Epidemiology of Thyroid Cancer in Oman
Abstract
Background
Over the past few decades, the incidence of thyroid cancer has substantially proliferated across the world. The rise is eminent only for Differentiated Thyroid Cancers (DTC).
Objective
The purpose of this study was to describe the characteristics of differentiated thyroid cancer among Omani men and women who were diagnosed and treated at National Diabetes and Endocrine Center (NDEC) and Royal hospital in Oman.
Methods
A retrospective analysis involving the data of all thyroid cancer cases at NDEC from 2006 to 2016 were carried out. We examined the changes specifically for the incidence of differentiated thyroid cancers by age, sex, histologic types, tumour size and stage. Sub-group analysis involving 116 DTC cases was also done to describe the response to treatments.
Results
Thyroid cancer increased 5.5-fold from 2006 and 2016. Salient features of 507 differentiated thyroid cancer showed that the median age of diagnosis was 36 years. Thyroid cancer was common amongst females (423) than males (84) with 5:1 ratio. There were 474 (93.5%) papillary thyroid cancer, 27 (5.32%) follicular thyroid cancer and 6 (1.1%) hurthle cell type cases. Tumour across all sizes and stages were increased over the study period. Of 116 DTC patients, the majority (75%) had no evidence of active disease at five years of follow-up.
Conclusion
The incidence of differentiated thyroid cancer has distinctly increased over the study period with the greatest rise occurring in papillary thyroid cancer and in females. Our findings are consistent with similar studies worldwide. Etiological factors promoting the rise in DTC must be investigated and may provide insight in developing suitable management strategies for the Omani population.
Keywords
Differentiated thyroid cancer, Incidence, Papillary, Oman.
Introduction
Thyroid cancer is the most common form of primary endocrine cancer: it occurs to patients worldwide and shows geographic variations in its respect to incidence rates, pathological types, affected age group, sex, race, etc. Reports from the United States and many other parts of the world shows a relentless increase in the number of incidents, particularly of differentiated thyroid cancers with a stable mortality rate [1-3].
It has also become one of the fastest growing malignancies in terms of incidence in several countries [4-6] and has since become the topic of interest for researchers, worldwide. The driving force for this tremendous increase in incidents is not well explained and is still being debated.
Some studies supported the thesis of "over diagnosis" by reporting more reservoirs of sub-clinical diseases and others demonstrated by a true increase in number of incidents [7-9]. However, the measures of incidence, survival and mortality-rates may be affected by many epidemiological factors and may not always reflect the true increase in incidence, success of treatment or screening efforts.
Amal Nasser Al Madouj and colleagues describe a significantly higher rate of thyroid cancer incidence among females in all Gulf Cooperation Council (GCC) countries. It remains the second-most common malignancy among women in this region; except in Bahrain where it ranks third in the list of malignancies. The highest number has been reported among Qatari women, followed by Kuwaitis, Bahrainis, Emiratis (United Arab Emirates), Saudis (Kingdom of Saudi Arabia) and Omanis (The Sultanate of Oman) [10]. As per this report, "the number of thyroid cancer continues to incline in GCC countries with a 24% increase among males and a 63% increase among females over the ten-year period".
Literature on thyroid cancer epidemiology from Oman is anecdotal. According to the National Cancer 2010 Report produced by Oman’s Ministry of Health (MoH), thyroid cancer accounts for 7.2% newly diagnosed cancers in females [11]. The overall Age Standardized Rate (ASR) was 1.3/100,000 (2.2/100,000 for females and 0.4/100,000 for males). The main pathological type identified was the papillary sub-type, which accounted for 85% of cases; and follicular thyroid cancer, which constituted for 3% of the known cases. Demonstrating the current trends in thyroid cancer and estimating the disease burdens in specific groups have immense implications for creating suitable changes in the management steps and surveillance of thyroid cancer.
The last study reported by Nooyi SC and Al Lawati was published in 2011 [12]. Addressing this information gap support the need for more research from the country. Royal Hospital is the largest tertiary care-center in Oman under Ministry of Health and NDEC is the endocrine division of Royal hospital providing care for diabetic and endocrine patients from all governorates across the Sultanate. The aim of this study was to examine the overall trends in the incidence of thyroid cancer from 2006 to 2016, with a detailed observation on differentiated thyroid cancer, based on age, sex, tumour size, extent and their response to treatment. This time frame was taken as our electronic data system (Al-Shifa) began operation in 2006. The literature was reviewed to understand the global trends.
Materials and Methods
We examined a retrospective cohort of patients at NDEC and Royal Hospital using the database of all Omani males, and females (14-years and above), with a diagnosis of thyroid cancer over a 10-year-period (2006-2016). Nineteen Omani patients who were diagnosed before 2006 and eighteen expatriate patients with thyroid cancer were excluded from the study.
A total of 518 patients were identified and the details of the patients were retrieved from their hospital records. We examined the overall incidence of thyroid cancer based on histology. Furthermore, the type of tumour was assessed from the pathology reports and classified mainly as papillary, follicular, hurthle cell, medullary and anaplastic. Papillary sub-type was also identified. Differentiated Thyroid Cancers (DTC) includes Papillary, follicular and hurthle cell were sub-stratified as per age, gender, tumour size and the extent. Based on the stage at diagnosis (TNM staging), patients were categorized into those with intra-thyroidal disease (confined to thyroid gland), regional metastasis (tumour deposits into local lymph nodes or peri thyroidal soft tissue), distant metastasis.
Data of 116 DTC cases were further evaluated for estimating the disease status at the fifth year of follow up. They were categorized into low, intermediate and high risk category as per the TNM staging [American Thyroid Association (ATA) 2009 initial risk stratification], until 2016 we were following the 2009 guidelines. The response to therapy was assessed every 6-12 months with stimulated or non-stimulated thyroid cancer profile [Thyroglobulin (Tg) and Tg autoantibody (Tg ab), TSH level], US neck and other imagings (iodine diagnostic scan, CT or PET CT if indicated) based on ATA recommendations.
Patients were labeled to have no active disease if Tg is negative (< 1 ug/L) in the absence of Tg ab and negative imaging studies. Patients with Tg between 1-10 ug/L or stable or dropping Tg ab levels or with non-specific US neck reports were labeled to have indeterminate response and those with Tg > 10 ug/L or rising Tg ab levels or suspicious US neck or other imagings, were with active disease (ATA 2009).
Ethical approval was obtained from Research and Ethics committee of the Royal Hospital. Data analysis was carried out using the SPSS statistical software package. Continuous variables are expressed as mean ±, SD, median and range. Chi-square test is used for comparison and p < 0.05 was considered significant.
Results
Overall incidence trends
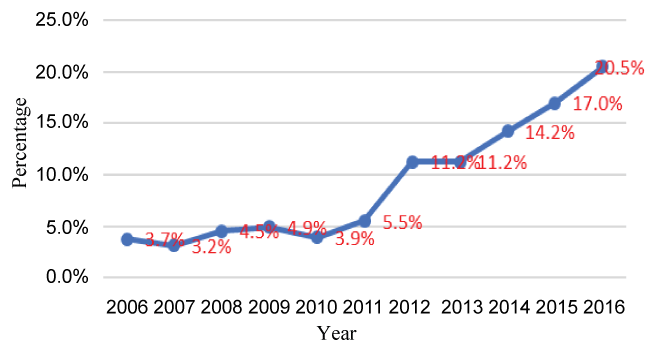
Overall, at NDEC and the Royal Hospital, 518 Omani patients were identified with thyroid cancer between 2006 and 2016. There was a 5.5-fold increase in the number of thyroid cancer-from 19 (3.7%) in 2006 to 105 (20.5%) in 2016 (Figure 1). The highest number of cases was detected from the Muscat Governorate, accounting for 43.28% of the total number, followed by the Batinah region (19.9%). The lowest was from the Musandam region (0.2%).
Histology
Summary of main histology and sub-types of thyroid cancer are depicted in Table 1.
Demographic data of differentiated cancer
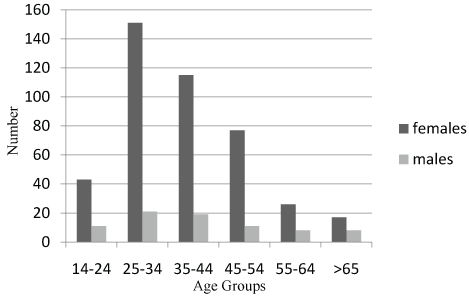
Of these 507 follicular cell derived cancers, after excluding 11 patients (8 cases of medullary and 3 cases of anaplastic), there were 423 (83.43%) females and 84 (16.56%) males The mean age at diagnosis in males was 40.48 ±15.96 and in females 38.61±12.63 (Figure 2).
Tumour stage (Extent of the disease)
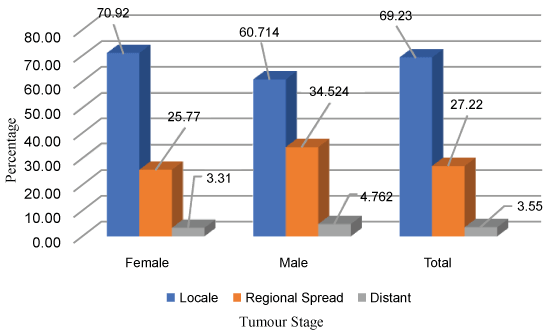
Seventy per cent of the females and males with DTC had intra-thyroidal disease. In a quarter of them the disease was extended to the regional Lymph nodes or adjacent peri-thyroid soft tissues and almost four percentages had spread to distant regions (Figure 3).
Tumour size of 507 DTC, 2006-2016
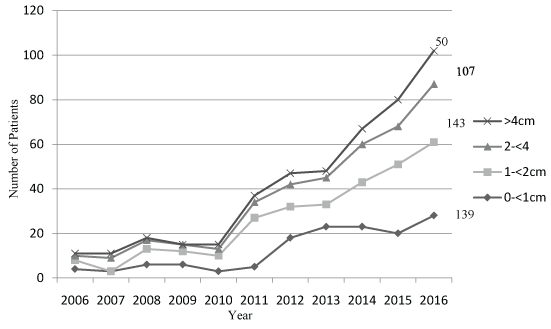
Differentiated thyroid cancer of all sizes increased between 2006 and 2016 (Figure 4). Small size cancers (< 2 cm) accounted for 55.62% of the total cases and around 10% was contributed by large size tumors (> 4 cm).
Disease status of DTC at 5-year follow-up
One hundred and sixteen (116) DTC patients were analyzed to determine the disease status (response to treatments). Out of 72 low risk and 38 intermediate risk groups, 90% and 58% (respectively) showed no evidence of active disease, but all (6/6) high risk patients had active disease (Table 2) at 5-year follow-up.
Discussion
This retrospective study reviewed the epidemiological characteristics of all thyroid cancer patients (518 in number) seen at NDEC & Royal Hospital from 2006 to 2016. Over this period, it was observed that there was a tremendous increase (5.5-fold) in the number of thyroid cancer cases. Medullary thyroid cancer accounted for 1.5% and anaplastic thyroid cancers 0.6% of the total cases. We analyzed only the salient features of 507 cases of Differentiated Thyroid Cancer (DTC). The upward trend in DTC was gradual (n = 103, 20.3%) in the beginning (between 2006 and 2010) but was sharp from 2010 to 2016 (n = 404, 79.7%, p < 0.05). There was a consistent increase in incidence of DTC across all ages and both genders during the study period. This rise was merely due to the growing number of Papillary Thyroid Cancer (PTC) cases, which rose from 97 (2006-2010) to 377 cases (2010-2016 p < 0.05). A majority of the cases were classic papillary sub-type (78.7%). Follicular variant PTC constituted 10.05% and less than 1% was by aggressive forms. The Follicular type of DTC was identified in 5.2% of DTC. There were 423 females and 84 males with a female-to-male ratio of 5:1 reflecting a significant prevalence of thyroid cancer among women. However, this ratio declined to 2:1 among older people (> 65 years). Researchers from different parts of the world showed a similar observation. In the United States (US) as well as the rest of the world-over the past 30 years-the incidence of thyroid cancer has increased (three-fold) and is expected to increase by 50 to 60% between 2010 and 2020 [13]. Louise Davies and Gilbert Welch highlighted this: since 1975 in the US, the overall thyroid cancer incidence has increased from 4.9 to 14.3 per 100,000 populations and the increased incidence in women (14.9 per 100,000 women) was almost four-times greater than that of men (3.8 per 100,000 men). Virtually, this was attributed to the increasing number of papillary thyroid cancer (3.5 to 12.4 per 100,000) [14]. Similarly, a twenty-year retrospective analysis from United Kingdom (UK) showed a rise in incidence of thyroid cancer of around 3.4% per annum, specifically due to increasing number of the papillary sub-type. Overall, there was a female to male excess of 3.4, with a higher female rate in all age-groups except those aged between 5-9 years [15]. The major population study-including data from five continents by Briseis A Kilfoy and colleagues-showed despite the wide inter-country variation in age adjusted incidence rates, a consistent female-to-male ratio of 3:1 among all patients [2].
71 per cent of the patients in the current study were young (< 45 years). The median age at diagnosis was 36 (14-88) years. In both men and women, the highest incidence of cancer was observed among age-groups of 25 to 44 years (33.90% between 25-44 and 26.40% between 35-44 years). This was much lower than the affected age groups in the US and UK. Information from SEER data showed that the median age at diagnosis was 51 years and the highest burden of the increase has been in those aged 65 years and over [16]. In the UK, the median age at diagnosis was 54.21 years (1987 to 2006). Interestingly, our result was very similar to the report from Saudi Arabia, which also showed predominance of thyroid cancer with patients under the age of 45. The median age of diagnosis was 40 years for females and 44 years for males [3].
The reason for the worldwide increase in incidence of thyroid cancer is not very clear but seems to be multi factorial [17]. Exposure to ionizing radiation, particularly during childhood is the only well-known risk factor for thyroid cancer. Furukawa K, et al. [18] demonstrated a persistent risk of thyroid cancer 50 years after childhood exposure to ionizing radiation [18]. Both obesity and diabetes have been implicated as risk factors for the increasing rates of thyroid cancer [19,20], however most reports are inconclusive and need further studies to determine a real correlation. Given the high prevalence of thyroid cancer among females, estrogen has historically been proposed as a cause of thyroid cancer [21]. But, pertinent literature in this area is limited. Other potential risk factors like dietary factors, iodine excess, nitrate, auto-immune thyroiditis [22,23] also showed some causal relationship, however, none have shown conclusive evidence, so warrant more investigation. Widespread use of diagnostic technologies [24] and increased pathological detection [25] has significantly contributed to the apparent increase in the incidence of thyroid cancer by detecting clinically insignificant micro-cancers.
Based on SEER 9 data between 1975 to 2009, Davies and Welch [14] reported that the greatest increase in thyroid cancer was among micro-cancers (39% of < 1 cm tumors). Although, most studies agree upon this, some state that this alone cannot explain the current trend. If sensitive diagnostic surveillance is the only reason for the upward trend, we expect a rise in small size thyroid tumours only. Conversely, in the present study (2006-2016) there was a marked increase in tumour of all sizes. The incidence of micro-cancers increased seven-fold from 2.9% to 20.1%, but with a similar rise in tumor size between 1-2 cm from 2.8 to 23.8%, 2-4 cm from 1.87 to 24.3% and > 4 cm from 2.0 to 20.5%. The American college of endocrinology and American association of Clinical endocrinologist stated that in the US, thyroid cancer of all sizes increased between 1983 and 2011. The incidence of the smallest cancers (2 cm or less) increased more than four-fold from 2 to 9.6 per 100,000 population. Cancers measuring 2.1 to 5 cm more than doubled in incidence, from 1.4 to 3.7 per 100,000 population. Those greater than 5 cm are quite rare, but their rates tripled, from 0.2 to 0.7 per 100,000 population [26]. Similarly, John D and colleagues using SEER 193-2006 described an increase across all tumor sizes and stages, suggesting that increased detection is not the only the cause [27]. Our result was consistent with these results. The information from SEER data [16] indicated that at the time of diagnosis 69.7% them had localized disease; this reflected in our study. The majority of the study population (69.2%) had cancer confined to the thyroid gland (intra-thyroidal), 27.2% had spread to regional lymph nodes or with minimal extra-thyroidal extension and 3.5% had spread to distant regions. The extent of the disease was identical in both men and women.
We also observed that there was an intra-regional geographical variation in the distribution of DTC. The highest number of cases (222/507) was identified in the capital region (Muscat). Perhaps this could be explained by accessibility to medical care. Nevertheless, further studies are required to support this explanation.
Based on ATA 2006 and 2009 recommendations, most of our DTC patients (98%) received surgery (total or near total thyroidectomy) ± Radioactive Iodine Therapy (RAI-131). At an average of five years, follow-up of 116 DTC patients showed 75% of them had no evidence of active diseases (clinically, biochemically and radiologically), 13% had indeterminate status and 12% had evidence of active disease. This was comparable to the recent literature from King Faisal Specialist Hospital and Research Center (KFSH & RC) in Saudi Arabia [28]. At a median of 7.6 years follow-up of 318 patients, 53.3% showed no active disease, 24.5% showed indeterminate status and 14.7% with active disease. Well-differentiated thyroid cancers often have indolent clinical course with low morbidity and mortality [29,30]. However, the prognosis of any primary cancer is affected by age, gender and tumor stage at diagnosis. In the current study, we aimed primarily at knowing the trends in incidence and pathological features. So, a detailed assessment of these prognostic variables in terms of disease outcome was not done at this time. Nevertheless, it was very obvious that most patients, with DTC confined to the thyroid gland at the time of diagnosis and more than half of those which spread to regional lymph-nodes or minimal extension to extra-thyroid tissues, remained disease free five years after the initial treatments. All patients with distant metastasis at the time of diagnosis had evidence of active disease-either biochemically or structurally-despite multiple surgeries and RAI doses. No deaths due to DTC were reported over the study period.
Conclusion
The incidence of thyroid cancer has exponentially risen from 2006 to 2016 at NDEC. The increase was almost exclusively due to rising number of papillary thyroid cancer. Though this upward surge may be partly augmented by the detection of sub-clinical disease (small cancers), a real increase is evident by the increase in large size tumours. Future epidemiological investigations describing the trends in prevalence of suspected etiological factors may yield more insight into the reason for the rise in DTC in Oman. This can create successful strategies for increasing awareness, early detection and management of thyroid cancer in the country.
Strength and Limitations
The present study has particular strengths: first, it included a large sample from a main tertiary care centre in Oman, where international standards have been followed for management of thyroid cancer; second, the study covered patients from all governorates of Oman. This may be seen as representation of the population of the country. However, we acknowledge the limitations of a retrospective study.
Competing Interests
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Mao Y, Xing M (2016) Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer 23: 313-322.
- Kilfoy BA, Zheng T, Holford TR, et al. (2009) International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control 20: 525-531.
- Hussain F, Iqbal S, Mehmood A, et al. (2013) The incidence of thyroid cancer in the Kingdom of Saudi Arabia, 2000-2010. Hematol Oncol Stem Cell Ther 6: 58-64.
- Darrin V Bann, Neerav Goyal, Fabian Camacho, et al. (2014) Increasing incidence of thyroid cancer in the commonwealth of Pennsylvania. JAMA Otolaryngol Head Neck Surg 140: 1149-1156.
- Ramírez-Vick M, Nieves-Rodríguez M, Lúgaro-Gómez A, et al. (2011) Increasing incidence of thyroid cancer in Puerto Rico,1985-2004. P R Health Sci J 30: 109-115.
- Wang Y, Wang W (2015) Increasing incidence of thyroid cancer in Shanghai, China, 1983-2007. Asia Pac J Public Health 27: 223-229.
- Morris LG, Sikora AG, Tosteson TD, et al. (2013) The Increasing Incidence of Thyroid Cancer: the influence of access to care. Thyroid 23: 885-891.
- N Pandeya, DS McLeod, K Balasubramaniam, et al. (2016) Increasing thyroid cancer incidence in Queensland Australia ,1982-2008 - true increase or overdiagnosis? Clinical Endocrinology 84: 257-264.
- Davies L, Ouellette M, Hunter M, et al. (2010) The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope 120: 2446-2451.
- Amal Nasser Al-Madouj, Abdelmoneim Eldali, Ali Saeed Al-Zahrani (2011) Ten-Year Cancer Incidence among Nationals of the GCC States 1998-2007. Gulf Center for Cancer Control and Prevention 28-31.
- Ministry of Health Sultanate of Oman (2010) Cancer incidence in Oman report.
- Nooyi SC, Al-Lawati JA (2011) Cancer incidence in Oman, 1998-2006. Asian Pac J Cancer Prev 12: 1735-1738.
- Weir HK, Thompson TD, Soman A, et al. (2015) The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 121: 1827-1837.
- Davies L, Welch HG (2014) Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140: 317-322.
- Olaleye O, Ekrikpo U, Moorthy R, et al. (2011) Increasing incidence of differentiated thyroid cancer in South East England: 1987-2006. Eur Arch Otorhinolaryngol 268: 899-906.
- (2016) Cancer of The Thyroid-SEER Stat Fact Sheets.
- Gabriella Pellegriti, Francesco Frasca, Concetto Regal buto, et al. (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. Journal of Cancer Epidemiology.
- Furukawa K, Preston D, Funamoto S, et al. (2013) Long-term trend of thyroid cancer risk among Japanese atomic bomb survivors: 60 years after exposure. Int J Cancer 132: 1222-1226.
- Pappa T, Alevizaki M (2014) Obesity and thyroid cancer: a clinical update. Thyroid 24: 190-199.
- Schmid D, Behrens G, Jochem C, et al. (2013) Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol 28: 945-958.
- Kamat A, Rajoria S, George A, et al. (2011) Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Arch Otolaryngol Head Neck Surg 137: 1146-1153.
- Cho YA, Kim J (2015) Dietary factors affecting thyroid cancer risk: a meta-analysis. Nutr Cancer 67: 811-817.
- Paparodis R, Imam S, Todorova-Koteva K, et al. (2014) Hashimoto's thyroiditis pathology and risk for thyroid cancer. Thyroid 24: 1107-1114.
- Zevallos JP, Hartman CM, Kramer JR, et al. (2015) Increased thyroid cancer incidence corresponds to increased use of thyroid ultrasound and fine-needle aspiration: a study of the Veterans Affairs health care system. Cancer 121: 741-746.
- Simon Grodski, Tani Brown, Stan Sidhu, et al. (2008) Increasing incidence of thyroid cancer is due to increased pathological detection. American Association of Endocrine Surgeons 144: 1038-1043.
- Louise Davies, Luc GT Morris, Megan Haymart, et al. (2016) The increasing incidence of thyroid cancer. Endocrine Practice 21.
- John D, Cramer BS, Pingfu Fu, et al. (2010) Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. American Association of Endocrine Surgeons 148: 1147-1153.
- AS Alzahrani, H Alomar, N Alzahrani (2017) Thyroid Cancer in Saudi Arabia: A Histopathological and Outcome Study. International Journal of Endocrinology 2017: 8423147.
- La Vecchia C, Malvezzi M, Bosetti C, et al. (2015) Thyroid cancer mortality and incidence: a global overview. Int J Cancer 136: 2187-2195.
- Colonna M, Uhry Z, Guizard AV, et al. (2015) Recent trends in incidence, geographical distribution, and survival of papillary thyroid cancer in France. Cancer Epidemiol 39: 511-518.
Corresponding Author
Dr. Fathimabeebi Pambinezhuth, Division of Endocrinology, National Diabetes and Endocrine Centre, Royal Hospital, Muscat, Oman, Tel: +968-93321093.
Copyright
© 2017 Pambinezhuth F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.