Evolution of Succinate Dehydrogenase Inhibitor (SDHI) Fungicides as Plant Protection Active Substances in Europe
Abstract
Succinate dehydrogenase inhibitors (SDHI) are EU crop protection active substances which are controversial regarding their toxicity and ecotoxicity. From the total number of SDHI fungicides known (24) up to twelve were approved at EU plant protection Regulation EC 1107/2009 between 2011 and 2016. All these active substances are listed in Part A, B and E of Regulation EU 540/2011. Two SDHI approvals were removed by end of approval in 2021 and withdrawal in 2022 and there are concerns that more may follow in 2023. The evolution of these substances and the corresponding global modifications in terms of employment, functions, uses, crops and maximum residue limits were analysed in this work.
Keywords
Regulation EC 1107/2009, Regulation EU 540/2011, SDHI, Active substance, Chemicals
Abbreviations
SDHI: Succinate Dehydrogenase Inhibitor; AS: Active Substances; PPP: Plant Protection Products; MRL: Maximum Residue Limit; GAP: Good Agricultural Practice; CfS: Candidate for Substitution; ADI: Acceptable Daily Intake
Introduction
Following our constant plant protection substances survey [1-4,27-30], succinate dehydrogenase inhibitors (SDHI) are crop protection active substances (a.s.) exclusively with one fungicide function [5,6]. A specific mode of action (MOA) is associated with this of class of molecules targeted to complex II in the mitochondrial respiration chain, the functional part of the TCA cycle. However, some controversial points of view regarding toxicity [7] and ecotoxicity [8-12] regarding this class of pesticides were raised in 2018 in France [13]. These toxicological concerns were recently correlated by a global survey showing exposure impact [14]. Moreover, SDHI fungicides are already encountering resistance problems [15,16].
There are currently 24 approved SDHI fungicides, with twelve previously approved in Europe (ten left and four pending) listed separately in Table 1 and Table 2. Most of them, except benzovindiflupyr, are in renewal procedure, including two issues for 2023. The evolution of these active substances in EU regarding their plant protection status is described in this study.
This study has been conducted at various levels: their own evolution in number and their entry/exit, the evolution of their regulatory status (prolongation, renewal, Parts (A, B or E) of the Implementing Regulation EU 540/2011) [4,17], and their uses in crop protection together with their MRLs [18].
Material and Methods
Legal support
European pesticides database: The raw data were retrieved from the European pesticides database. This database lists all the substances approved as well as those not approved and those where an approval is pending [19].
Directives and regulations: Regulation (EC) No 1107/2009 [20] is the main and original document dealing with plant protection products (PPP) and substances (pesticides) since 2009. Implementing Regulation (EU) No 540/2011 [17] is the main companion of the PPP regulation as regards the list of approved active substances. Following, Regulation (EC) No 396/2005 [21] manages the rules on maximum residue levels of pesticides in or on food as well as plant and animal feed. Subsequently, all the information on one active substance is centralized on the Pesticide database, including Review Reports which contain the GAP usage tables.
The evolution of active substances was published in the European Union Official Journal. Raw data were extracted from the European Commission pesticide database rev 2.2 website [19] dealing with the Implementing Regulation (EU) No 540/2011 active substance management [17].
Definitions
Active substances: Active substances are all substances, including micro-organisms which have a general or specific action against harmful organisms or on plants, parts of plants or plant products. These active substances are further classified into categories that correspond to the parts of Implementing Regulation (EU) No 540/2011. All substances in Part A of Implementing Regulation (EU) No 540/2011 [17] came directly in 2011 from previous Directive 91/414/EEC. Substances in Part B are coming from Part A via renewals or from direct approvals after 2011. Substances candidates for substitution (CFS) are approved according to Article 24 of Regulation (EC) No 1107/2009 and listed in Part E directly or during renewals. These active substances meet one or more of the additional criteria laid down in point 4 of Annex II of Reg. No 1107/2009.
Agricultural uses or usages in plant protection are defined by cultivation practices and roughly managed by the couple crop vs. bioagressor/pathogen, linked to their unique fungicide function, and are listed in the corresponding Good Agricultural Practices (GAP) Table in the Review Reports in the EU Pesticide database.
Health and safety Hazards/Risks phrases: Hazard statements form part of the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). They are intended to form a set of standardized phrases about the hazards of chemical globally linked to the toxicity of substances and mixtures.
H301: Toxic if swallowed.
H331: Toxic if inhaled.
H400: Very toxic to aquatic life.
H410: Very toxic to aquatic life with long lasting effects.
H411: Toxic to aquatic life with long lasting effects.
Results
SDHI active substances
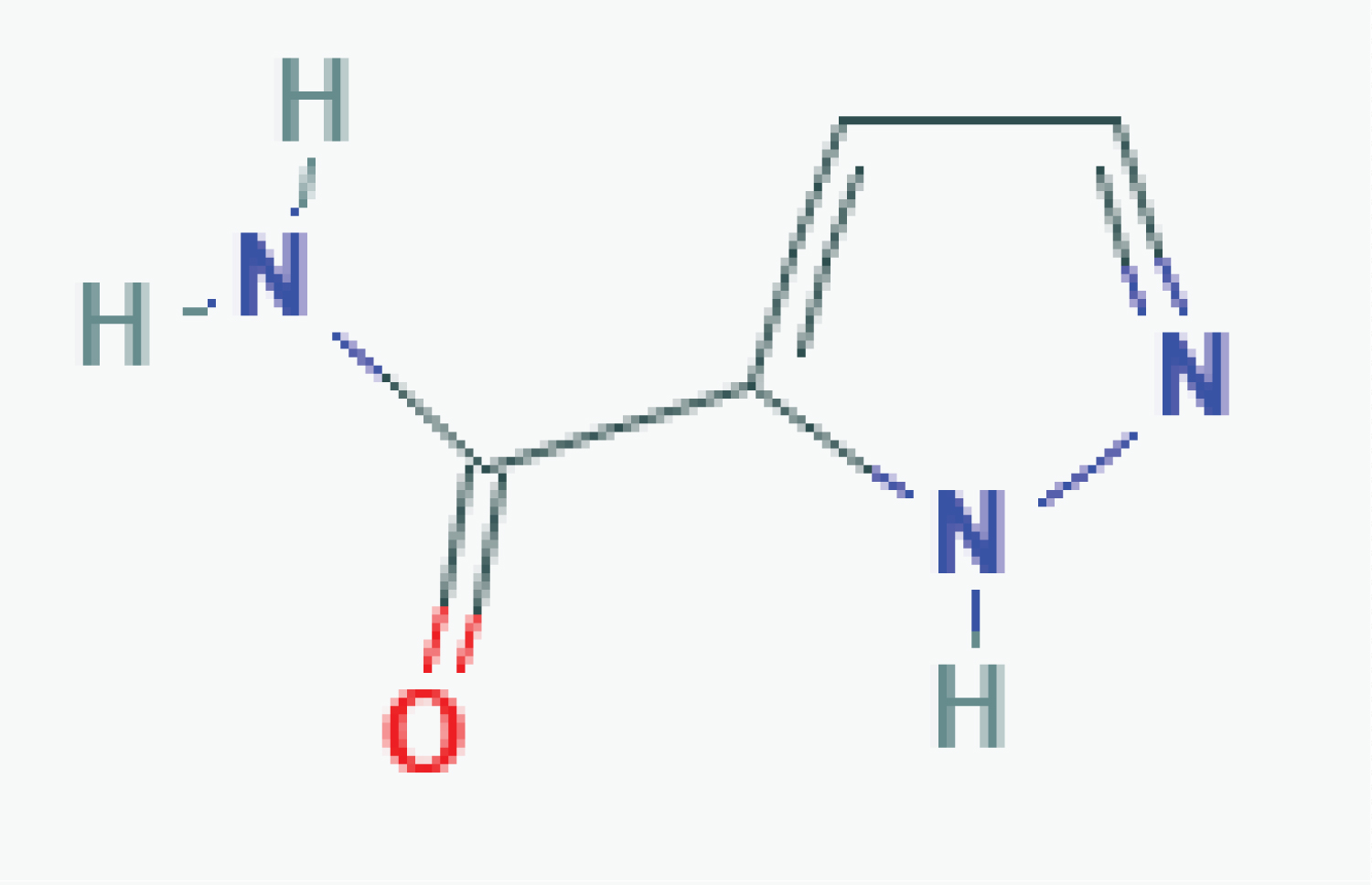
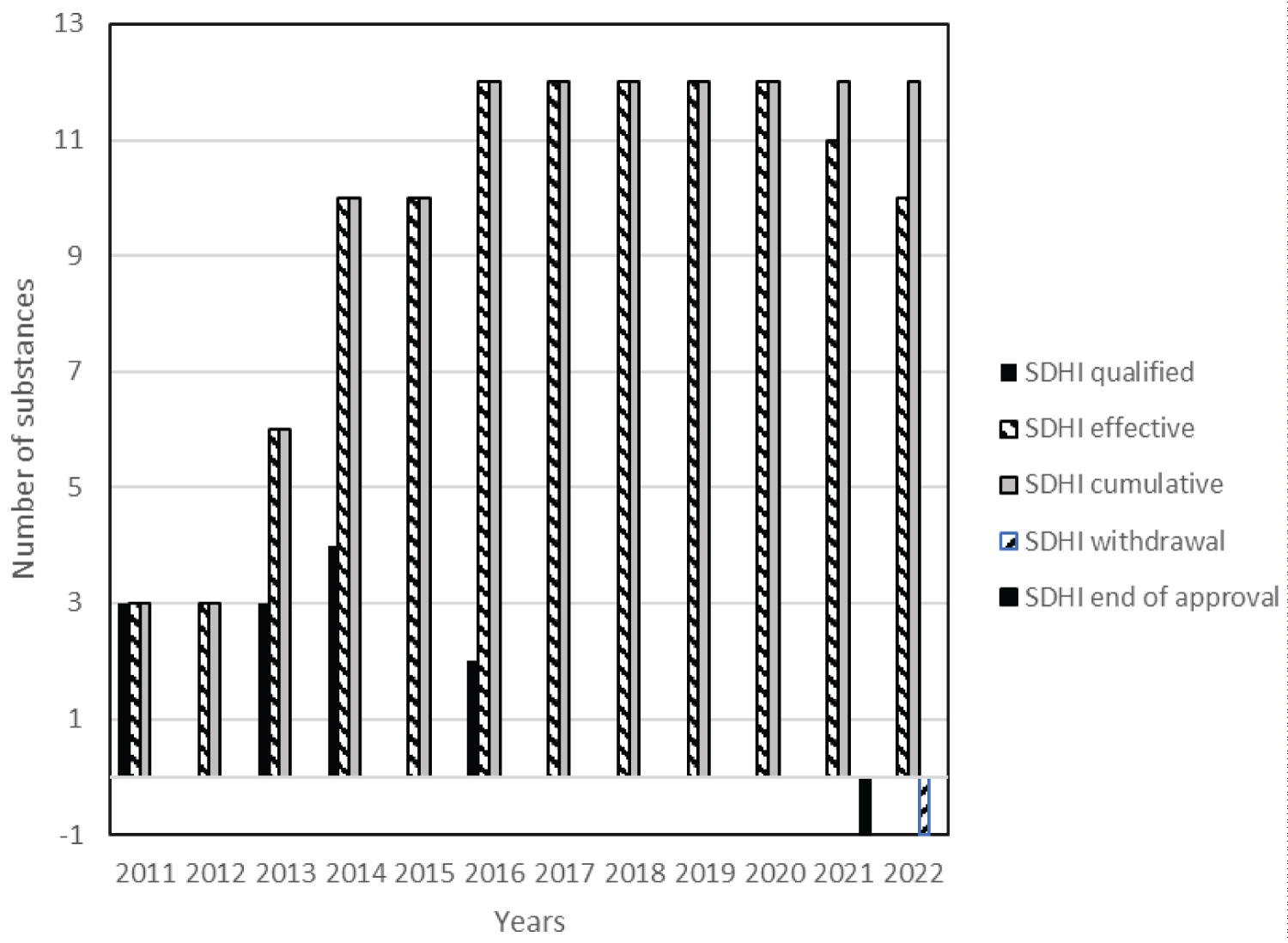
From the 24 SDHI fungicides currently known, half (twelve) were considered in Europe (ten still approved) and are listed as plant protection products (PPP) in Table 1. Others are considered in Table 2. None are low-risk substance [22] while two were considered by EU PPP regulation as candidate for substitution (CfS) (refereeing to Article 24 of Regulation EU No 1107/2009 [20]) with one of them already no longer approved [23]. The large majority of them (18, 75%) are from the carboxamide family (Figure 1), especially pyrazole carboxamide, including the most recent approvals, pending and EU application deposit or admissibility.
Other regulatory and toxicological considerations are included in Table 1 including those at the end of their approval at the EU level. The four candidates pending in EU, from pyrazolecarboxamide family, are fluindapyr, inpyrfluxam, isoflucypram and pydiflumetofen.
Evolution of the SDHI active substances since 2011
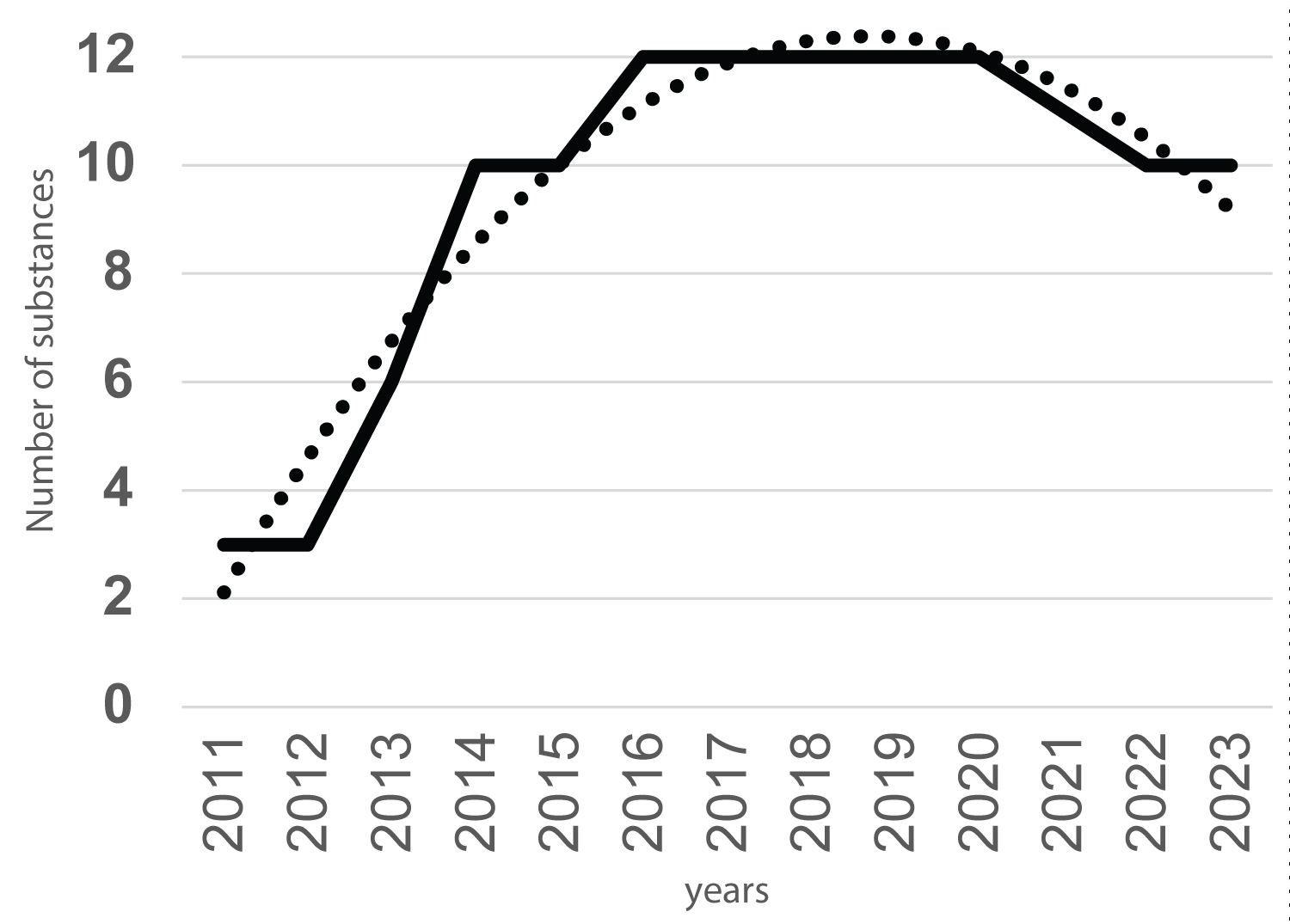
This global bell curve corresponds to total amount of SDHI substances managed over time, shown in years, is the result of the difference between approvals, withdrawals, renewals and disappearance of active substances without regulatory acts. Thus, most of all SDHI are subject to revaluation procedure in the coming years (2023-2026) except the recently renewed benzovindiflupyr (as CfS) and the recent removal of two SDHI in 2021 and 2022 (Figure 2 and Figure 3).
This evolution is indeed guided by the approval regulatory acts for all a.s., the withdrawal for isopyrazam in 2022 and the end of approval for carboxin in 2021 (Figure 3).
A positive evolution is expected starting in 2023, since admissibility was given for four new SDHI fungicides since 2016, now pending with full evaluations having been performed for two candidates without comes released for isoflucypram and pydiflumetofen and ongoing for two others (fluindapyr and inpyrfluxam) at EU level (Table 2) with EFSA.
Regulatory repartitions of the SDHI active substances
Following the modification of substances positions in the different Parts of the Regulation 540/2011 [23], all remaining SDHI are all in Part B or E after approval or renewal as exhibited in (Table 1). Previous SDHI (carboxin) listed in Part A was not renewed. All pending, if approved, will be listed in Part B or E depending toxicological evaluation, although isoflucypram and pydiflumetofen are not considered as endocrine disruptors and would probably be listed in Part B.
Consideration of EU SDHI crop usages
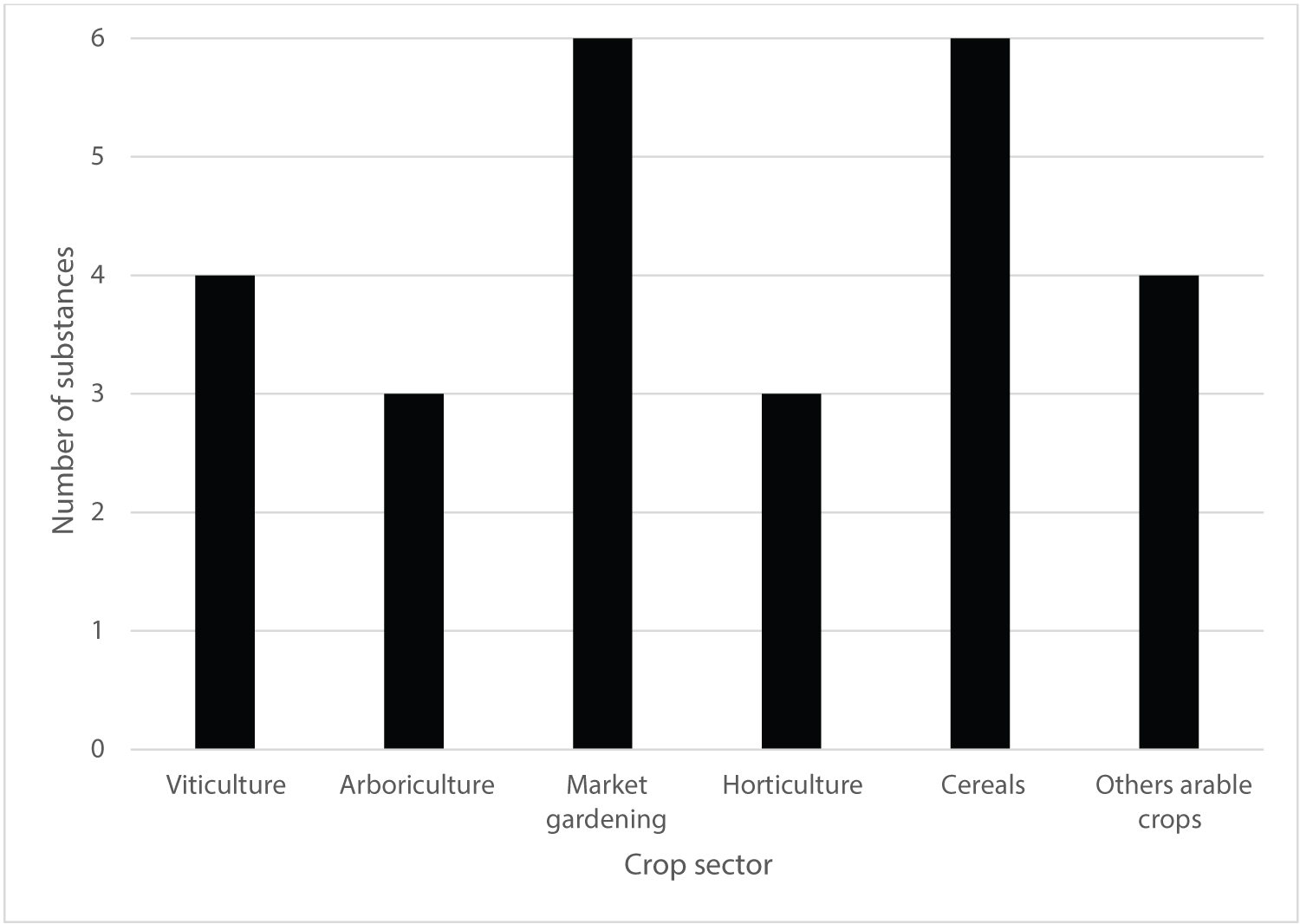
All crop classes [2,3] are covered by SDHI fungicides (Figure 4) although with only few active substances (from three to seven) and three crop usages were affected by the loss of the two a.s. (market gardening, cereals and others arable crops).
Discussion
Evolution of the SDHI and regulatory considerations
As mentioned above, eleven SDHI active substances are due to be following renewal process during the coming years (Table 1), namely AIR programmes. Suppressed crop usages are likely to increase in the future considering CfS substances and actual struggle for chemical PPP to be renewed (March and, submitted 2022) while global controversies which started in 2019 still generate debate and toxicological trials.
Possible evolution of the SDHI amount
The first observation from existing SDHI is the presence of toxicological concerns for existing SDHI, which will handicap their renewal. In addition, toxicological and ecotoxicological work is continuing which could lead to restrictions or withdrawals [9,24]. However, four new SDHIs are pending approval in the EU, which are already approved in North America, with no endocrine disruptors (ED) properties having been identified and are therefore not likely to become CfS (Art. 24) at EU PPP Regulation. Thus, with the recently published EFSA outcome, two of them, isoflucypram and pydiflumetofen may be approved in 2023 [25,26].
Evolution of the SDHI crop usages
Decrease of crop usages were observed with the disappearance of two a.s.: two usages for cereals were lost with one for market gardening, horticulture and others arable crops respectively while new potential usages for market gardening (two), arboriculture (two), horticulture, viticulture, cereals (two) and others arable crops were identified.
Conclusions
Following a rapid increase until 2017, SDHI active substances are now quite stable. Despite criticisms and controversies, only few SDHI a.s. were removed from approval and new family members are coming. At the same time, the evaluation of new SDHI members with modern evaluation criteria will allow progress from the point of view of their human and animal toxicity. Most already approved SDHIs will undergo renewal at this point, and objections and controversies will be resolved, although it is probable that some may not be renewed.
Acknowledgements
This work about phytochemical regulation was initiated and supported through French Ministry of Ecology, French Ministry of Agriculture, via “Plan de Relance” (RACAM, 2022). The Authors would like to thank Dr. Trevor M. Fenning of Forest Research (UK) for providing the helpful advice on the editing and writing of the manuscript.
References
- Marchand PA (2015) Basic substances: An opportunity for approval of low-concern substances under EU pesticide regulation. Pest Manag Sci 71: 1197-1200.
- Marchand PA (2023) Evolution of plant protection active substances in Europe: Disappearance of chemicals in favour of biocontrol agents. Environmental Science and Pollution Research.
- Robin DC, Marchand PA (2019) Evolution of the biocontrol active substances in the framework of the European pesticide regulation (EC) No. 1107/2009. Pest Manag Sci 75: 950-958.
- Robin D, Marchand PA (2019) Evolution of Regulation (EU) No 540/2011 since its entry into force. Journal of Regulatory Science 7: 1-7.
- FRAC (2011) Succinate Dehydrogenase Inhibitor (SDHI) Working Group, 4th Meeting on December 1, 2010. Protocol of the discussions and use recommendations of the SDHI Working Group of the Fungicide Resistance Action Committee (FRAC) 1-18.
- FRAC (2020) Understanding the SDHI (FRAC group 7) fungicides.
- Bénit P, Kahn A, Chrétien D, et al. (2019) Evolutionarily conserved susceptibility of the mitochondrial respiratory chain to SDHI pesticides and its consequence on the impact of SDHIs on human cultured cells. PLoS One 14: e0224132.
- Avenot HF, Luna M, Michailides TJ (2019) Phenotypic and molecular characterization of resistance to the SDHI fungicide fluopyram in populations of Alternaria alternata from pistachio orchards in California. Crop Prot 124: 104838.
- Azpiazu C, Bosch J, Martins C, Sgolastra F (2022) Effects of chronic exposure to the new insecticide sulfoxaflor in combination with a SDHI fungicide in a solitary bee. Sci. Total Environ 850: 157822.
- He F, Wan J, Li X, et al. (2021) Toxic effects of benzovindiflupyr, a new SDHI-type fungicide on earthworms (Eisenia fetida). Environ Sci Pollut Res 28: 62782-62795.
- Wu S, Lei L, Liu M, et al. (2018) Single and mixture toxicity of strobilurin and SDHI fungicides to Xenopus tropicalis embryos. Ecotoxicol Environ Saf 153: 8-15.
- Yanicostas C, Soussi Yanicostas N (2021) SDHI fungicide toxicity and associated adverse outcome pathways: What can zebrafish tell us? Int J Mol Sci 22: 12362.
- Anses (2019) Opinion of the French agency for food, environmental and occupational health & safety on the "assessment of a warning signal regarding the toxicity of Succinate dehydrogenaseinhibitor (SDHI) fungicides", Request n° 2018-SA-0113: 1-83.
- Herbert C, Döll P (2022) Analyzing the informative value of alternative hazard indicators for monitoring drought risk for human water supply and river ecosystems at the global scale. Nat Hazards Earth Syst Sci.
- Sang H, Hyang Burm Lee B (2020) Molecular mechanisms of succinate dehydrogenase inhibitor resistance in phytopathogenic fungi. Res Plant Dis 26: 1-7.
- Samaras A, Hadjipetrou C, Karaoglanidis G (2020) Bacillus amyloliquefaciens strain QST713 may contribute to the management of SDHI resistance in Botrytis cinerea. Pest Manag Sci 77: 1316-1327.
- EU (2011) Commission Implementing Regulation (EU) No 540/2011 of 25 May 2011 implementing regulation (EC) no 1107/2009 of the European parliament and of the council as regards the list of approved active substances. Official Journal of the European Union L 153/1.
- Charon M, Robin D, Marchand PA (2019) The major interest for crop protection of agrochemical substances without maximum residue limit (MRL). Biotechnol Agron Soc Environ 23: 22-29.
- EU (2022) EU Pesticides database v2.2.
- EC (2009) Regulation (EC) No 1107/2009 of the European parliament and of the council of 21 October 2009 concerning the placing of plant protection products on the market and repealing council directives 79/117/EEC and 91/414/EEC. Official Journal of the European Union L 309/1.
- EC (2005) Regulation (EC) No 396/2005 of the European parliament and of the council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending council directive 91/414/EEC. Official Journal of the European Union L70/1.
- Robin D, Marchand PA (2022) Expansion of the low-risk substances in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Eur J Risk Regul 13: 514-531.
- Robin D, Marchand PA (2021) The slow decrease of the active substances candidates for substitution in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Eur J Risk Regul 13: 1-22.
- Stojkovic D, Ivanov M, Ciric A (2022) Synthetic and natural antifungals-desirable and hazardous effects. Int J Mol Sci 23: 9608.
- EFSA (2019) Conclusion on the peer review of the pesticide risk assessment of the active substance pydiflumetofen. EFSA Journal 17: e05821.
- EFSA (2022) Conclusion on the peer review of the pesticide risk assessment of the active substance is oflucypram. EFSA Journal 20: e07328.
- Marchand PA (2016) Basic substances under EC 1107/2009 phytochemical regulation: Experience with non-biocide and food products as biorationals. J Plant Prot Res 56: 312-318.
- Marchand PA (2017) Basic Substances under EU Pesticide Regulation: An opportunity for organic production? Organic Farming 3: 16-19.
- Marchand PA (2018) Novel plant protection regulation: New perspectives for organic production? Organic Farming 4: 3-6.
- Marchand PA (2019) Synthetic Agrochemicals: A necessary clarification about their use exposure and impact in Crop Protection. Environ Sci Pollut Res Int 26: 17996-18000.
Corresponding Author
Patrice Marchand A, Institut de l'agriculture et de l'alimentation biologiques (ITAB), 149 rue de BERCY F-75595 PARIS CEDEX 12, France, Tel: +33-140-045-075; 33-140-045-063
Copyright
© 2022 Taylor A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.