Effects of Functional Single Nucleotide Polymorphisms on Plant Phenotypes
Abstract
Single nucleotide polymorphisms (SNPs) describe a change in a single nucleotide within the genome. SNPs are known as the markers of choice due to their desirable properties such as co-dominant in nature, robust, widely distributed throughout the genome, highly multiplexable, easily automated with high throughput techniques, have high reproducibility, and high efficiency for detection of polymorphism. Several studies have been successfully done for the identifications of the functional SNPs in the plant crops. However, the effects of the identified functional SNPs on plant phenotypes are not yet reviewed so far. Functional SNPs located in the coding regions of the gene can change the first or the second nucleotide sequence in the cordon which in turn alters the amino acid, its sequence, and protein structure that change the activities of the enzymes and finally results in the formation of new traits in the plants. The various desirable phenotypic variations produced within individuals of plant species include grain yields, quality traits, fruit size and shape, tolerance, resistance and adaptation to different abiotic and biotic factors, different colors of fruits and plants, flowering and ripening time. These important phenotypic traits could be improved through breeding using MAS. SNPs that are located in the coding sequence or found near to functional gene controlling desirable traits can help molecular breeder select targeted plant at the early growth stage. This makes the crop improvement program very efficient and rapid. Therefore, the application of marker technology particularly SNPs can increase the speed and efficiency of crop improvement. Therefore, the present seminar paper was targeted to review the application of SNPs in crop phenotypic traits.
Keywords
Functional SNPs, Marker assisted selection, Marker technology, Plant breeding, Plant phenotypes, SNPS
Introduction
Molecular markers are fragments or nucleotide sequences of DNA showing variations that can be used to detect polymorphism between different genotypes or alleles of a gene for a particular sequence of DNA in a population. The polymorphisms can be produced by different types of mutation such as insertion, deletion, substitution, duplication, and translocation of bases [1]. Molecular markers can be generally divided into three different categories based on criteria such as their mode of gene action, method of detection, and mode of transmission. Based on their mode of gene action (dominant such as RAPD, ISSR or co-dominant markers such as RFLP, SSR, and SNP), method of detection (hybridization-based, e.g., RFLP or polymerase chain reaction-based such as RAPD, AFLP, ISSR, and SSR), and mode of transmission either paternal organelle inheritance or maternal organelle inheritance [2,3].
Molecular markers are used for genetic diversity analysis, cultivar identification or assessment of purity, hybrid testing, marker-assisted selection, construction of genetic linkage maps, quantitative trait locus (QTL) mapping, and characterization of transformants in plants [4,5].
Furthermore, molecular markers can be also classified into two based on the determination of allele size. These are gel based and sequence based molecular markers. According to Sushil [6], RAPD, AFLP, ISSR, and SSR are gel based molecular markers because it uses gel electrophoresis for the determination of allele size. The bands formed indicate the length variation of DNA and show polymorphisms. On the other hand, the length variation of DNA fragments may not show the exact difference among genotypes because their DNA sequence is unknown. Even equal lengths of DNA may have different base sequences. Furthermore, according to Gupta, et al. [7], gel-based molecular markers are expensive, time-consuming that limits their utility.
However, SNPs are the sequence-based variation that solved the limitations of gel-based molecular markers because they are fast, cost-effective, reduce labor and provide a higher rate of accuracy during selection [7,8]. Additionally, SNPs are also known as the markers of choice and used in genetic studies due to their desirable properties such as robust, widely distributed throughout the genome of plants, highly multiplexable, and easily automated with high throughput techniques [9].
Furthermore, SNPs have been used to detect the presence of polymorphism without gel-based electrophoresis utilizing high-throughput methods, such as microarray technology [10]. SNPs can be detected using sequencing such as NGS (next-generation sequencing) technologies (Roche/454, Illumina, SOLiD) [11], genotyping by sequencing (GBS) [12]. Unlike the other DNA-based molecular markers, SNPs are based directly on known sequence polymorphisms [13]. Therefore, SNPs are the most superior molecular markers for detecting the polymorphisms among different organisms using the sequence of nucleotides of the genomes.
SNPs describe a change in a single nucleotide within the genome. If SNPs occur in a coding region of the gene, it can change the phenotype of an individual within the same species and produce desirable or undesirable characteristics in plants [14]. The most important phenotypic variation produced within individuals of plant species includes grain yields, quality of crops, fruit size and shape, disease resistance, tolerance and adaptation to different abiotic and biotic factors, the various color of fruits and plants, flowering and ripening time [15,16]. These desirable phenotypic traits can be improved using marker-assisted selection (MAS). MAS is the molecular breeding method which involves the use of DNA based markers that are closely linked to a phenotype to assist the selection of economic trait in a breeding program [17]. DNA based markers that are closely linked a gene controlling useful trait can serve as a chromosomal land mark to select plants that carry desirable traits.
SNPs have various applications in studying genetic diversity analysis, cultivar identification, detecting relationships between allelic forms of a gene and phenotypes, high-resolution genetic map construction, linkage disequilibrium based association mapping, genetic diagnostics, and phylogenetic analysis, creates great potential for characterization of genetic resources, and becoming the preferred marker system in marker-assisted breeding programs [18,19].
Several studies have been conducted on the identification of functional SNPs in various plants and their importance in plant breeding program [19-24]. The main objective of this seminar paper is to review the effects of functional SNPs on plant phenotype and their applications in crop improvements using marker-assisted selection.
Definition of SNPs Marker and Its Distributions in the Plant Genome
Single nucleotide polymorphism can be defined as a single base-pair change found in the genome sequence of an individual with an allele frequency of greater than 1% in the population. It is caused by point mutations such as insertion, substitution, deletion, and indels [25]. SNP is a recent type of molecular marker which represents the site where the DNA sequence shows a variation on the gene by single base pair in a plant genotype [14] also defined SNP as the most common form of DNA sequence variation between alleles that shows polymorphisms in plant species. According to Devesa-PeirÓ, et al. [26], SNPs were first introduced in 1998 to determine the linkage between a disease locus and a chromosomal region for the genotype-phenotype association.
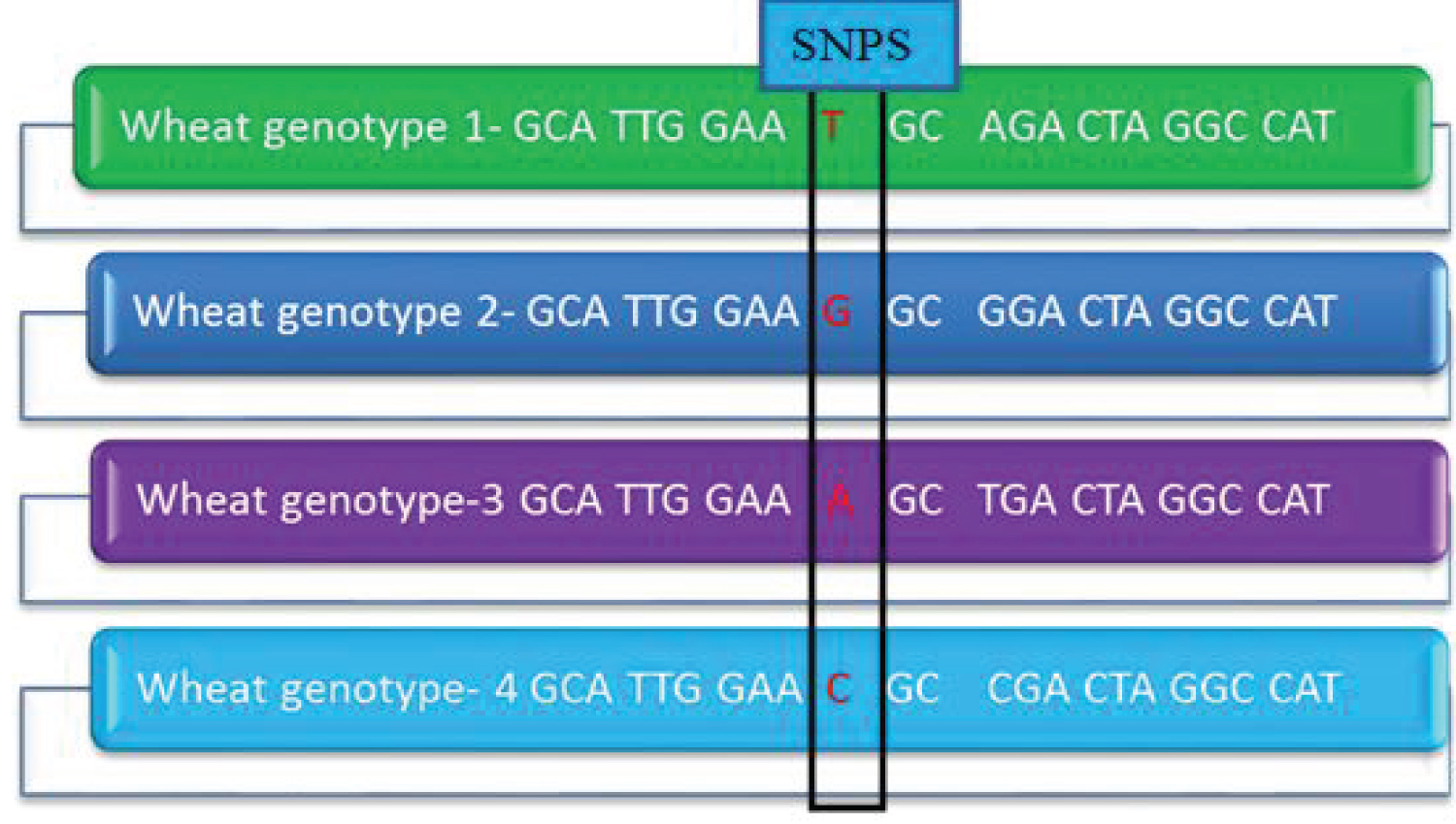
As indicated in Figure 1 above, SNPs found in four wheat genotypes (1-4) at locus 10 created variation among wheat with a nucleotide change from T to G, A, and C producing cysteine, glycine, serine, and arginine respectively.
According to Xu [3], SNPs are widely distributed within the plant genome and can be found in exons (coding regions) or intron (non-coding regions) of genes or between two genes (intergenic region) with different rates of frequency. Zhang, et al. [27] reported that the distribution of SNPs in the plant genome are widely distributed and it can be found in any region of a gene, mRNA, and intergenic region. However, most SNPs identified in plants are located in the introns and may not be functional.
Working mechanisms of functional SNPs
Sarkar, et al. [28] reported that functional SNPs are found in various pathways such as amino acid metabolism, purine, and pyrimidine biosynthesis pathway, cellulose and lignin biosynthesis, in line with breeding strategies that target pathways governing carbon and energy partition.
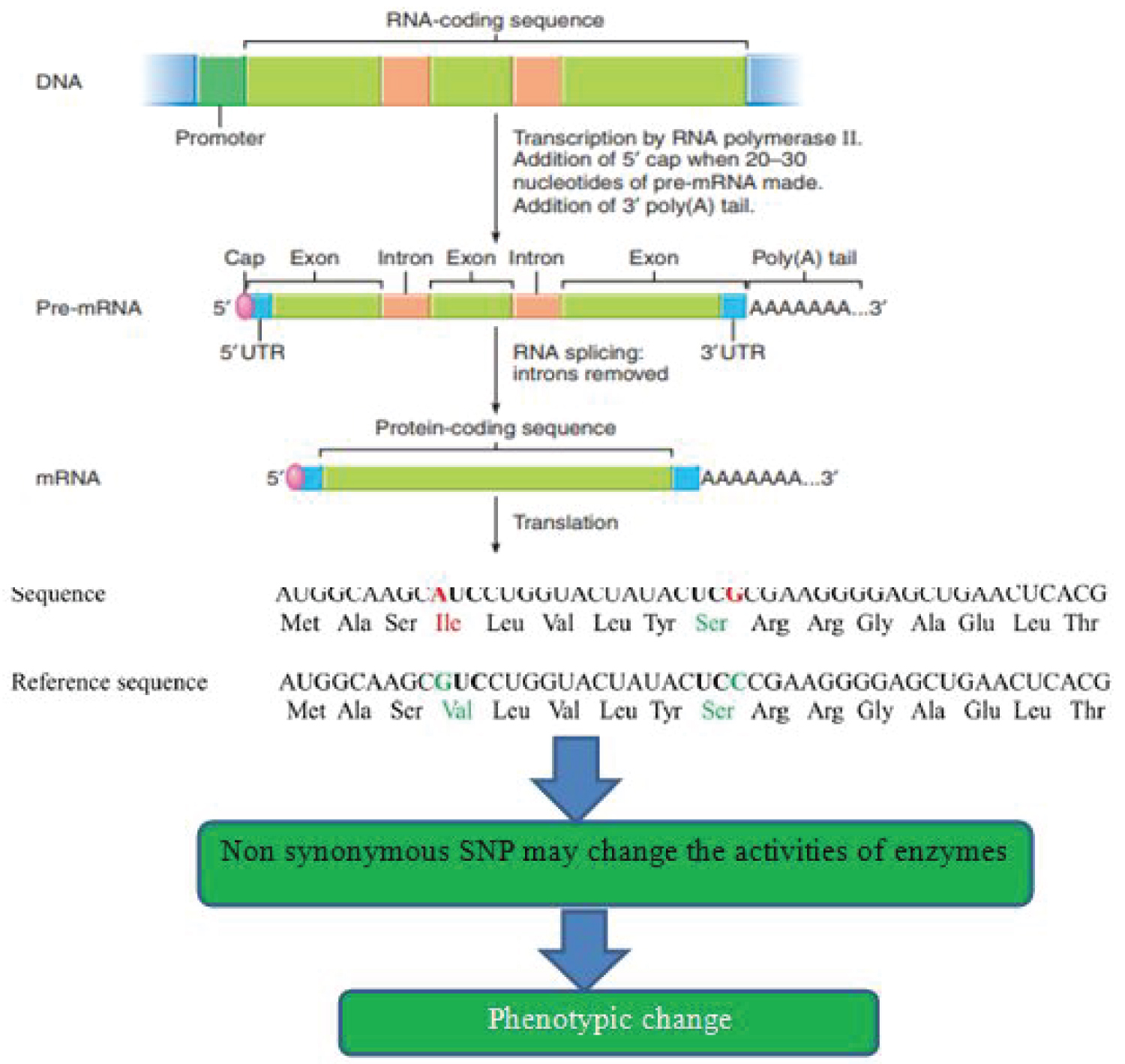
According to Aerts, et al. [29], in addition to non-coding regions, SNPs are located in genes. During post-transcription, introns are removed and exons are joined together. SNPs that are occurred in the intron regions of the gene have no effect on the plants. SNPs in the coding region are further classified into two as synonymous and non-synonymous SNPs. Synonymous SNPs do not affect the amino acid sequence and remain silent whereas non-synonymous SNPs change the amino acid sequence of the protein and may influence the enzyme activity. Non-synonymous SNPs are functional SNPs and very important because they affect the function of the gene. Therefore, functional SNPs may influence the activity of promoters for gene expression and produce functional protein through translation which results in the variations of plant phenotype.
Zhang, et al. [27] also reported that functional SNPs affect the levels of translation or gene expression, efficiency to enhance or inhibit mRNA stability, splicing, and protein function, which results in the formation of new traits in the plants as shown in Figure 2 below.
This concept was taken from Russell (2014) and Huq, et al. [16] with minor modification.
Effects of Functional SNPs on Plant Phenotypes
Crop production can be highly affected by various abiotic factors such as drought, salinity, and biotic factors such as diseases and insects. Functional SNPs are the key to overcoming the problems through developing improved crop varieties that can resist these factors and give a high yield. Some of the effects of functional SNPs on plant phenotypes are described as follows.
Flowering time adaptation
In plant breeding, flowering time is one of the most important key traits for the life cycles of plants for the accomplishment of successful reproduction either through self or cross-pollination among plants [22]. However, different environmental conditions, such as precipitation, photoperiod, and temperature affect flowering time and could decrease crop yields by 10-20% [30]. To overcome these problems and guarantee the success of plant reproduction, plants have changed flowering times to adapt to global warming [22].
Several studies have reported that many wild plant species flower earlier to mitigate the negative effects of climate change on the annual and insect-pollinated plants in a short rainy season [31]. Early flowering strategy is an effective adaptive phenological shift in response to climate change in some wild species such as barley cultivars [22].
Flowering time can be regulated through different flowering regulator genes such as SVP, FT, and FLC that are linked to flowering time variation work together to orchestrate early flowering to adapt to the different climatic changes in plants [32]. Méndez-Vigo, et al. [33] reported that a single amino acid substitution in SVP using the method of transgenesis and directed mutagenesis caused the loss of amino acid function and resulted in the early flowering of Arabidopsis thaliana. CO1 regulates both the integrator FT1 and the repressor VRNH2 in barley causing early flowering. CO1 performs its activities by promoting the transcription of FT1, which in turn promotes flowering [34]. These findings could have great importance for crop improvement by providing instructive genetic information to overcome the continuous climatic changes.
Tolerance to biotic and abiotic stresses
Functional SNPs located in the exon parts of the gene can control the level of biotic and abiotic stresses and improve the variety of abiotic and biotic stress-tolerant crops by changing expressed regions [35]. 196 loci with 230 candidates of SNPs associated with drought resistance were identified from common bean (Phaseolus vulgaris L.) for nine quantitative traits. ABA-responsive element-binding protein family, MYB, the protein kinase superfamily, and NAC are linked to drought resistance. These findings showed promising alleles linked to root traits or drought resistance, providing insights into the genetic basis of roots and drought resistance, which will be valuable for common bean improvement [36].
Seven of the 57 SNPs were located in reported genes whose homologs in the common bean are involved in response to drought, ABA, and osmotic stresses [27,37]. The TaMYB94 transcription factor activates cuticular wax biosynthesis in Arabidopsis thaliana, and it is important in the plant response to environmental stress, including drought [20], which lays basic genetic information for common bean improvement through molecular marker-assisted selection. Wang, et al. [38] reported that seven functional SNPs found in MeMYB26 gene are drought-responsive transcription factor in cassava plants which plays an important role in the expression of drought resistance and cassava storage roots. The seven SNPs in MeMYB26 were significantly associated with drought tolerant crops (DTCs) of catalase (CAT), aboveground fresh weight (AGFW), superoxide dismutase activity (SOD), and storage root number (SRN). According to Prathap, et al. [39], it had a significantly up-regulated expression for starch accumulation in the sink and might play a positive role in cassava drought resistance under drought stress. Therefore, these functional SNPs are used for marker-assisted selection in the breeding of drought-tolerant cassava without loss of biomass storage.
Similarly, the functional SNPs found in the BrpHAIRY LEAVES1a (BrpHL1a) gene of Brassica rapa are very important for the formation of the tryptophan-92 residue at 274T/C and 403T/G, which produces leaf hairs phenotype but the substitution functional SNP of the tryptophan-92 residue at 274C and 403G produced hairless leaves using mutagenesis. The hairy phenotype could be used as a molecular marker for insect resistance in Brassica spp. and facilitate the molecular breeding of many crops [27]. A leaf hair (trichome) is an epidermal hair that helps as a physical barrier on plant surfaces against abiotic and biotic stresses including excessive transpiration, pathogenic microorganisms, insect herbivores, UV light, and freezing [40,41].
Disease resistance: Pathogenic microorganisms such as viruses, fungi and bacteria can cause plant disease and affect crop production. However, several researchers have been doing various studies to tackle these problems using functional SNPs and develop disease-resistant crop varieties. Yundaeng, et al. [42] identified functional SNP found in the TAF5 gene from mung bean (Vigna radiata L.) that enables plants to develop disease resistance against Cercospora leaf spot, fungus disease. Two populations of mung bean namely "V4718" (resistant) and "Kamphaeng Saen 1" (KPS1; susceptible) were developed from F2 X BC1F1 using the backcross method. Inserts/deletions of SNPs found in exon 8 of the VrTAF5 gene caused the amino acid change at residue position of 250 from serine (S) to threonine (T) in V4718 but not in KPS1. VrTAF5 gene encodes TAF5. The In Dels mutation in the VrTAF5 (LOC106765332) gene causing an amino acid change in structure and function is responsible for Cercospora leaf spot resistance in V4718 probably by invoking a transcriptional response in the host plant through the histone acetylation. This enables the breeder to develop Cercospora leaf spot disease-resistant mung bean variety using MAS.
Wu, et al. [43], reported that three natural functional SNP found in the gene TraesCS2B01G51310 of wheat namely Rv-680 changed a nucleotide a C to a T, AX108806204 a G to an A and AX-111730867 a G to an A respectively causes amino acid change from alanine to valine, alanine to threonine, and glutamate to lysine respectively. These SNPS encodes a serine/threonine-protein kinase (STPK) were responsible for combating yellow rust caused by Puccinia striiformis f. sp. tritici (Pst) that affect the wheat production. Mutation analysis and gene expression approved that STPK performed an important function in yellow rust resistance.
According to Serrano, et al. [44], 92 functional SNPs observed in seven genes of olive (Olea europaea L.) showed disease resistance against Verticillium wilt that could be useful to distinguish germplasm collections regarding Verticillium wilt resistance and thus providing aid in the selection process of breeding programs. Furthermore, Gharbi, et al. [45] described that TLP1 belongs to the PR5 protein family related to stress resistance response that inhibits sporulation of fungal pathogens and hyphal growth. A previous study indicated that genes coding for PR5 found in olive plants were highly expressed in resistant cultivar after Verticillium dahliae infection.
According to Shi, et al. [46], soybean cyst nematode is the most economically destructive pathogen of soybean. To solve this problem, the researcher identified three functional SNPs, two from the Rhg1 locus and one from the Rhg4 locus of soybean genes that could be responsible for disease resistance against soybean cyst nematode. The SNPs were found in the coding sequences of resistance genes (Glyma18g02590 and Glyma08g11490) and create changes in protein sequences. Thus, they can be used for the resistance of cyst nematode race3 in soybean and applied in high throughput marker-assisted selection with high accuracy. Furthermore, Liu, et al. [47] explained a new mechanism of plant resistance to soybean cyst nematode. A single gene at the Rhg4 locus encoded a serine hydroxymethyl transferase (SHMT, Glyma08g11490) that was responsible for soybean cyst nematode (SCN) resistance. Two SNPs have been identified in the coding sequence of SHMT, 389 G/C in the first exon changes amino acid from Arginine (R) to Proline (P) and 1,165 A/T in the second exon, changes amino acid from Tyrosine (Y) to Asparagine (N). These results in the change of the enzyme property and may correlate with Rhg4-dependent resistance and susceptibility. Therefore, the result could be used for marker-assisted selection of plants that will exhibit SCN resistance for accelerating its breeding accurately and efficiently.
Ripening of fruits
Functional SNPs have great role in regulating the ripening of different fruits. Xu, et al. [3] identified functional SNPs in eight genes that regulate early berry ripening and coloring in SBBM grapes. The expression of ERF3 and ERF5, involved with ethylene production, increased during ripening time to increase the production of ethylene that might promote early berry ripening in SBBM. The CHS gene was potentially related to early berry ripening by participating in the anthocyanin biosynthesis pathway and produce anthocyanin in the skin of the berry of grape to give deepen color indicating the ripening of grapes. NCED6 gene expression has participated in the ABA biosynthesis of grape. The production of ABA promotes anthocyanin accumulation in the grapes.
Quality of yields
SNPs also have significant contribution quality improvement of crops. In line with this, Nishio, et al. [23] identified a new functional SNP from Japanese Pear (Pyrus spp.) on chromosome 11 that had large effects on sugar conversion from fructose to glucose and vice versa. The SNP found on chromosome 4 was associated with fructose that would be useful for increasing the contents of fructose and total sugar content without affecting other sugar contents. Therefore, these SNPs would potentially enable breeders to select genotypes with high fructose and low glucose for improving the sweetness of the fruits of Japanese Pear (Pyrus spp.). Similarly, Orchard, et al. [24] identified two functional single nucleotide polymorphisms (SNPs) from tomato that most likely cause high β-carotene through their influence on transcription of Beta allele (B) of the Cyc-B gene that is elevated in ripening fruit. Beta carotene is very important in food and provides an essential nutrient to animals that consume them.
Moreover, Udoh, et al. [19] identified functional SNPs found in the exon region of the LYCE gene at the 1295 nucleotide position of the reference gene (cassava43823.valid.ml) nucleotide variation G/T. G (guanine) was found in the reference sequence and T (thymine) in high carotene accession (07/0539) that occurred only in high carotene accession. This change was resulted in an amino acid change from Trp (tryptophan) to Leu (leucine) and increased levels of β-carotene in cassava plants. These findings could solve vitamin A deficiency in several cassava varieties. Genes such as β-carotene hydroxylase (HYD), Phytoene synthase (PSY), lycopene β and ε cyclase (LYCB and LYCE) play an important role in increasing the levels of β-carotene in plants. Bang, et al. [48] reported that a single functional SNP found in the exon region of the LYCB gene changed an amino acid and produced red and yellow-fleshed watermelon (Citrullis vulgaris).
Jang, et al. [15] reported that the qHS1 found in the soybean cultivar gene encodes an endo-1,4-β-glucanase that controls the permeability of seed coat in soybean by the accumulation of carbohydrates containing β-1,4-glucan at the outer layer of seed. New cultivar Tachinagaha was developed by transferring the genomic region of qHS1 from the non-permeable NIL into the permeable cultivar Kariyutaka which finally resulted in the accumulation of β-1,4-glucan in the outer layer of palisade cells and results in the production of hard seeds. SNP in the endo-1,4-β-glucanase gene of seed in a new cultivar changed an enzyme function and resulted in increasing the amounts of β-1,4-glucan which is responsible for the formations of rigidity and impermeability for the seed.
Furthermore, Kharabian-Masouleh, et al. [49] confirmed that two functional SNPs identified from rice were located in the coding regions of GBSSI and SSIIIa one in each gene of rice and cause amino acid changes. SNP in GBSSI (waxy gene) gene controls starch properties such as trough viscosity, final viscosity, set back, retrogradation (Martin test), and amylose content while SNP in SSIIIa controls physiochemical properties of cooking such as pasting temperature, peak time, and breakdown viscosity of the starch properties of rice.
Shirasawa, et al. [50] reported that some functional SNPs controlled substantial effects on phenotypic variations of cultivated tomatoes. The SNPs located in FAS, SP, and U genes are responsible for creating phenotypic variations among cultivated tomatoes. SNPs at SL1_00sc6004_2094360_solcap_snp_sl_44897 FAS were responsible for fruit size and locule number, SL2.40ch06_42601581W SP responsible for plant height, SL2.40ch06_42601581W SNP located at SP genes determine the number of leaves between inflorescences and plant habit (indeterminate or determinate), U gene causes green shoulder of tomato. These functional SNPs could be used as powerful selection markers for marker-assisted selection in tomato breeding (Table 1).
Conclusion
Currently, SNPs have become the most preferred molecular markers in various applications in genetic research, including genetic diversity study, cultivar identification, detecting relationships between allelic forms of a gene and phenotypes, genetic diagnostics, high-resolution genetic map construction. It is also crucial in the fields of genetic engineering to produce genetically modified plants. Furthermore, functional SNPs are powerful tools to accelerate crop improvement and help breeders develop improved crop varieties for productivity and resilience. SNPs that are associated with desirable agronomic phenotypes can provide a better understanding of gene function and can be used efficiently in plant breeding programs. These desirable agronomic phenotypic traits could be utilized for plant breeding using marker-assisted selection. Therefore, functional SNPs could be used for the improvement of crops for the selection of high-yielding varieties, high-quality crops, disease-resistant crops, flowering time adaptation, ripening of fruits, abiotic and biotic stress-tolerant crop varieties.
Functional SNPs have the potential to revolutionize the field of plant breeding in the future to overcome the fluctuating climatic conditions and diseases that might reduce or devastate crop production. Currently, plant breeders are using marker-assisted selection for developing improved crop varieties, which is better than conventional breeding. However, the applications of functional SNPs are limited to some crops, fruits, and vegetables. Therefore, further research is needed to widen its scope to commercial crops, fruits, and vegetables using extra advanced molecular tools such as gene-editing technology.
Acknowledgements
Not applicable.
Authors' Contributions
Fekadu Korsa reviewed different article, summarize and wrote the review paper by developing introduction, objectives and the whole main bodies of the review. Tileye Feyissa served as supervisors of Fekadu Korsa and played great role in guiding the review starting from its writing up to finalizing it by editing, and commenting the review paper of Fekadu Korsa. This manuscript was extracted from the review paper of Fekadu Korsa. Both authors read and approved the final manuscript.
Financial Support
Not applicable.
Availability of Data and Materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
References
- Jiang GL (2013) Molecular markers and marker-assisted breeding in plants. Plant breeding from laboratories to Fields 45-83.
- Semagn K, Bjørnstad Å, Ndjiondjop MN (2006) An overview of molecular marker methods for plants. African Journal of Biotechnology 5: 25.
- Xu Y (2010) Molecular plant breeding. Cabi. 1-10.
- Mandal L, Verma SK, Sasmal S, et al. (2018) Potential Applications of Molecular Markers in Plant. Curr Trends Biomedical Eng & Biosci 12: e555844.
- Nickle T, Barrette-Ng I (2021) Applications of molecular markers.
- Sushil K (2013) Potential of molecular markers in plant biotechnology. International Journal of Plant Sciences 8: 426-444.
- Gupta PK, Roy JK, Prasad M (2001) Single nucleotide polymorphisms: A new paradigm for molecular marker technology and DNA polymorphism detection with emphasis on their use in plants. Curr Sci 524-535.
- Udoh LI, Willie P, Uzoebo C (2021) Single nucleotide polymorphisms: A modern tool to screen plants for desirable traits. In plant breeding-current and future views. Intech Open.
- Valliyodan B, Qiu D, Patil G, et al. (2016) Landscape of genomic diversity and trait discovery in soybean. Sci Rep 6: 1-10.
- Pindo M, Vezzulli S, Coppola G, et al. (2008) SNP high-throughput screening in grapevine using the SNPlex™ genotyping system. BMC Plant Biol 8: 12.
- Kircher M, Kelso J (2010) High-throughput DNA sequencing–concepts and limitations. Bioessays 32: 524-536.
- Elshire RJ, Glaubitz JC, Sun Q, et al. (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One 6: e19379.
- Young PR, Vivier MA (2010) Genetics and genomic approaches to improve grape quality for winemaking. Managing Wine Quality 316-364.
- Morgil H, Gercek YC, Tulum I (2020) Single nucleotide polymorphisms (SNPs) in plant genetics and breeding. In The Recent Topics in Genetic Polymorphisms. Intech Open.
- Jang SJ, Sato M, Sato K, et al. (2015) A single-nucleotide polymorphism in an endo-1, 4-β-glucanase gene controls seed coat permeability in soybean. PLoS One 10: e0128527.
- Huq MA, Akter S, Nou IS, et al. (2016) Identification of functional SNPs in genes and their effects on plant phenotypes. J Plant Biotechnol 43: 1-11.
- Singh BD, Singh AK (2015) Marker-assisted plant breeding: principles and practices. New Delhi: Springer India.
- Freudenthal JA, Ankenbrand MJ, Grimm DG, et al. (2019) GWAS-Flow: A GPU accelerated framework for efficient permutation based genome-wide association studies. Bio Rxiv.
- Udoh LI, Adesoye A, Gedil M (2017) Identification and molecular analysis of pro-vitamin a carotenoid genes in cassava (Manihot esculenta Crantz). Mol Plant Breed 8.
- Lee J, Davari H, Singh J, et al. (2015) Industrial Artificial Intelligence for industry 4.0-based manufacturing systems. Manufacturing Letters 18: 20-23.
- Xu Y, Gao Z, Tao J, et al. (2016) Genome-wide detection of SNP and SV variations to reveal early ripening-related genes in grape. PLoS One 11: e0147749.
- Qian C, Yan X, Shi Y, et al. (2020) Adaptive signals of flowering time pathways in wild barley from Israel over 28 generations. Heredity 124: 62-76.
- Nishio S, Hayashi T, Shirasawa K, et al. (2021) Genome-wide association study of individual sugar content and sugar conversion in fruit of Japanese pear (Pyrus spp.).
- Orchard CJ, Cooperstone JL, Gas Pascual E, et al. (2021) Identification and assessment of alleles in the promoter of the Cyc-B gene that modulate levels of β-carotene in ripe tomato fruit. The Plant Genome 14: e20085.
- Nadeem MA, Nawaz MA, Shahid MQ, et al. (2018) DNA molecular markers in plant breeding: current status and recent advancements in genomic selection and genome editing. Biotechnol Biotechnol Equip 32: 261-285.
- Devesa Peiró A, Sánchez Reyes JM, Díaz Gimeno P (2020) Molecular biology approaches utilized in preimplantation genetics: real-time PCR, microarrays, next-generation sequencing, karyomapping, and others. In human reproductive genetics. Academic Press 49-67.
- Xueyong Z, Jian Y, Li X, et al. (2018) The first report of Cryptosporidium spp. in Microtus fuscus (Qinghai vole) and Ochotona curzoniae (wild plateau pika) in the Qinghai-Tibetan Plateau area, China. Parasitol Res 117: 1401-1407.
- Sarkar D, Maranas CD (2020) SNP effect: identifying functional roles of SNPs using metabolic networks. Plant J 103: 512-531.
- Aerts J, Wetzels Y, Cohen N, et al. (2002) Data mining of public SNP databases for the selection of Intragenic SNPs. Human Mutation 20: 162-173.
- Ray DK, Gerber JS, MacDonald GK, et al. (2015) Climate variation explains a third of global crop yield variability. Nat Commun 6: 1-9.
- Bock A, Sparks TH, Estrella N, et al. (2014) Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Glob Chang Biol 20: 3508-3519.
- Li J, Carlson BE, Lacis AA (2014) Application of spectral analysis techniques to the intercomparison of aerosol data-Part 4: Synthesized analysis of multisensor satellite and ground-based AOD measurements using combined maximum covariance analysis. Atmos Meas Tech 7: 2531-2549.
- Méndez Vigo B, Martínez Zapater JM, Alonso Blanco C (2013) The flowering repressor SVP underlies a novel Arabidopsis thaliana QTL interacting with the genetic background. PLoS Genet 9: e1003289.
- Mulki MA, Von Korff M (2016) Constans controls floral repression by up-regulating vernalization2 (VRN-H2) in barley. Plant Physiol 170: 325-337.
- Xavier A, Muir WM, Rainey KM (2016) Impact of imputation methods on the amount of genetic variation captured by a single-nucleotide polymorphism panel in soybeans. BMC Bioinformatics 17: 1-9.
- Wu L, Chang Y, Wang L, et al. (2021) Genetic dissection of drought resistance based on root traits at the bud stage in common bean. Theor Appl Genet 134: 1047-1061.
- Sura W, Kabza M, Karlowski WM, et al. (2017) Dual role of the Histone variant h2a. z in transcriptional regulation of stress-response genes. Plant Cell 29: 791-807.
- Wang B, Guo X, Zhao P, et al. (2021) MeMYB26, a drought-responsive transcription factor in cassava (Manihot esculenta Crantz). Crop Breeding and Applied Biotechnology 21.
- Prathap V, Ali K, Singh A, et al. (2019) Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol Biochem 142: 440-451.
- Nafisi M, Stranne M, Fimognari L, et al. (2015) Acetylation of cell wall is required for structural integrity of the leaf surface and exerts a global impact on plant stress responses. Front Plant Sci 6: 550.
- Hegebarth D, Buschhaus C, Wu M, et al. (2016) The composition of surface wax on trichomes of Arabidopsis thaliana differs from wax on other epidermal cells. Plant J 88: 762-774.
- Yundaeng C, Somta P, Chen J, et al. (2021) Fine mapping of QTL conferring Cercospora leaf spot disease resistance in mungbean revealed TAF5 as candidate gene for the resistance. Theor Appl Genet 134: 701-714.
- Wu J, Yu R, Wang H, et al. (2021) A large-scale genomic association analysis identifies the candidate causal genes conferring stripe rust resistance under multiple field environments. Plant Biotechnol J 19: 177-191.
- Serrano A, León L, Belaj A, et al. (2020) Nucleotide diversity analysis of candidate genes for Verticillium wilt resistance in olive. Sci Hortic 274: 109653.
- Gharbi Y, Barkallah M, Bouazizi E, et al. (2017) Differential biochemical and physiological responses of two olive cultivars differing by their susceptibility to the hemibiotrophic pathogen Verticillium dahliae. Physiol Mol Plant Pathol 97: 30-39.
- Shi Z, Liu S, Noe J, et al. (2015) SNP identification and marker assay development for high-throughput selection of soybean cyst nematode resistance. BMC Genomics 16: 314.
- Liu S, Kandoth PK, Warren SD, et al. (2012) A soybean cyst nematode resistance gene points to a new mechanism of plant resistance to pathogens. Nature 492: 256-260.
- Bang H, Yi G, Kim S, et al. (2014) Watermelon lycopene β-cyclase: Promoter characterization leads to the development of a PCR marker for allelic selection. Euphytica 200: 363-378.
- Kharabian Masouleh A, Waters DL, Reinke RF, et al. (2012) SNP in starch biosynthesis genes associated with nutritional and functional properties of rice. Sci Rep 2: 1-9.
- Shirasawa K, Fukuoka H, Matsunaga H, et al. (2013) Genome-wide association studies using single nucleotide polymorphism markers developed by re-sequencing of the genomes of cultivated tomato. DNA Res 20: 593-603.
Corresponding Author
Fekadu Korsa, Institute of Biotechnology, Addis Ababa University, Addis Ababa, P.O. Box, 1162, Ethiopia
Copyright
© 2022 Korsa and Feyissa. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.