series was 279.55 nmol/l ± 128.68 (min 96.1332 nmol/l; max 665.0521 nmol/l). Sixty-nine patients (86.3%) had baseline cortisol ≤ 413.79nmol/l, suggesting an insufficient response to stress. No statistically significant association was found between serum cortisol and disease severity.
Conclusion: The absence of a marked rise of cortisol during COVID-19 suggests possible involvement of the hypothalamic-pituitary-adrenal axis in this infection.
Keywords
COVID-19, Serum cortisol, Severity
Abbreviations
ACE: Angiotensin Converting Enzyme; ACTH: Adrenocorticotropic Hormone; RNA: Ribo Nucleic Acid; Covid+: Confirmed Case of COVID-19; COVID-19: Disease Caused by the New Coronavirus (2019-nCoV or SARS-CoV-2); ELISA: Enzyme Linked Immuno Sorbent Assay; HTN: Hypertension; MERS: Middle East Respiratory Syndrome; RT-PCR: Real Time Polymerase Chain Reaction; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SARS: Severe Acute Respiratory Syndrome
Background
Rapidly declared as a pandemic, COVID-19 is a highly contagious disease with a clinical presentation ranging from mild, moderate to severe [1]. The cytokine storm track is cited as the reason for the severity and mortality during COVID-19 [2]. However, the determinants of severity and mortality during COVID-19 are still poorly understood. While it is true that the elemental lesion is thromboembolic and affects the pulmonary parenchyma, it cannot be excluded that the adrenal gland is affected. Moreover, in the recovery trial published in June 2020, the benefit of dexamethasone in the management of COVID-19 has been demonstrated, with a reduction in mortality of critical patients by one third [3]. This beneficial effect of dexameth as one is linked to its action on alveolo-interstitial pulmonary lesions but possibly to a supplementation of the corticotropic axis with respect to severe infection. An induced adrenal response during critical illness is a determinant of survival. During sepsis, the hypothalamus-pituitary-adrenal axis is stimulated by the systemic inflammatory response (SIRS) [4]. The increase in cortisol is proportional to the severity of sepsis. However, in 30 to 70% of sepsis, the adrenal response is insufficient, or there is peripheral resistance to glucocorticoids which is associated with a poor prognosis and requires supplementation [5]. Few data exist on the adrenal response induced by COVID-19 infection. The purpose of this preliminary study was to assess baseline adrenal function during primary infection with the new coronavirus and to correlate it with disease severity.
Methods
Setting and participants
The study was conducted in the isolation unit for the Covid positive patient at the Yaoundé central hospital. This is a unit created as part of the Covid pandemic since March 2020 when Cameroon recorded its first positive case of Covid 19. The department has a capacity of 26 beds with an intensive care unit suitable for critical patients requiring oxygen.
Study period
Patients were recruited from March 2020 to April 2020.
Study design
We conducted a cross-sectional study of Covid-19 management centres in Cameroon.
Inclusion criteria
We included consenting participants followed up in covid-19 management centers with positive virological RT-PCR results.
Exclusion criteria
Patients on corticosteroids, regardless of the route of administration taken during the 3 months prior to recruitment, patients with hypothalamic-pituitary-adrenal axis pathology and pregnant women were excluded.
Procedure
Data were collected from the medical records of the patients concerned. Age, sex, co-morbidities, clinical presentation, ambient oxygen saturation and resort oxygen therapy were recorded.
Specimens collection
RNA Extraction and RTPCR amplification: Nasopharyngeal aspirates samples were collected from patients and frozen at 80 ℃ before RNA extraction by using the NucleoSpin Dx Virus (Ref: Macherey Nagel 740895.50), according to manufacturer's instructions at the Centre Pasteur, Yaoundé, Cameroon. RNA extracted from 100 µl of original sample, is eluted in 100 µl of elution buffer. RT-PCR was performed by using Invitrogen Superscript™ III Platinum® One-Step qRT-PCR system (ref: 11732-088) in a final volume of 25 μL containing 0.4 μM of each primer, 0.8 mM MgSO4, 3 mM Mg of reaction mix 2X, 0.2 μM of probe and 5 μL of RNA extract. A Light cycler fi 480 (Roche) system was used with the following cycling conditions: 55 ℃ for 20 min, 95 ℃ for 3 min, 50 cycles of 95 ℃ for 15s, 58 ℃ for 30 s, and 40 ℃ for 30s [6].
Quantitative determination of cortisol: Blood samples were then taken at 8 a.m. to respect the nycthemeral cycle of cortisol secretion after curbing the stress of direct venipuncture. The assays were performed by competitive ELISA in a serial manner according to laboratory procedures on a spectrophotometer (UV Mini 1240) using cortisol ELISA from IBL International GmbH, part of TECAN group kits (catalog Number RE52061). The biological data were interpreted as follows: A cortisol threshold ≤ 413.79 nmol/l was considered to be an insufficient cortisolic response to infection and > 413.79 nmol/l normal. Patent adrenal insufficiency was defined by a cortisol level ≤ 137.93 nmol/l.
Statistical analyses: Patients were then classified into 2 subgroups according to severity: Subgroup with oxygen therapy and subgroup without oxygen therapy. Data were compiled and analyzed using SPSS version 23 software. Quantitative variables were reported as means and standard deviation. These were compared using student t-test. Qualitative variables were presented as percentages and these were compared with a chi-square test. The statistical significance level was set at 0.05.
Results
Socio-demographic characteristics
We recruited 80 participants, the majority of whom were male 45/80 (56.3%). The average age was 43 ± 13 years. The most represented age group was over 40 years (44, 55%).
Clinical characteristics
All participants recruited were positive for RT-PCR. The main co-morbidities found were hypertension (9/80, 11.3%), diabetes mellitus (7/80, 8.8%), cardiovascular disease (8/80, 10%) and obesity (4/80, 5%). The symptoms found included asthenia (44/80, 55%), fever (36/80, 45%), cough (49/80, 61%), respiratory distress (14/80, 17.5%), rhinorrhea (12/80, 15%) and diarrhea (3/80, 3.8%). Most of the patients presented less than 03 symptoms (74/80, 92.5%). Half of the patients recruited were sampled within an average of 5 days from the onset of symptoms. The non-severe form was the most common (70/80, 87.5%) and 12.5% (10) of the patients received oxygen therapy (Table 1).
Biological data
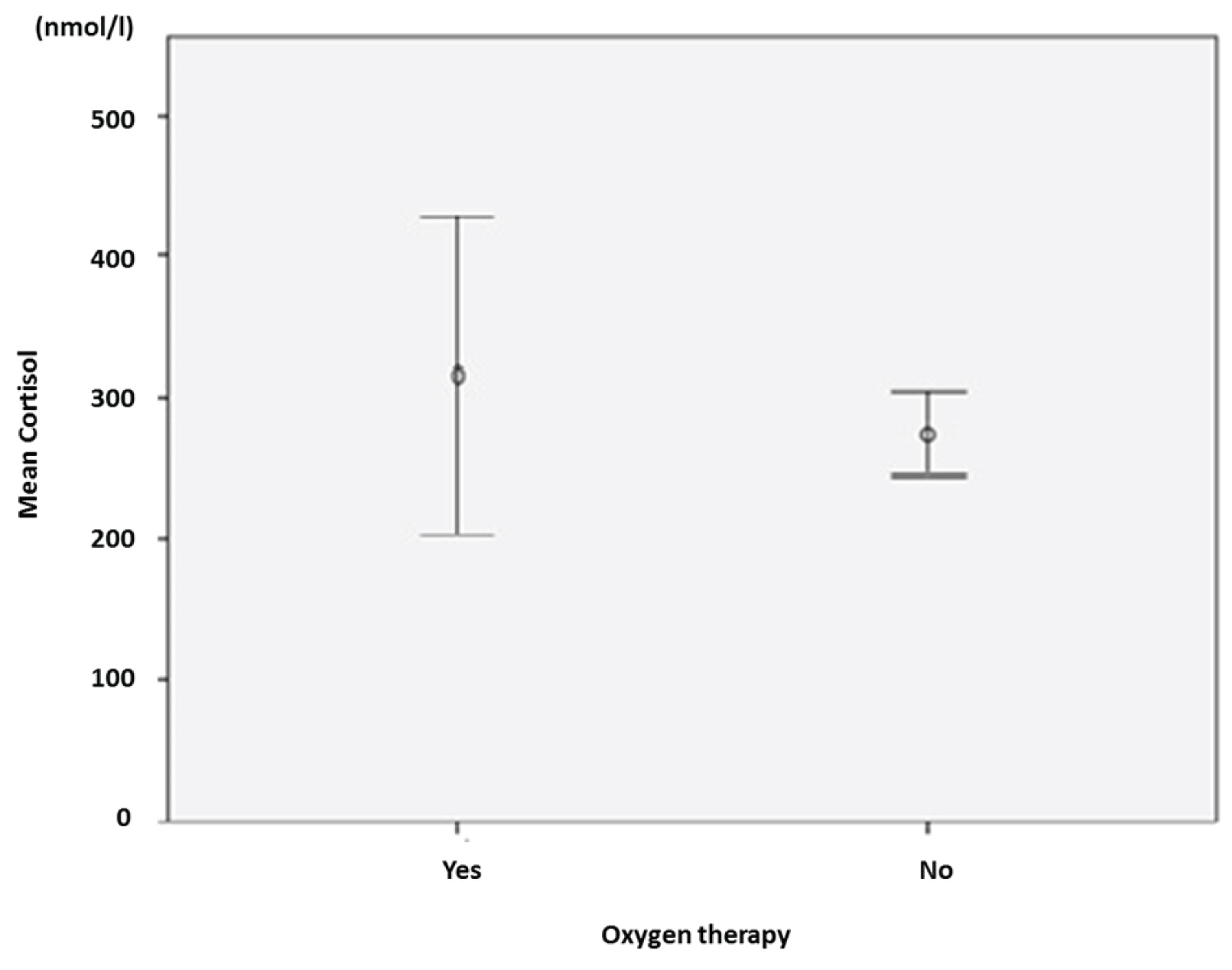
The mean baseline total cortisol level in the series was 279.5 nmol/l ± 128.68 (min 96.1 nmol/l; max 665.0 nmol/l). Sixty-nine patients (86.3%) had baseline cortisol ≤ 413.8 nmol/l and eleven (11.8%) had > 413.8 nmol/l. The mean cortisol level was higher in the group with oxygen therapy versus no oxygen therapy (316 nmol/l ± 49.7 vs. 124.54 nmol/l ± 14.9). Nine patients had patent adrenal insufficiency with serum cortisol ≤ 137.9 nmol/l (11.2%) (Figure 1).
Association between total baseline cortisol and clinical features of Covid-19
We did not find a statistically significant association between serum cortisol and oxygen therapy, asthenia, comorbidities (hypertension, diabetes mellitus and obesity), number of patient symptoms and duration of symptoms for a P < 0.05 value. In contrast, serum cortisol level was associated with patient age (Table 2).
Discussion
The objective of this study was to evaluate baseline total serum cortisol in covid positive patients. We therefore perfomed an 8 a.m. serum total cortisol in all patient positive to Covid 19 who were hospitalized in the isolation unit for Covid-19 patients. We found that the mean baseline total cortisol level was 279.5 nmol/l ± 128.7. The mean cortisol level was higher in the group with oxygen therapy versus no oxygen therapy (316.0 nmol/l ± 49.7 versus 124.54 nmol/l ± 14.9). Nine patients had patent adrenal insufficiency with serum cortisol ≤ 137.9 nmol/l. We did not find a statistically significant association between serum cortisol and oxygen therapy. In contrast, serum cortisol level was associated with patient age.
The diagnosis of adrenal insufficiency requires ACTH stimulation test which is the most specific test for the diagnosis of adrenal insufficiency. However, Covid-19 infection in itself could constitute a stimulating stress for the HPA axis by the induced inflammatory systemic response. As a preliminary study, baseline adrenal function should be assessed in all COVID+ patients regardless of clinical disease status.
The technique used for the bioassay was the competitive ELISA method. Although this is not the reference method, it is easy to perform and suitable for screening. Its threshold of sensitivity is 88% and it is accessible in our context.
The socio-demographic and clinical characteristics of our study population are consistent with those found in the Wuhan cohort in China. Men are the most affected by the disease and have diabetes mellitus and hypertension as the main co-morbidities. The clinical presentation is by far dominated by asthenia and fever [7].
Males are believed to be most vulnerable to SARS-CoV-2 due to the greater expression of ACE receptors in men than in women. In addition, men are more susceptible to co-morbidities (hypertension, diabetes, cardiovascular disease) and therefore to severe forms of the disease [8].
Diabetes is an independent predictor of morbidity and mortality in COVID-19 [9,10]. Regardless of the time of discovery, it predisposes to severe forms through its susceptibility to infection. This is due to impairment of immune mechanisms, dysfunction of immune cells, oxidative stress caused by hyperglycemia and hypersecretion of counter-regulatory hormones [11]. However, SARS-CoV-2 as a risk factor for glycemic dysregulation is yet to be demonstrated. Some authors have raised the hypothesis of viral colonization of the pancreas through ACE receptors expressed in islets of Langerhans, however pancreatic viral particles have not been isolated in the autopsy series [9].
The mean baseline cortisol level (279.5 nmol/l ± 128.7) with 69 patients (86.3%) with baseline cortisol ≤ 413.79 nmol/l and 09 patients with cortisol less than 137.93 nmol/l represent an insufficient adrenal response to the stress induced by 2019-n CoV infection. Tricia Tan, et al. in 2020 found an association between baseline cortisol threshold value ≥ 744 nmol/l and worsening morbidity/mortality in COVID-19. They compared the value of the patient's baseline cortisolaemia within 03 days following the onset of symptoms and its fate during the following 42 days. This suggests integrity and an adapted response of the corticotropic axis in the acute phase of infection [12].
Nevertheless, these results are not applicable in our context since our patients were enrolled later in the infection (beyond 03 days). The cytokine storm characteristic of SARS-CoV-2 infection and predictor of disease severity occurs from day 6 [2,13]. This overly inadequate inflammatory response to viral aggression would also be incriminated in the syndrome of multivisceral failure with damage to the corticotropic axis in COVID-19 by destruction of the adrenal parenchyma [4]. This suggests an alteration of the cortisolic response later in the infection. In our series, it is clear that the mean baseline cortisol level decreases with the duration of symptoms (293.3 nmol/l ± 115.2 vs. 269.24 nmol/l ± 142.2).
The cortisolic response is inadequate in both the oxygen therapy group and the non-severe group. This suggests an insufficiency of basal cortisol secretion in response to stress in all phases of the disease, from less symptomatic patients to patients on respiratory assistance. During the SARS outbreak in 2003, adrenal insufficiency was described in patients infected with SARS-CoV-1 [4,14,15]. Three mechanisms were incriminated:
1. Necrosis of the adrenal tissue found in autopsy series by viral colonization of the adrenals through ACE receptors expressed in this tissue and the cytokine tornado triggered by the viral infection and directed to the adrenals.
2. Viral infiltration of the hypothalamo-pituitary axis through ACE receptors expressed in this tissue and resulting in hypopituitarism.
3. Corticotropic insufficiency caused by antibodies to ACTH. This is explained by the molecular analogy between viral proteins and ACTH [16].
SARS-CoV-2, a virus of the same family as SARS-CoV-1, could also trigger the same mechanisms and be responsible for adrenal insufficiency.
Preliminary results from the RECOVERY study published in June 2020 showed a significant reduction in mortality in severe patients with dexamethasone [10]. This potent corticosteroid is believed to be effective in reducing the cytokine storm characteristic of severe and lethal forms of COVID-19. Hence the benefit of corticosteroid therapy in severe forms of the disease which will act as an anti-inflammatory and could also have a benefit in hormone supplementation if there is associated cortisol deficiency. In our cohort, there is evidence of an insufficient and inadequate cortisol response that may require supplementation for effective control of COVID-19.
Conclusion
The absence of a marked rise in serum cortisol during COVID-19 would suggest possible involvement of the hypothalamic-pituitary-adrenal axis during this infection.
The need to perform cosyntropin stimulation tests and ACTH assays keeps its place to better characterize organ damage in the covid+ patient.
Declarations
Ethics approval and consent to participate
Before starting the study, an authorization was obtained from authorities ofthe Yaoundé Central Hospital, and an ethical clearance was delivered by the Institutional ethic committee of the Université des Montagnes (CIE-UdM) N° 2020/057/UdM/PR/CIE. All the procedures used in the present study were in conformity with the current revision of the Helsinki Declaration. All participants were informed of the various aspects of the study, and they were enrolled only after providing a signed consent form.
Consent for publication
All the authors approved the final version of the manuscript and consented for its publication.
Availability of data and materials
Data will be made available by the corresponding author upon request.
Competing interests
All the authors declared no competing interest.
Funding
None.
Authors' contributions
Study conception: ES, MCEE, AHI; Data collection: AHI, JANM; Laboratory analysis: AHI, MGF, VAM; Data analysis and Manuscript drafting: MCEE, AHI, ES; Criticalrevision of manuscript: ES; ABO, MD, MGF LKM, CK, PJF, VAM; All authors read andapproved the final manuscript.
Acknowledgements
To all the patients who participated to this study.
Authors' information
MCEE: Department of clinical sciences; faculty of medicine and pharmaceutical sciences; University of Douala and National Obesity Center, Yaoundé Central Hospital. AHI: Department of internal medicine and specialties, faculty of medicine and biomedical sciences, University of Yaoundé 1. MGF, VAM: Department of biochemistry, faculty of medicine and biomedical sciences, University of Yaoundé 1. JANM: Higher institute of sciences and health, Université des Montagnes Baganté. ABO: National Obesity Center, Yaoundé Central Hospital. MD, PJF, CK, ES: National Obesity Center, Yaoundé Central Hospital and Department of internal medicine and specialties, faculty of medicine and biomedical sciences, University of Yaoundé 1.
We declared that no patients have been reported in a previous study in our context.
References
- Aylward B, Liang W, Dong X, et al. (2019) Report of the WHO-china joint mission on coronavirus disease (COVID-19).
- Soy M, Gökhan K, Atagündüz P (2020) Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clinical Rheumatology 39: 2085-2094.
- Recovery Collaborative Group (2020) Effect of dexamethasone in hospitalized patients with COVID-19-preliminary report.
- Rimesh P (2020) COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 68: 251-252.
- William F Paolo Jr, Nosanchuck JD (2006) Adrenal Infections. International Journal of Infectious Diseases 10: 343-353.
- Institut Pasteur, Paris. Protocol: Real-time RT-PCR assays for the detection of SARS-CoV-2.
- Huang C, Wang Y, Li X, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet.
- Kaijin X, Yanfei C, Jing Y, et al. (2020) Factors associated with prolonged viral RNA shedding in patients with COVID-19. Clin Infectious Disease 71: 799-806.
- Bonny V, Maillard A, Mousseaux C, et al. (2020) COVID-19: Physiopathologie d'une maladie à plusieurs visages. La Revue de Médecine Interne-Elsevier 40: 375-389.
- Xiaochen L, Shuyun X, Muqing Y, et al. (2020) Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 146: 110-118.
- Huin C (2020) COVID-19 et diabète un enjeu de santé publique qui doit s'inspirer de l'expérience des pandémies virales. Diabète et Obésité.
- Tan T, Khoo B, Mills E (2020) Association between high serum total cortisol concentrations and mortality from COVID-19. Lancet Diabetes Endocrinol 8: 659-660.
- Yajing F, Yuanxiong C, Yuntao W (2020) Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virologica Sinica 35: 266-271.
- Yanqing D, Li H, Qingling Z, et al. (2004) Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol 203: 622-630.
- Ksiazeck T, Erdman D, Goldsmith C (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348: 1953-1966.
- Wheatland R (2004) Molecular mimicry of ACTH in SARS-implications for corticosteroid treatment and prophylaxis. Medical Hypothesis 63: 855-862.
Corresponding Author
Martine Claude Etoa Etoga, Department of Clinical Sciences, Faculty of Medicine and Pharmaceutical Sciences, University of Douala; National Obesity Center, Yaoundé Central Hospital, Cameroon.
Copyright
© 2021 Etoga MCE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.