Quantitative Structure-Activity Relationship (QSAR) Study of A Series of 2-Thioarylalkyl Benzimidazole Derivatives by the Density Functional Theory (DFT)
Abstract
In this work, we used the quantum density theory (DFT), B3LYP / 6-311G (d, p) to establish a QSAR (Quantitative Structure Activity Relationships) model on a series of molecules derived from 2-thioarylalkyl-1H -Benzimidazole. This model is built with molecular descriptors and anthelmintic activities against the Haemonchus contortus. This model has as statistical indicators: the coefficient of determination , a standard deviation S, the Fisher coefficient F and the cross-validation coefficient . The statistical parameters of the model are efficient.
The quantum descriptors responsible for the anthelmintic activity of 2-thioarylalkyl-1H-Benzimidazole derivatives are the dipole moment (µ), the energy of the highest occupied orbital (EHOMO), the smallest negative charge of the molecule (q -).
The acceptance criterion of Eriksson et al. used for the test series is verified. For the external validation, the values of the ratio of theoretical activity and experimental activity tends to unity.
Keywords
2-Thioarylalkyl-1H-Benzimidazole, QSAR, Anthelmintic activity, Quantum descriptors
Introduction
The fight against infectious diseases remains a public health problem, which is explained by the high mortality and morbidity rate caused by these diseases [1].
Indeed there are three main families of anthelmintic available on the market. Unfortunately, the frequent use of its molecules has led to the appearance of resistance to its drugs. In this context, it is imperative to design and prepare new drugs with a reinforced anthelmintic aim.
Consequently, the pharmaceutical industry is moving towards new research methods, which consist in predicting the properties and activities of molecules before they are even synthesized. In recent years, the use of technologies allowing to synthesize a very large number of molecules simultaneously and to test their actions on therapeutic targets has given very attractive results. This is the main objective of QSAR (Quantitative Structure Activity Relationships) studies. These studies are based on the search for similarities between molecules in large databases of existing molecules whose activities are known. The discovery of such a relationship linking both activities and molecular descriptors makes it possible to predict the activities of new compounds, and therefore to guide the syntheses of new molecules.
Material and Methods
Database
This QSAR study concerns a series of sixteen molecules derived from 2-thiarylalkyl-1H-Benzimidazole with twelve molecules (75% of database) used for the training set and four molecules (25% of the database) for the test set. These compounds have been synthesized and tested for their nematocidal activities by Akpa et al [2].
Calculation material and methods
All of the sixteen molecules used in our study have larvicidal concentrations ranging from 0.005 to 424 µg / ml. This concentration range does not allow a quantitative relationship to be defined between anthelmintic activity and theoretical descriptors.
Biological activities are generally expressed as the opposite of the base 10 logarithm of the activity so as to obtain higher mathematical values when the molecule is biologically effective. The anthelmintic activity is then expressed by the anthelmintic potential pCL100 defined by the relationship:
Where M is the molecular mass (g / mol) and CL100 the larvicidal concentration, it is the concentration necessary to eliminate 100% of the larvae of Haemonchus contortus.
Calculation level
The relationship between the values of the biological activity of the molecules studied and the molecular structures was highlighted by calculations of theoretical chemistry using the software Gaussian 09 [3]. The density functional theory DFT was used for our calculations with its functional B3LYP with the base 6-311G (d, p) in order to determine the molecular descriptors [4].
Indeed, DFT is known to generate a variety of molecular properties in a QSAR study [5,6]. This method makes it possible to reduce the calculation time, increases predictability, and involves a lower cost in the design of drugs [7,8]. The model is obtained using the multi linear regression (RML) method using the XLSTAT [9] and EXCEL [10] software.
Quantum descriptors
For the development of the QSAR model, several theoretical descriptors derived from the conceptual DFT were determined. These descriptors all determined following the optimization of the geometry of the molecules followed by the frequency calculation.
The calculation of the partial correlation coefficient between the descriptor pairs (aij) must be less than 0.70 which shows that the descriptors are independent of each other [11].
Estimation of the predictive capacity of the QSAR model
The quality of a QSAR model is determined based on the analysis of certain statistical criteria including the coefficient of determination , the standard deviation S, the Fisher coefficient F and the cross-validation coefficient.
The statistical parameters, F and S relate to the adjustment between the experimental values and the calculated values. The cross-validation coefficient measures the accuracy of the model's prediction on the data from the training set [12].
The coefficient of determination measures the share of experimental variance explained by the model in relation to the total variance. Its value is between 0 and 1. The closer its value is to 1, the more observed and predicted values are not correlated [13].
Where
: Experimental value of anthelmintic activity
: Theoretical value of anthelmintic activity
: The average of the experimental values of the anthelmintic activity
The variance is determined by the following relation:
Where k is the number of independent variables (descriptors) of the equation of the model, n is the number of molecules in the test set and n-k-1 is the degree of freedom.
The standard deviation S is another statistical parameter, it provides information on how the distribution of data is distributed around the average [14].
The Fisher coefficient F allows to test the global significance of the linear regression [15]
The cross-validation coefficient measures the accuracy of the prediction on the data from the training set. It is calculated using the following relation:
The performance of the model according to the Erickson et al criterion is characterized by the value of for a satisfactory model and for an excellent model must be close to 0.9 [16]. The training set of the model will be acceptable if the criterion is respected.
However, the predictive power of the model can be obtained by the ratio for the test set. The model is acceptable when the ratio of the values of theoretical activity to experimental activity tends towards unity for the validation set. The model is acceptable when the ratio of the values of theoretical activity to experimental activity tends towards unity.
Results and Discussion
Training set and test set
The molecules of the training set as well as those of the validation test are presented in table 1 as well as the different calculated descriptors.
The partial correlation coefficients aij between the descriptor pairs shows that they are less than 0.70, which demonstrates the independence of the descriptors used to develop the model.
Validation of the QSAR model
The positive or negative sign of the coefficients of the descriptors of the model reflects the effect of proportionality between the evolution of the anthelmintic activity and the descriptors in the equation of the model. The negative sign indicates that when the value of the descriptors is large, the biological activity decreases,the positive sign translates the opposite effect.
The best QSAR models obtained for the anthelminthical activity against Haemonchus contortus as well as statistical indicator are given below :
pCL100 = -42, 73511-0, 52477*µ -173, 94520*EHOMO-21, 29665*q- (7)
Negative signs of dipole moment, HOMO energy and the smallest negative charge in the molecule indicate that anthelmintic activity can be improved for low values of its coefficients. The cross-validation coefficient =0.916. This model is acceptable because the value
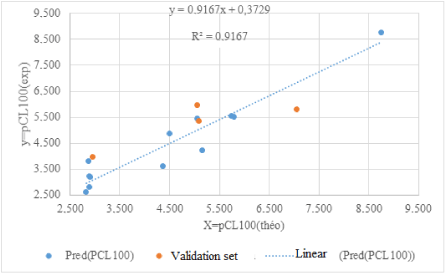
is less than 0.3. The external validation of the model is obtained by the ratio is presented in table 3.
All the values of the ratio tend towards the unit. This indicates the good correlation between the experimental and theoretical values of the anthelmintic potential of 2-thioalkylaryl-1H-Benzimidazole derivatives. This model is therefore acceptable for predicting anthelmintic activity against Haemonchus contortus in the series of 2-thioalkylaryl-1H-Benzimidazole derivatives.
The line of regressions between the theoretical and experimental anthelmintic activities between the training set and the test set is illustrated in figure 1.
Analysis of the contribution of descriptors in the model
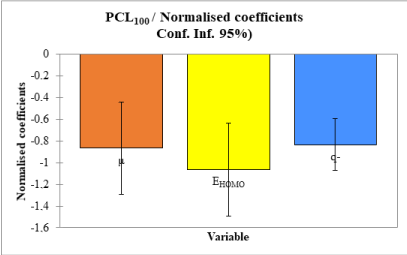
The relative contribution of the descriptors in predicting the anthelmintic activity of the compounds is presented in figure 2.
The energy of the highest occupied molecular orbital has the largest contribution followed by the dipole moment and the smallest negative charge in the molecule.
Conclusion
QSAR methodology and theoretical chemistry methods were used to establish a predictive model of the anthelmintic activity of a series of 2-thioalkyl aryl benzimidazole derivatives against Haemonchus contortus. We determined the theoretical descriptors using the DFT method with the B3LYP/6-311G (d,p) level of theory.
The model obtained takes into account three descriptors including the dipole moment, the energy of the highest occupied orbital and the smallest negative charge of the molecule. The proposed model tells us that the energy of the highest occupied orbital is the descriptor that enhances anthelmintic activity for this series of molecules.
This study represents an orientation for the design of new molecules active against Haemonchus contortus. This study will play an important role in understanding the relationship between the physico-chemical parameters of molecular structures and biological activity.
References
- DS Richard , C Joanna and Bull (2002) WHO 80: 126.
- Akpasagne and Thèse de Doctorat (2013) Synthèse de 2-Thiobenzyl (Thiomethylbenzimidazolyl) Benzimidazoles et analogues structuraux à visée anti-infectieuses.
- MJ Frisch, GW Trucks and HB Schlegel (2009) Gaussian 09, Revision A.02.
- Hohenberg P and Kohn W (1964) Inhomogeneous electron gas. Phys Rev 136: B864.
- Chattaraj PK, Cedillo A and Parr RG (1995) Variational method for determining the Fukui function and chemical hardness of an electronic system. Chem Phys Let 103: 7645.
- De Proft F, Martin JML and Geerlings P (1996) QSAR Study of a Serie of Benzimidazolylchalcone Derivatives by the Density Fonctional Theory (DFT) Method. Chem Phys Let 256: 400.
- Hansch C, Sammes PG and Taylor JB (1990) Computers and the medicinal chemist. In: Comprehensive Medicinal Chemistry, Pergamon Press, Oxford 4: 33-58.
- R Franke (1984) Theoretical Drug Design Methods. Elsevier, Amsterdam.
- (2018) XLSTAT and Addin software Registrered Trademarks of Addinsoft.
- (2013) (15.0.4420.1017) MSO (15.0.4420.1017) 64 Bits Partie de Microsoft Office Professionnel Plus.
- Vessereau A (1988) Méthodes statistiques en biologie et en agronomie. Lavoisier (Tec and Doc). Paris 538.
- Golbraikh and Tropsha A (2002) Beware of Q !A J.Molecular Graphics and Modelling, 20: 267-276.
- M Lejeune (2010) Statistiques :la théorie et ses applications, Springer Verlag, Paris 2004.
- Siegel AF (1997) Pratical Business Statistic, IRWIN.
- Cook RD and Weisberg S (1994) An introduction to regression graphics ,Wiley Series in Probability and Statistics.
- Eriksson L, Jaworska J, Worth A, et al. (2003) Methods for Reliability and Uncertainly Assessment and for Applicability Evaluations of Classification and Regression-Based QSARs. Environmental Health Perspectives 111: 1361-1375.
Corresponding Author
Mawa Koné, Laboratoire de Constitution et de Réaction de la Matière de l'UFR SSMT, Université Félix Houphouët Boigny, 22 BP 582, Abidjan 22, Côte d'Ivoire, Email: kone_m2001@yahoo.fr.
Copyright
© 2022 Béké DE. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.