Response of Kenaf Varieties to Different Threshing Methods and Storage Environments
Abstract
A study was conducted in the Seed Testing laboratory of the Institute of Agricultural Research and Training to determine the response of kenaf to different threshing and packaging materials. Experiment design was a 2 × 3 × 4 × 2 factorial arrangement in CRD with 3 replications. The factors were variety, threshing methods, packaging materials and storage environments. The results showed that kenaf seed stored in both Jute bag and muslin cloth under ambient condition recorded significantly higher germination percentage with 34% and 32.02% after six months in storage, respectively. Similar trend was followed under cold storage. Seed stored under jute bag recorded higher germination percentage of about 47% after six months in storage but not statistically significant from those stored under muslin cloth that recorded about 42% germination percentage. The rate of seed deterioration was lowest when both varieties were stored using jute bag with values of 18.03% (Cuba 108) and 17.87% (Ifeken 400). This was also followed by muslin cloth which recorded 31.2% (Cuba 108) and 25.6% (Ifeken 400). The result also showed that seed threshed mechanically and stored using jute bag obtained the highest level of germination in six-month.
Keywords
Kenaf, Threshing method, Germination, Storage period, Packaging material
Introduction
Kenaf (Hibiscus cannabinus) is an herbaceous, annual, short-photoperiod plant that contains high quality cellulose. The genus Hibiscus has a cosmopolitan distribution and contains more than 400 species. Kenaf is a commercially important fiber crop next to cotton and jute. Both jute and kenaf constitute raw jute as it goes in trade and industry. The use of quality seeds in cultivation is one of the most important factors that can increase farm level yield. Although seed quality is governed by genetic makeup, seed storage and retention of viability are important for seed vigor [1]. Seed availability is a fundamental factor to ensure successful development of a novel crop. Insufficient and irregular supply of seed will obstruct planning and can cause setback to development of any industrial or agricultural project. The quality of kenaf seeds depends on many pre and post harvest factors, such as time of cultivation and method, time of seed maturity and harvest, threshing, processing, and drying operation and storage condition. Mechanical damage is one of the major factors which reduce the seed quality. In traditional method, some amount of kenaf seed losses due to inefficient harvesting and threshing practices. Manual threshing methods are labour intensive as compared to mechanical threshing. However, harvest of kenaf seed is another major problem as bristles from kenaf capsules can cause extreme itchiness and irritation to human skin and farmers face an acute problem of threshing kenaf seed. The seed injuries are caused from the weathering, fungi, insects, artificial drying and mechanical damage during harvest, handling, threshing and storage [2]. Dharmaputra, et al. [3] studied post harvest quality improvement of sorghum grain and showed that method of threshing of sorghum seeds have significant effects on grain damage in terms of cracked and broken grains and germination. The percentage of damaged grain of sorghum threshed using a wooden stick was higher and significantly different from that threshed with a paddy thresher. Also, the percentage of seed germination was higher in sorghum threshed using paddy thresher (93.3%) and significantly different from that of sorghum threshed using a wooden stick (91.04%). Mechanical damage is one of the major causes of seed deterioration during storage [4]. Damaged to grains pose less resistance against pests and diseases and have the minimum storage life [5]. In areas were kenaf is being planted, low yield have been attributed to poor seed quality, among others. Mbora, et al. [6] reported that seeds of the best quality will result in crops of the best quality in the field which will result to yields of the highest value. Contributory factors to such low seed quality are inappropriate threshing methods and storage. During threshing, internal crack may occur along embryo of the seeds. These fissures may retard the germination potential, viability and vigour of the seeds leading to poor establishment. They may also serve as entry points for pathogen to destroy the seeds in storage.
Seed storage generally aims to maintain seed viability and protect it from factors that cause a decline in viability so that high quality seeds remain available for the next planting season [7]. Storability of seeds is mainly a genetically regulated trait which is influenced by the seed quality at the time of storage, history of seed before storage (environmental factors during pre and post-harvest stages), moisture content of seed, ambient relative humidity and temperature of storage environment, storage period and biotic agents [8,9]. Damage of seed during storage is inevitable [10]. Seeds undergo deterioration during storage with the rate dependent on storage temperature, moisture and storage period [11]. These environmental conditions are very hard to maintain during storage. The seed storage environment highly influences the period of seed survival. After planting of deteriorate seeds, seedling emergence may be poor and transmission of pathogens to the new crop may occur. Lower temperature and humidity result in delayed seed deteriorative process and hence prolonged viability period [12]. Therefore, the present study was undertaken with a view to determine the effects of threshing methods and packaging materials on the germination potential of kenaf cultivars before and after storage.
Materials and Methods
The design of the laboratory experiment was a 2 × 3 × 4 × 2 factorial arrangement in CRD with 3 replications. The factors were variety at 2 levels (Ifeken 400 and Cuba 108), threshing methods at 3 levels (Hand, Manual and Machine), 4 levels of packaging materials (Envelop, Jute bag, Muslin cloth and Aluminum foil) and 2 levels of storage environments (Cold room and Ambient). The field experiment was planted in August 2020 and all agronomic practices for good seed yield were observed. Threshing was carefully done on the harvested plant in order to reduce the risk of damaging the seed or mixing them with other variety. The 3 threshing methods were applied on 50 kg of harvested capsules of each of the variety. One variety was threshed at a time and the threshing equipment and environment were properly cleaned. After threshing the seed, moisture content and purity analysis were determined and three replicates of 500g per each treatment were weighed and placed into different packaging materials for storage under ambient and cold room storage environments. Throughout the period of the experiment, the cold room had at least four hours of light on daily basis from Monday to Friday due to epileptic power supply in the country. At zero storage, sample of each treatment was collected for seed testing to determine the initial viability of the seed. For storage, the containers were placed on a wooden creates in the laboratory (ambient) and the other inside the seed storage facility of the Institute for six months. The temperature and relative humidity readings of the storage environment were recorded daily at 10 am and 3 pm for a period of six months ranging from February 2021 to July 2021. Laboratory seed quality analysis was carried out at two months interval during the period of the experiment.
Standard germination
Standard germination test was carried out in three replications of 50 seeds per replicate. Plastic germination bowls were filled with moistened sharp sand and seeds were evenly spaced on the sand and thereafter thinly covered with moistened sand and lightly pressed for a good seed-substratum contact. The bowls were covered with nylon sheets to conserve moisture and kept at ambient room temperature. Germination counts were made daily from the 3rd to 7th day after planting. On the 7th day, seedling analysis was carried out and the numbers of normal and abnormal seedlings were recorded. Germination was interpreted as the percentage of seeds producing normal seedlings [13].
Data that were collected were subjected to combined ANOVA separately for each storage conditions and combined using general linear model procedures in statistical analysis system, SAS software version 9.2 [14] to compute mean squares for each character. Mean separation was done using Duncan Multiple Range Test (DMRT).
Results and Discussion
The mean monthly minimum and maximum temperature and relative humidity ranges in the ambient and cold storage environments used during the study were presented in Table 1. Temperature values both minimum (T1) and maximum (T2) for ambient and cold storage environments ranged from 30.76 ℃ T1to 26.29 ℃ T2 and 31.90 ℃ T2 to 23.82 ℃ for ambient and 19.73 ℃ T1 to 21.84 ℃ T1 and 18.94 ℃ T2 to 19.81 ℃ T2 for cold storage condition. Relative humidity ranged from 52.4% RH1 to 37.58% RH2 and 49.2% RH2 to 66.12% RH2 for ambient and 53.77% RH1 to 37.58% RH1 and 47.64% RH2 to 31.67% RH2. Mean values of kenaf seed stored under different storage conditions and packaging materials is presented in Table 2. Kenaf seed stored in both Jute bag and muslin cloth under ambient condition recorded significantly higher germination percentage with 34% and 32.02% after six months in storage, respectively. Seeds stored using envelops recorded the lowest germination percentage statistically significant from other three packaging materials. The reduction in germination in seeds stored with both aluminum and envelop under ambient temperatures could be as a result of non porosity of the materials. Additionally, an increase in storage periods under high temperature and humidity also caused a reduction in viability of the stored seeds. This observation could be due to non exchange of gases between the seeds and their ambient environment which could bring about rapid deterioration of the seeds as a result of rapid respiration. This negates the findings of Tonin and Perez [15]. They reported that the type of packaging at the time of seed storage becomes extremely relevant on the quality indicators when the packaging can minimize the rate of seed spoilage, and continue to regulate the initial water content of seeds in storage, preventing the speed at which the seeds respire. Similar trend was followed under cold storage. Seed stored under jute bag recorded higher germination percentage of about 47% after six months in storage but not statistically significant from those stored under muslin cloth that recorded about 42% germination percentage. Germination percentages for seed stored with both envelop and aluminum are not statistically significant. It was observed that the germination decreased with increase in storage period. Although, seed stored under cold condition lose viability slowly over storage periods as compared to those stored under ambient condition using any of the packaging materials, This gradual loss of viability for seeds stored under cold condition may be attributed to maintenance food reserves as a result of low respiration rate and low metabolic activity due to controlled temperature, relative humidity in the storage environment. This finding is in line with Balesevic-Tubic, et al. [16], who reported that seed germination of soybean declines more in storage under convectional conditions due to variability in temperature and relative humidity than seeds stored under controlled conditions.
Initial average germination percentages for all the packaging material was 61.78% (Table 3). Average germination percentage at two, four and six months after storage was highest with kenaf seeds stored using jute bags and lowest with seed stored using aluminum. Jute bag recorded 57.9%, 52.89% and 46.69% while aluminum recorded 52.17%, 20.28% and 12.22%, respectively 2, 4 and 6 months after storage. The low viability of kenaf seed at the initial stage of the study could be as a results of delayed threshing and also pre-harvest sprouting of the seeds on mother plants prior harvesting [17]. Highly significant (P < 0.01) difference was observed in mean germination percentage in kenaf seeds using different packaging materials after six months in storage. Response of kenaf seeds during storage using both jute and muslin cloth are very close though statistically different from one another. The seeds stored in jute bag had the highest germination percentage (46.69%) six month in storage. Seeds stored in envelop recorded the lowest average germination percentage (12.22%) six months after storage. The packaging materials had a significant effect on seed germination under the two storage environments. Lower germinations recorded by both aluminum and envelop six months after storage could be as a result of low air movement in stored seed which caused heat accumulation resulting from respiration [18].
Response of kenaf varieties to different packaging materials is presented in Table 4. Initial germination percentage for the two varieties used were 62.89% and 60.67% for Cuba 108 and Ifeken 100. Two months after storage, average germination percentage was highest when the two varieties were stored using jute bags with values of 58.50% (Cuba 108) and 57.33% (Ifeken 400). No statistical difference between germination values when Ifeken 400 was stored with either jute or muslin cloth 2 months after storage but lowest with aluminum. For Cuba 108, no statistical differences among muslin cloth, envelop and aluminum two months after storage. At four months after storage, both varieties recorded highest germination percentage of 54.625% (Cuba 108) and 54.67% (Ifeken 400) with jute. Storage with jute also recorded highest values for both varieties at 6 months after storage period. Reduction in average germination percentage was pronounced for the two varieties when aluminum was used during the storage period. From the results above, the two varieties followed similar trend in their rates of deterioration irrespective of the packaging materials used. The longer the storage period, the more the catabolic reaction. The difference could be as a result of continuous catabolic reaction resulting in depletion of food reserve. This corroborates the findings of Talipata [19]. Gbolami and Golpayegani [20] also reported that by increasing the storage period of soybean and rice seeds, germination rate coefficient are decreased. Rate of seed deterioration for the two varieties were similar using different packaging materials. Deterioration rates was highest when both varieties were stored using aluminum with values of 79.3% (Cuba 108) and 81% (Ifeken 400), respectively. This was followed by aluminum 56.17% (Cuba 108) and 56% (Ifeken 400). The rate of seed deterioration was lowest when both varieties were stored using jute bag with values of 18.03% (Cuba 108) and 17.87% (Ifeken 400). This was also followed by muslin cloth which recorded 31.2% (Cuba 108) and 25.6% (Ifeken 400). The similarity between the varieties might be due to similar genetic factors and seed chemical composition. This present study is in line with the findings of Shelar, et al. [21] who stated that irrespective of genotypes, the germination potential of soybean seeds decreased during storage.
Interaction effects
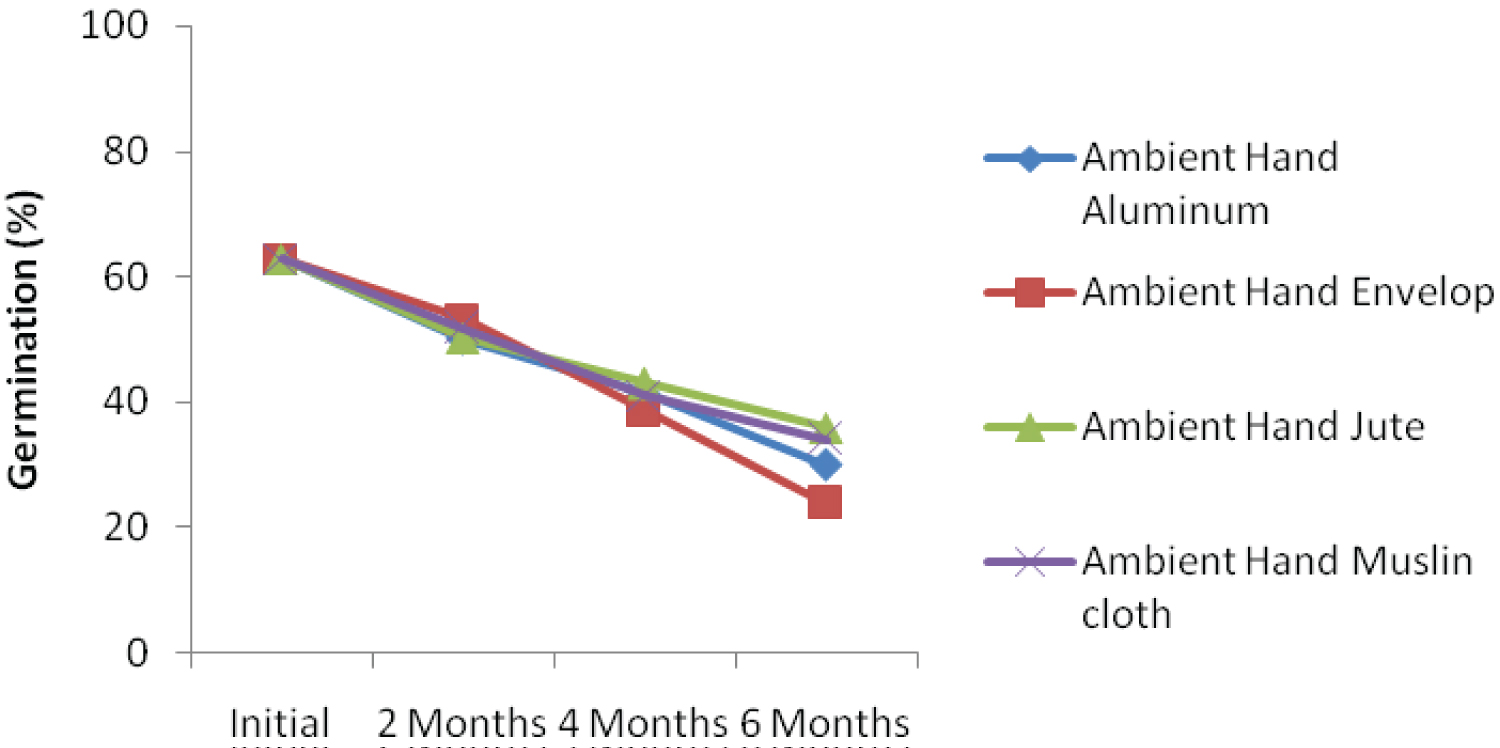
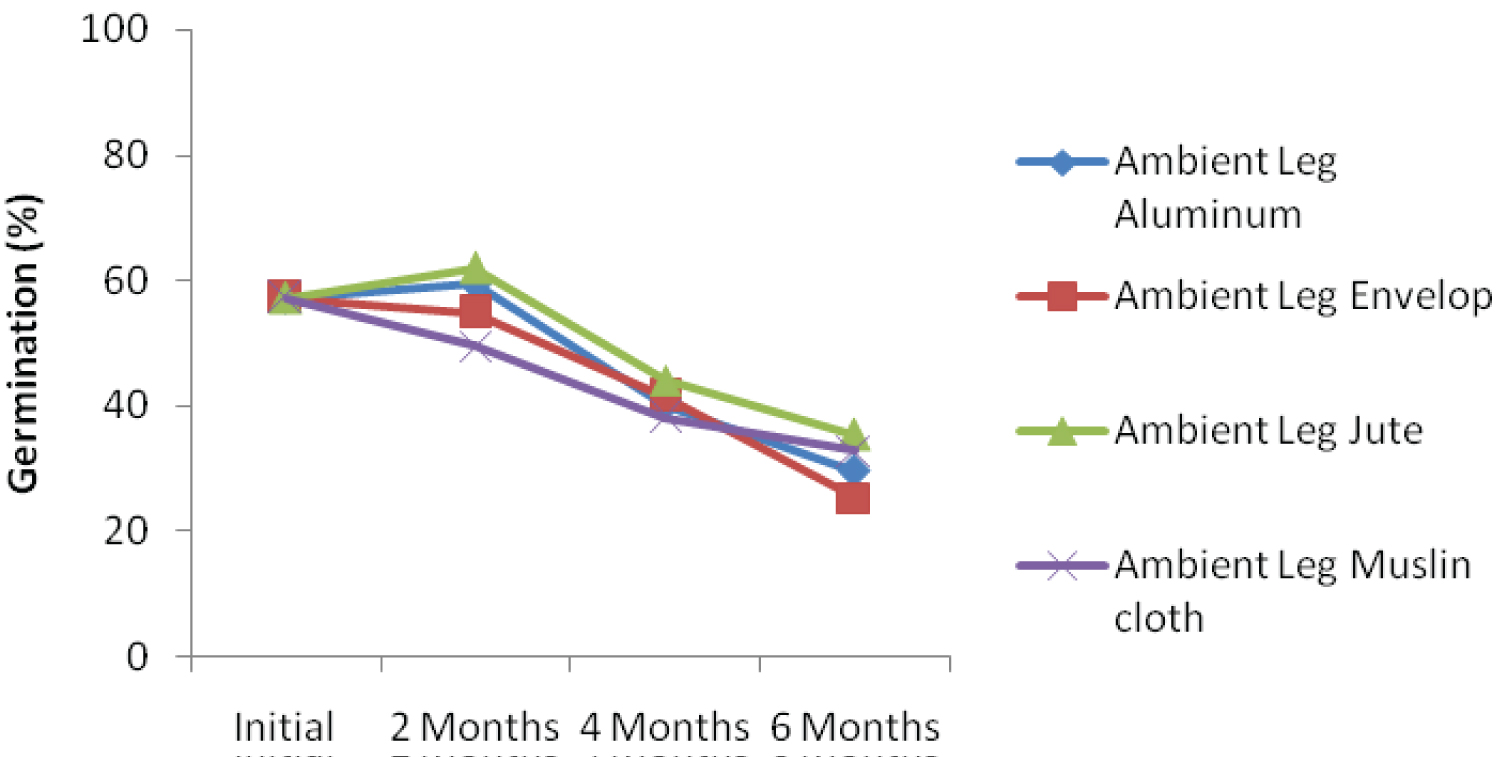
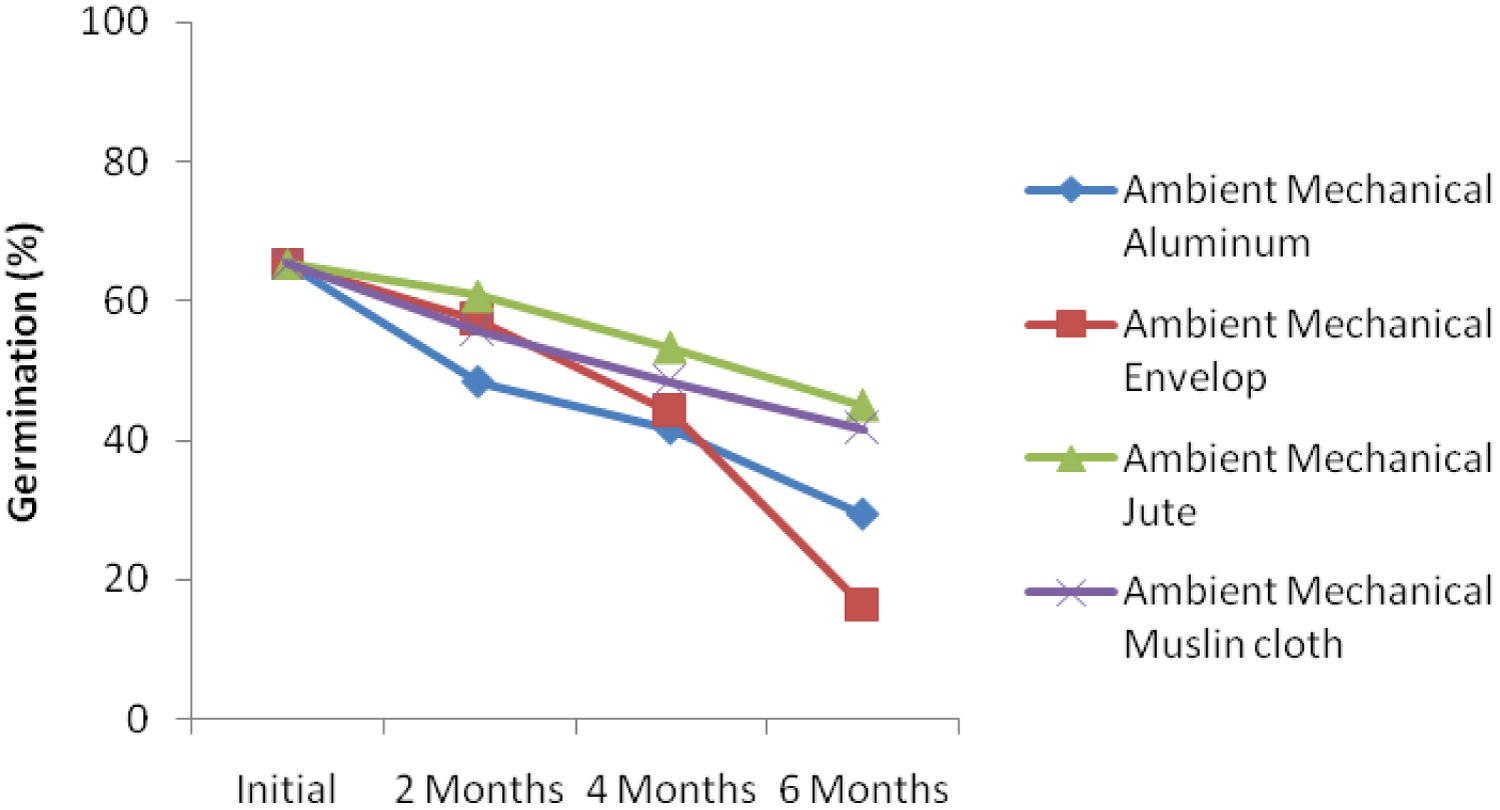
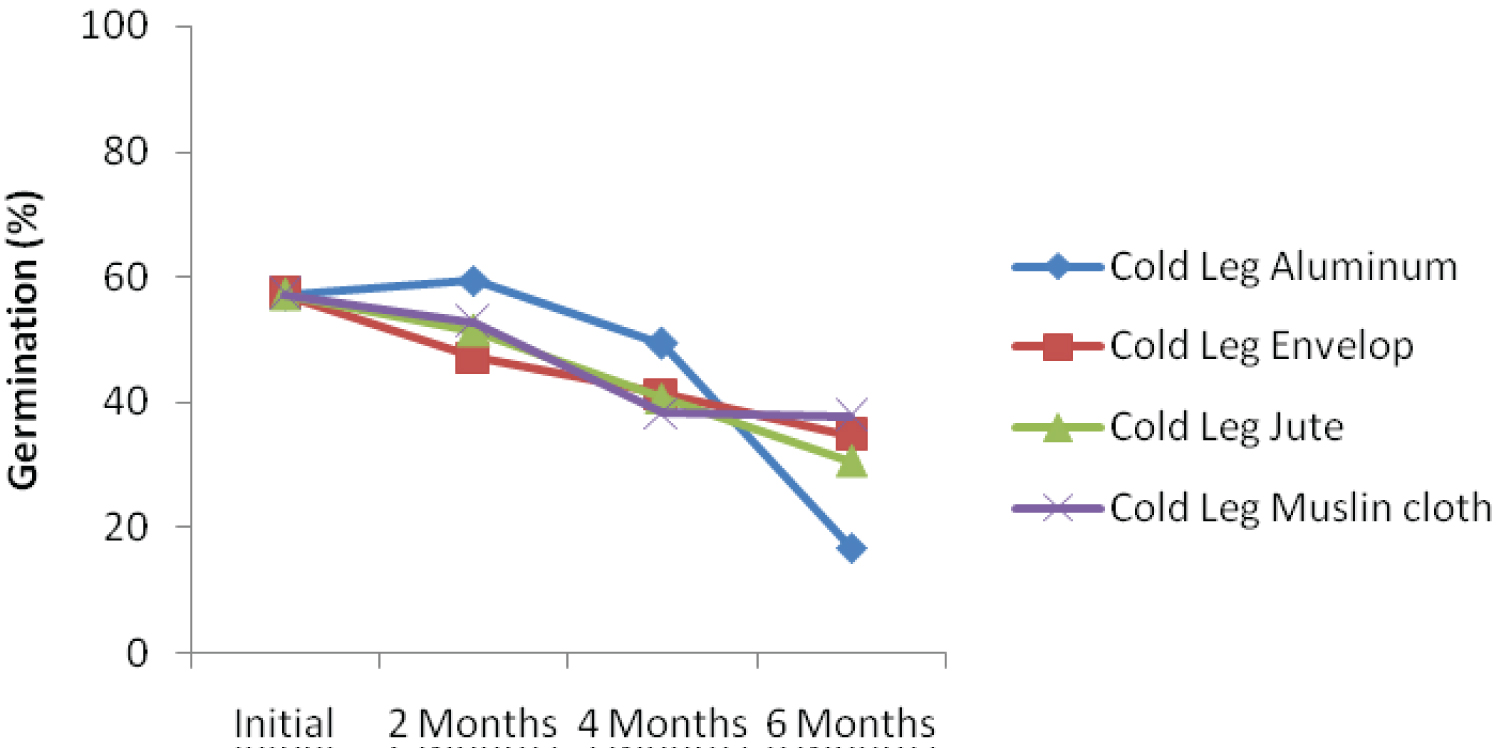
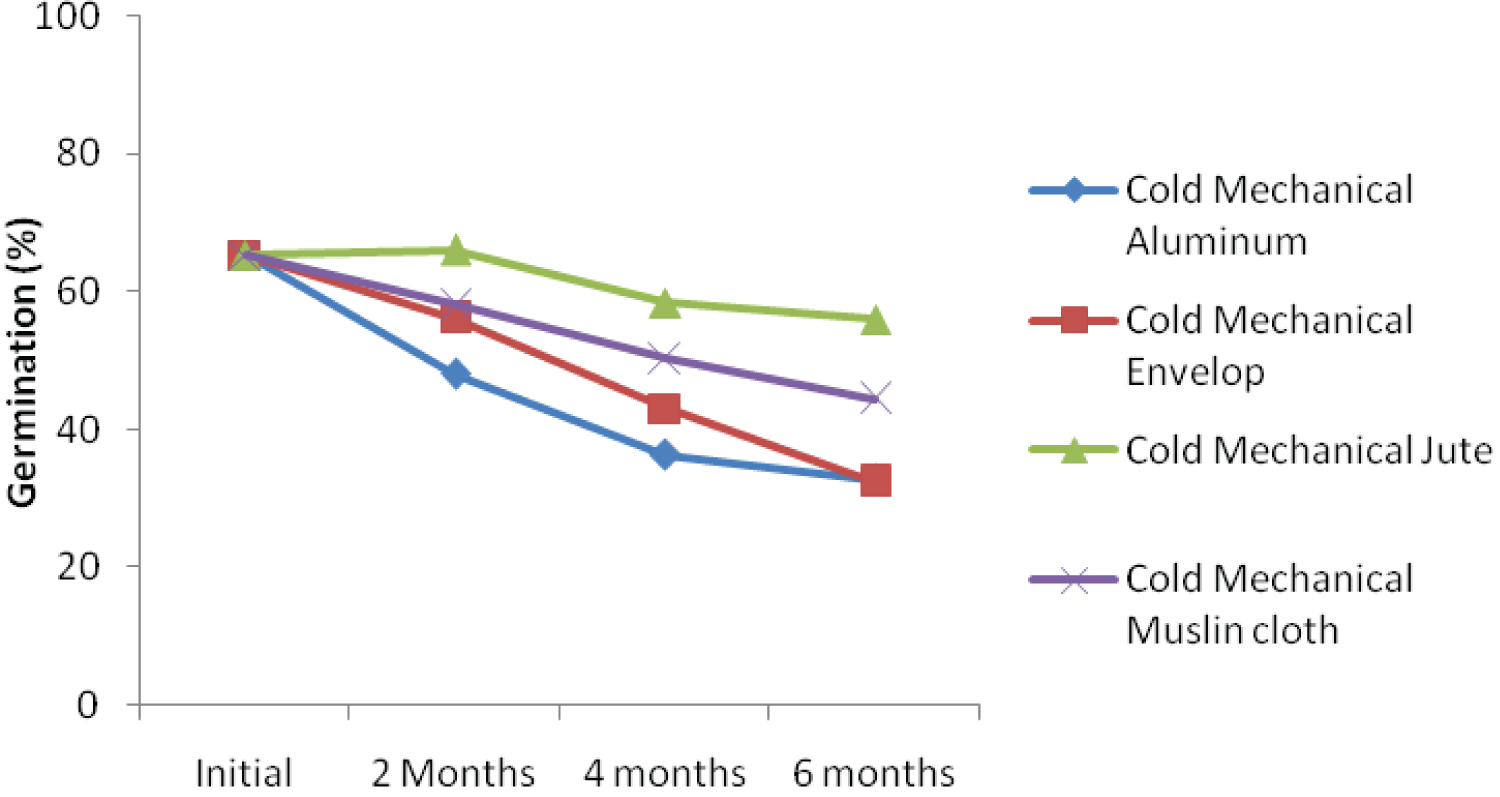
The interaction effect among ambient storage, hand threshing and packaging materials is presented in Figure 1a. The result showed that kenaf seed germination declined over storage periods. At 6 months after storage, seed threshed using hand and stored in jute bag under ambient condition recorded the highest germination percentage. Similar trend was also recorded when kenaf seed was threshed using leg and stored with jute bag (Figure 1b). Interaction effect among ambient storage, mechanical threshing and packaging materials is presented in Figure 1c. Despite decreasing in germination percentage over storage periods, the seed threshed mechanically and packaged with jute bag recorded the highest germination percentage. This could be as a result of subtle damage to the seed during mechanical threshing because the whole plant with the seed capsules were fed into the machine. This finding was not in agreement with the findings of Dharmaputra, et al. [3] and Jyoti and Malik [4].
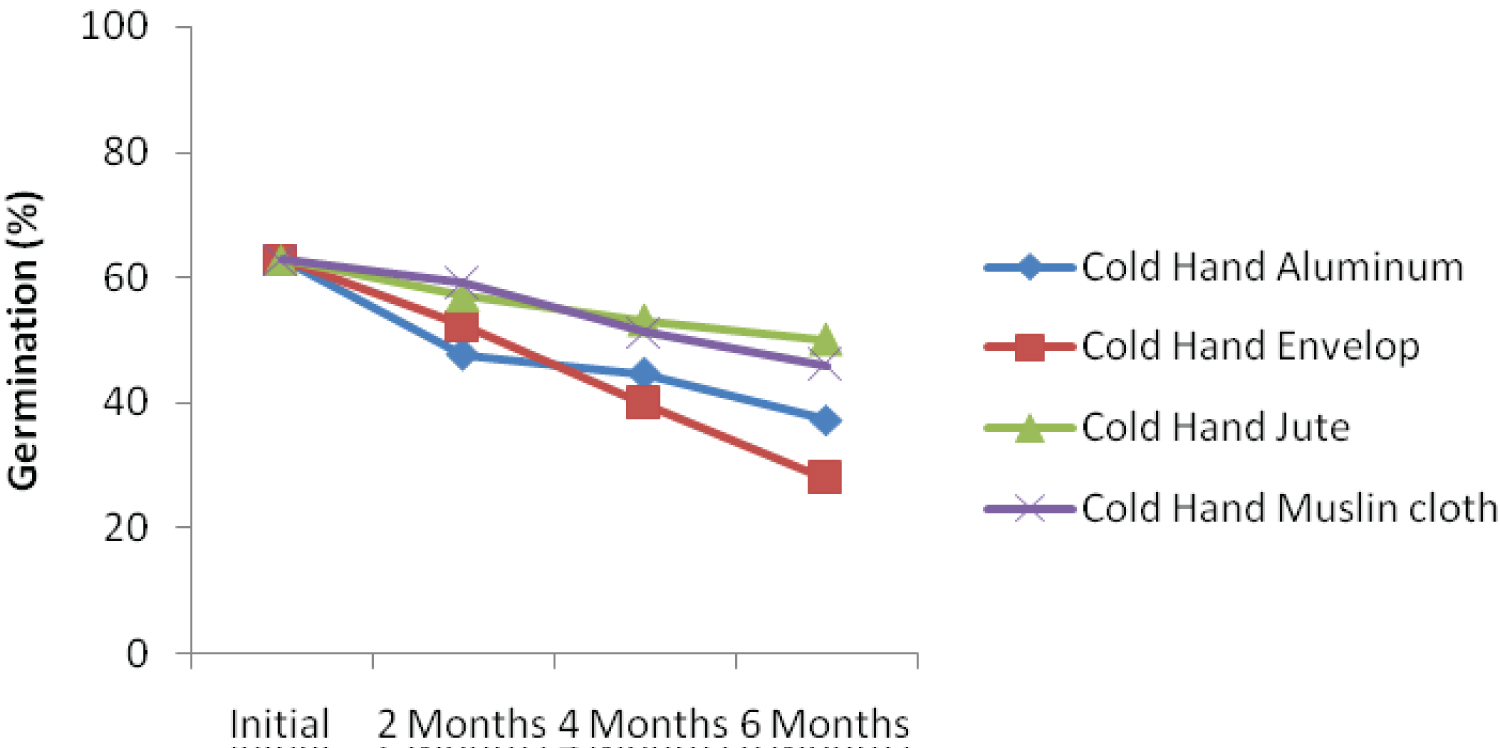
Under cold storage condition, germination of the seeds in different containers and threshed with hand decreased with storage durations (Figure 1d). The rate of deterioration was highest for seed stored with envelop after six-month (28.00%). Seed stored in jute bag recorded the highest germination percentage of 50 six-month after storage. Figure 1e showed that kenaf seed threshed with leg and stored using different storage materials deteriorate faster, Initial germination percentage was also the least when compared with other threshing methods. The decline in germination could be as a result of direct contact of the leg with the seeds during threshing. This corroborates the findings of Dharmaputra, et al. [3] Seed threshed mechanically and stored using jute bag obtained the highest level of germination (56.00%) in six-month (Figure 1f), which was significantly different from all other packaging materials. The rate of deterioration over the period of storage was very small when seed was threshed mechanically and packaged with jute bag. The rate of deterioration 2, 4, and 6 months after storage are 0.5%, 10% and 4.01%, respectively. The result showed that initial germination was also the highest (65.33) when threshed mechanically.
Conclusion
Results from this study have shown that highest germination percentage and lowest rate of seed deterioration were recorded when kenaf seeds was threshed mechanically and stored in jute bag followed by muslin cloth. The more extended storage period caused decreasing kenaf quality in all kenaf varieties and packaging materials used. Kenaf seed threshed mechanically and packaged using jute bag gave the best performance.
Acknowledgment
We are profoundly grateful to the Institute of Agricultural Research and Training, Obafemi Awolowo University, Moor Plantation, Ibadan, Nigeria for the research grant.
References
- Deepa GT, Chetti MB, Khetaagoudar MC, et al. (2013) Influence of vacuum packaging on seed quality and mineral contents in Chilli (Capsicum annuum L). Journal of Food Science Technology 50: 153-158.
- Henning A, Francisco K, França Neto C, et al. (2006) Technologies that add value to soybean seed. Seed News, The International Seed Magazine.
- Dharmaputra O, Ambarwati S, Retnowati I (2012) Postharvest quality improvement of sorghum (Sorghum bicolor (L.) Moench) grain. Biotropia 19: 115-129.
- Jyoti, Malik CP (2013) A review on seed deterioration. International Journal of life Science Biotechnology and Pharma Research 2: 374-385.
- Salari K, Amiri Chayjan R, Khazaei J, et al. (2013) Optimization of independent parameters for chickpea threshing using response surface method (RSM). Journal of Agricultural Science and Technology 15: 467-477.
- Mbora A, Schmidt L, Angaine P, et al. (2009) Tree seed quality guide.
- Desheva G, Petrova S, Deshev M (2017) Germinability of soybean seeds stored more than 30 years in the Bulgarian National Seed Genebank. World Scientific News 69: 29-46.
- Khatun A, Kabir G, Bhuiyan MAH (2009) Effect of harvesting stages on the seed quality of lentil (Lens culinaris L.) during storage. Bangladesh J Agric Res 34: 565-576.
- Biabani A, Boggs LC, Katozi M, et al. (2011) Effects of seed deterioration and inoculation with Mesorhizobium cicerion yield and plant performance of chickpea. Aust J Crop Sci 5: 66-70.
- Tubic B, Malencic Ð, Tatic M, et al. (2005) Influence of aging process on biochemical changes in sunflower seed. HELIA 28: 104-114.
- Ellis RH, Roberts EH (1980) Improved equations for the prediction of seed longevity. Ann Bot 45: 13-30.
- Mohammadi H, Soltani A, Sadeghipour HR, et al. (2011) Effect of seed aging on subsequent seed reserve utilization and seedling growth in soybean. International Journal of Plant Production 5: 65-70.
- ISTA (1993) International rules for seed testing. Seed Sci Technol 21.
- (2002) SAS Institute. SAS/STAT user’s guide, Version 8, SAS Inst, Inc, Carg.
- Tonin GA, Perez SCJGD (2006) Qualidade fisiologicade sementes de Ocotea porosa (Nees et Martius ex. Nees) apos diferentes codicoes dearmazenamento esemeadura. Revista Brasileira de sementes.
- Balesevic-Tubic S, Tactic M, Dordevic V, et al. (2010) Seed viability of oil crops depending on storage conditions. HELIA 33: 153-160.
- Olasoji JO, Aluko AO, Adeniyan ON, et al. (2012) Effect of time of harvest on physiological maturity and kenaf (Hibiscus cannabinus) seed quality. Afr J Plant Sci 6: 282-289.
- Chattha SH, Jamali LA, Ibupoto KA, et al. (2012) Effects of different packaging materials and storage conditions on the viability of wheat seed (TD-1 variety) Science. Technology and Development 31: 10-18.
- Talipata A (2009) Effect of seed moisture content, packaging and storage period on mitochondria membrane of soybean seed. Journal of Agricultural Technology 5: 51-64.
- Gbolami TH, Golpayegani A (2011) Effect of seed ageing on physiological and biochemical changes in rice seed (Oryza sativa L). Int J Agric Sci 1: 138-143.
- Shelar VR, Shaikh RS, Nikam AS (2008) Soybean seed quality during storage: A Review. Agric Rev 29: 125-131.
Corresponding Author
Julius Oluseyi OLASOJI, Institute of Agricultural Research and Training, Obafemi Awolowo University, Moor Plantation Ibadan, Nigeria.
Copyright
© 2023 OLASOJI JO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.