The Effect of Silicon on Salinity Toxicity in Tomatoes Plants: The Role of Antioxidants in Alleviating Oxidative Stress Caused by Salt Conditions
Abstract
Salinity stress is a major environmental factor which adversely affects plant growth and development. Silicon (Si) is an abundant element which, when supplied to plants, increases tolerance to abiotic stresses, such as salinity and drought stress. This study aimed to investigate the roles of silicon (Si) in the amelioration of salinity in two tomato (Lycopersicon esculentum Mill) cultivated varieties cv bacioty, a cherry type cultivar, and cv. de barao chornyy, a blach cherry type, which is known as a plant sensitive to saline conditions. Despite, tomato is classified as a plant with low capacity of accumulating metalloids, when Si supplied to tomato plants, it improves growth under abiotic stress conditions by enhancing the yield of fruits or by improving vegetative growth through the modulation of physiological parameters. Chlorophyll fluorescence, CO2 absorption, transpiration rate and water efficiency were measured on the leaves of the two varieties, while qualities characteristics (antioxidants, lycopene concentration, total sugars, color and juice acidity) were measured in tomato fruit. For most of the parameters measured, it was found that 5 mM Si was more effective than 2.5 mM of Si. Furthermore, it was observed that high Si concentration alleviates salinity of the plants grown in saline soil by preventing oxidative membrane damage and H2O2 accumulation. Overall, silicon may improve antioxidant defence ability by increasing the activity of antioxidants and also silicon may contribute to osmotic adjustment, increased photosynthetic activity and leading to decreased reactive oxygen species (ROS).
Keywords
Antioxidants, Salinity, Lipid peroxidation, Proline, Lycopene concentration, Tomato, Silicon
Introduction
Tomato is one of the most important agricultural fruits. It is the second most commonly consumed fruit and vegetable in Europe. Its economic importance is undoubted, due to its great nutritional value [1]. The benefits of tomato have been attributed mostly to their vitamins, carotenoids and phenolic compounds. Lycopene is a biologically occurring carotenoid, that has been found to be important for antioxidant activities [2] and exhibits the highest physical quenching rate constant with singlet oxygen.
Proline accumulation has been reported to occur after salt, drought, high temperature, low temperature, heavy metal, pathogen infection, an aerobiosis, nutrient deficiency, atmospheric pollution and UV irradiation [3-5]. The level of Pro accumulation in plants varies from species to species and can be 100 times greater than the control situation [6]. Noticed that also proline has been shown to protect plants against free-radical induced damage by quenching the singlet oxygen. Many researchers claim that the increase in proline content comes as an effect to stress [7], while others support that increased proline is a result of stress tolerance [8].
Osmotic stresses, which include treatments lowering the osmotic potential component of the water potential, are by far the most studied ones, as they represent a major concern in agriculture. Salinity is a restrictive kind of abiotic stress that affects plant growth and development. Therefore, there is a high interest in improving plant osmotolerance in agriculture. Almost one-half of the irrigated areas in the world are affected by high salinity. Proline accumulation is one of the adaptations of plants to salinity [9,10].
Nowadays many researchers proposed that salt tolerance can be also improved by the addition of silicon. Although our understanding of the role of Si in abiotic stress resistance is still limited, important advances with regard to salinity stress have been made [11]. In fact, it has been widely reported that the provision of Si increases salt tolerance and hence biomass in many important crops grown under different conditions, such as barley [12], wheat [13-15], rice [16,17], soybean [18], sugarcane [19], tomato [20-22], and cucumber [23,24], among others.
Our, hypothesis is that there are specific mechanisms that can be co-related with the role of Si in the modulation of physiological parameters. Most of the studies have focused on the mechanism of the effect of Si on the transportation from root to shoot of Na and Cl, but little information is known on Si-mediated toxicity relating to oxidative damage in plants and antioxidants.
The objective of this paper is to determinate the effects of Si on the growth and quality of salinity stressed tomato cultivars and also explore the possible mechanisms by which Si alleviates salinity stress and improves growth under abiotic stress conditions.
Materials and Methods
Growth conditions
Two tomato (Lycopersicon esculentum Mill) cultivated varieties were used. The first one was bacioty cultivar and the second was de barao chornyy cultivar. The plants were grown in greenhouse with natural light (PAR 500-700 μmol m2 s-1), relative humidity 70-80%, mean day and night temperatures of 32 ± 2 and 27 ± 2 ℃, respectively, and cultivated on sandy soil 18% slurry, 5.6% clay, 70.4% and, 0.88% organic matter, 0.9% CaCO3, and 1.5 μS cm-1 electrical conductivity) and pH (1:2 H2O) 7.4. Exposure of the plants, by foliar spraying, to NaCl (150 mM) and to Si concentrations (2.5 and 5 mM) lasted seven months, and the growing season of the tomato.
Chlorophyll and carotenoid estimation
The third from the top fully expanded leaf was sampled for analysis of chlorophylls and carotenoids, on the 60th day from the initiation of Pb and Cu stress. For estimation of photosynthetic pigments, fresh plant material (0.1g) was placed in 25 mL glass test tubes and 15 ml of 96% (v/v) ethanol was added to each tube. The tubes with the plant meterial were incubated in a water bath at a temperature of 79.8 ℃ until complete discoloration of samples, after about three to four hours. The absorbance of chlorophylls a and b was measured at 665 and 649 nm, respectively. Total chlorophyll was determined according to [25]. Carotenoids concentration was estimated on a vis spectrophotometer, as described by [26] and modified by [27].
In vivo chlorophyll fluorescence measurements
In vivo chlorophyll fluorescence was measured on the upper leaf surface of the third from the top fully expanded leaf, using a Plant Analyser (PEA, Hansatech Ltd King's Lynn, Norfolk, England) as described by [24] and the Fv/Fm ratio was determined. Measurements were conducted at 23 ℃, on intact leaves of four replicate plants from the nine treatments.
Photosynthesis measurements
Gas exchange was measured on the third, from the top fully expanded leaf with Li-Cor 6400, USA). Calculations of net photosynthetic rate (A), transpiration rate (E), water use efficiency (WUE = A/E), intercellular CO2 concentration (Ci) and stomatal conductunce (gs) from gas exchange measurements were conducted according to [28]. Leaf temperature was about 25 ℃, the relative humidity (RH) was 60-70%, CO2 concentration was 400 μl L-1, and light intensity was 1000 μmol m-2 s-1.
Lycopene determination
Extraction was performed according to [29]. Samples were first chopped and homogenized in a laboratory homogenizer. Approximately 0.3-0.6g samples were weighed and 5 mL of 0.05% (w/v) BHT in acetone, 5 mL of ethanol and 10 mL of hexane were added. The absorbance of the hexane layer (upper layer) was measured at absorbance 503 nm spectrophotometry using the re-determined extinction coefficients [30].
Fruit quality analysis
Ten representative fruits of each plot were analyzed for fruit quality parameters. Hunter color values parameters: L* (brightness), a* (chroma) (redness) and b* (yellowness) were measured on the soluble solids (TSS) contents as was described by [31] by employing an Atago N1 refract meter (Atago Co. Ltd., Japan). Acidity was determined by potentiometric titration with 0.1 M NaOH up to pH 8.1 using 10 ml of juice, and the results were expressed as percentage of malic acid in the juice.
Determination of malondialdehyde (MDA) and H2O2 concentrations
Lipid peroxidation was expressed as the concentration of MDA that was determined by the thiobarbituic acid (TBA) test. About 0.5g leaf segments were homogenized in 5.0 ml of 5% (w/v) trichloroacetic acid (TCA), and centrifuged at 12,000 g for 10 min. The supernatant was assayed for MDA concentration spectrophotometrically according to the method of [32]. All spectrophotometric analyses were performed using an Hitachi 7500 spectrophotometer (Hitachi Ltd, Tokyo, Japan).
Hydrogen peroxide levels were determined according to [32]. Briefly, leaf tissues (500 mg) were homogenized in ice bath with 5.0 ml 0.1% (w/v) TCA. To 0.5 ml of the supernatant, a reaction mixture was added of 0.5 ml 10 mM potassium phosphate buffer (pH 7.0) and 1.0 ml 1.0 M KI. The absorbance of the supernatant was read at 390 nm and the content of H2O2 was calculated from a standard curve.
Sample preparation for antioxidants
Fresh fruits were accurately weighed (0.1g) and cut into small pieces before extraction. material put in a mortar. And mixed with 1 mL of 80% aqueous methanol. The homogenates were centrifuged at 12,000 rpm at 4 ℃ for 20 min.
Determination of antioxidants
For non-enzymatic antioxindants determination it was measured the ability of the fruits to act as hydrogen or electron donors in the transformation of DPPH into its reduced form DPPH-H. Total antioxidant activity was determined following the method of [33]. Fruit extract (50 μL) added to 0.1 mM DPPH solution (2.95 mL). After 1 h the absorbance of the reaction mixture was measured in triplicate at 517 nm on a spectrophotometer The control solution was prepared by adding absolute ethanol (50 μL) to the DPPH solution. Measurements were expressed as scavenging activity %. The antioxidant activity was determined by the following formula:
Scavenging Activity (%) = {(Abs control-Abs sample)/Abs control} × 100
Where Abs is the absorbance at 517 nm.
Determination of phenolics
Total phenolics content was determined by the Folin-Ciocalteu method, as was described by [34] with some modifications. The solution of the reaction consists of 2400 μl Folin-Ciocalteu (1:10 v/v), 80% (v/v) methanolic extract (100 μl) and nanopure water (500 μl). These chemicals were combined in tubes and then mixed via magnetic stirring. The mixture was allowed to react for 3 min and then 2 mL of Na2CO3 (7.5% w/v) solution was added and mixed well. The solution was incubated at 37 ℃ for 5 minutes. The phenolic compounds in the sample are oxidised using the Folin-Ciocalteu reagent.
The tubes were left to be cooled at room temperature (23 ℃). The absorbance was measured at 760 nm using a spectrophotometer and the results were expressed in gallic acid equivalents (GAE; mg/100g fresh mass) using a gallic acid standard curve.
Proline determination
Proline was determined according to the method described by [35] Approximately 0.5 g of fresh plant material was homogenized in 10 mL of 3% aqueous sulfosalicylic acid and filtered through What man's no. 2 filter paper. Two milliliters of filtrate was mixed with 2 mL acid-ninhydrin and 2 mL of glacial acetic acid in a test tube. The mixture was placed in a water bath for 1 h at 100 ℃. The reaction mixture was extracted with 4 mL toluene and the chromophore containing toluene was aspirated, cooled to room temperature, and the absorbance was measured at 520 nm with a Shimadzu UV 1601 spectrometer. Appropriate proline standards were included for calculation of proline in the sample.
Experimental design and data analysis
Eighteen experimental plots (three replications for each treatment) for each cultivar were set up randomly inside the greenhouse using a randomized complete block design. Each plot contained three rows with 7 plants per row spaced 50 cm apart within each row. Distance between rows was 90 cm while distance between plots was 100 cm. Analysis of regression and variance were performed using the SPSS 11.0.1 for Windows statistical package (SPSS, Chicago, USA). For comparison of the means, Duncan's multiple range tests (p ≤ 0.05) were employed.
Results
This study aimed to investigate the protective effects of Si on two tomato varieties exposed to the NaCl (salt) stress. Silicium is known as an essential element that has been shown to give increased yield and quality characteristics under salt stress conditions in tomato. In both tomato varieties cultivated, plant height decreased as a response to the increase of salinity stress. Specifically, salinity stress decreased plant height by 27% and 46% of the control in cv bacioty and de barao chornyy, respectively (Table 1).
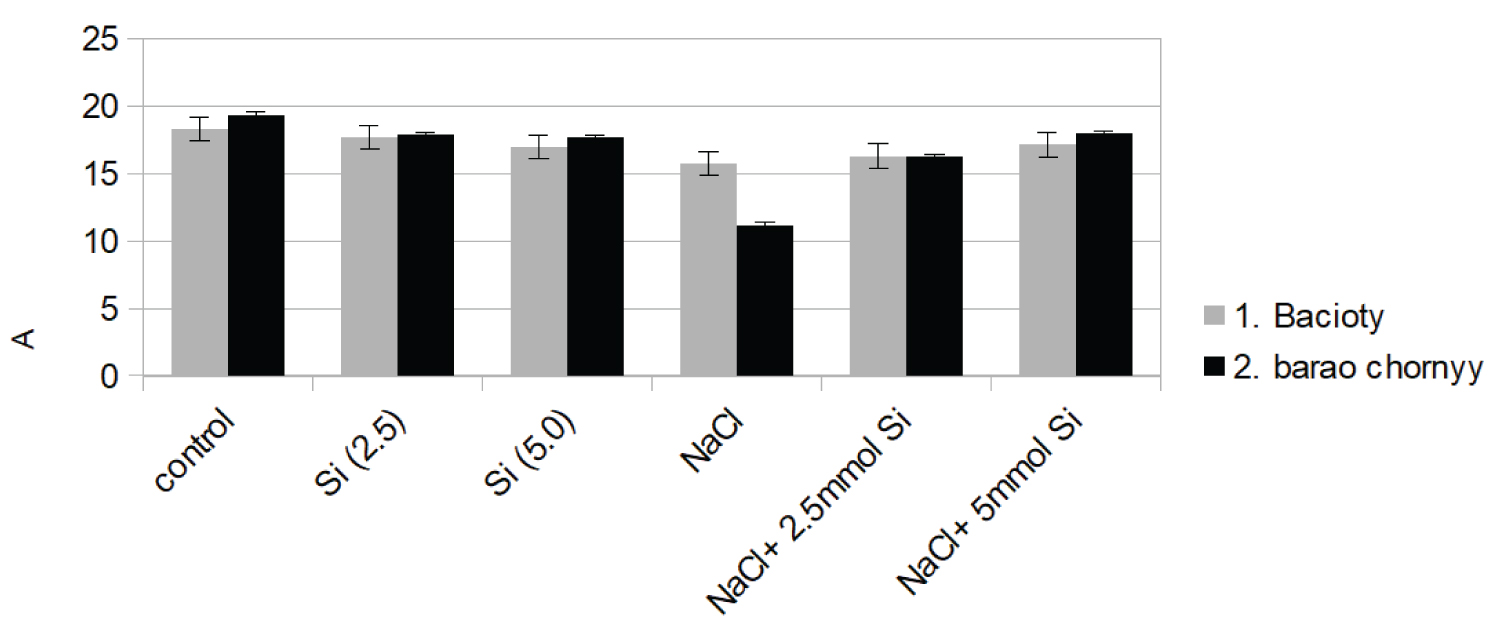
Changes in gas exchange parameters under salinity and salinity stress with Si application were observed in both tomato cultivars (Figure 1a, Figure 1b, Figure 1c and Figure 1d). The cultivation under Si application decreased CO2 assimilation by 18% and 21%, respectively, in comparison to control. However, when the plants were exposed to salinity stress, net assimilation was severely suppressed only in the second variety. The reductions were 15% and 43% below the control rate for bacioty and de barao chornyy, respectively (Figure 1a).
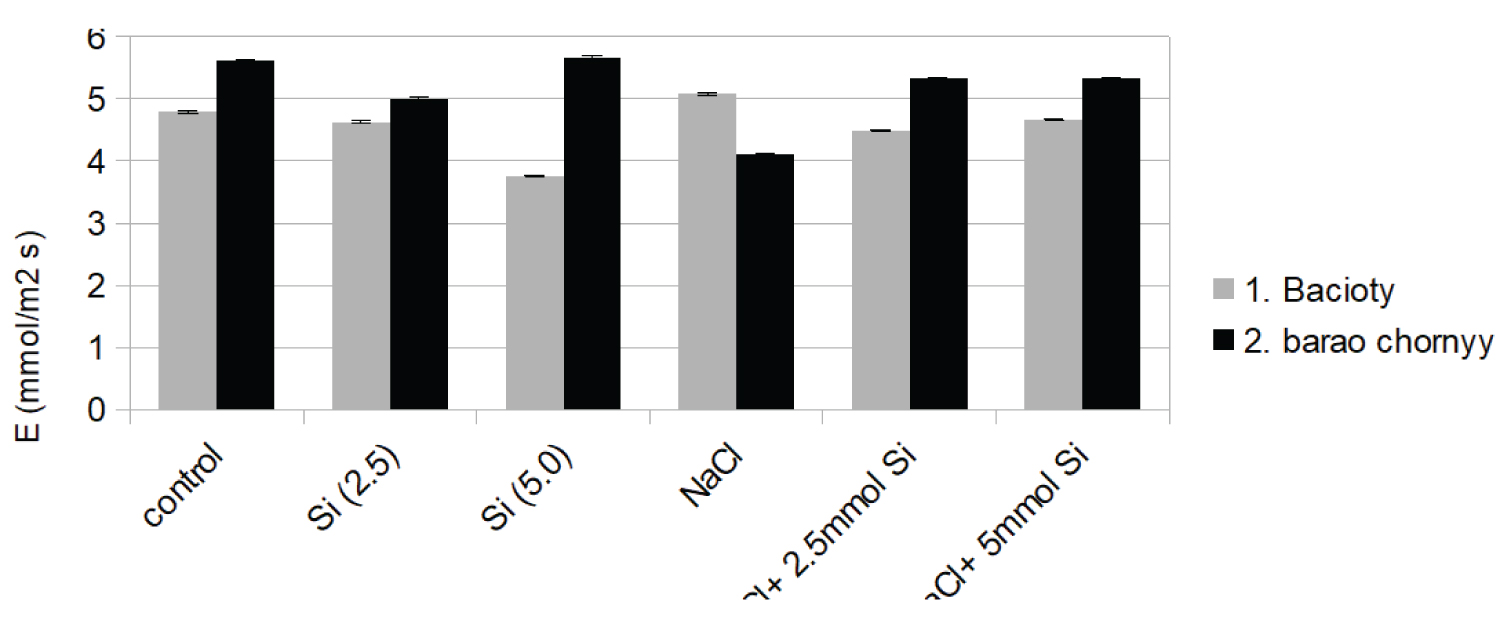
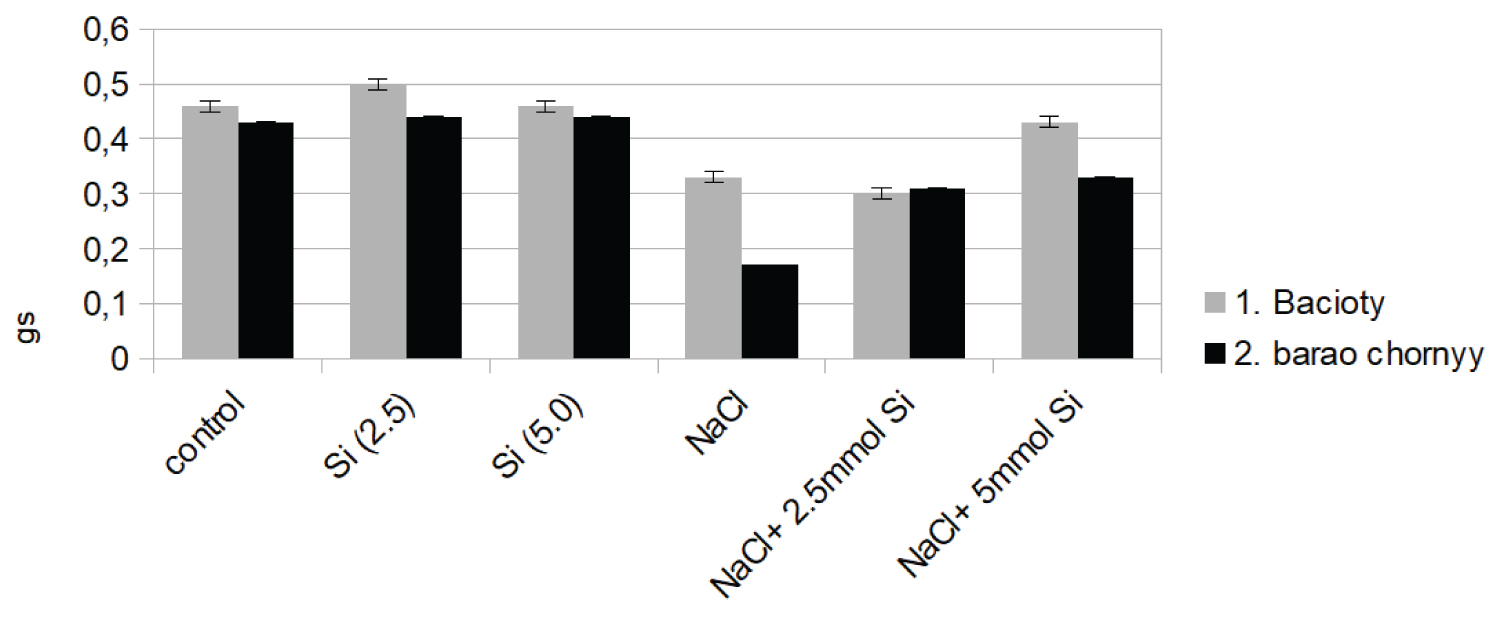
Similar results were observed in transpiration rate of both cultivars (Figure 1b). Although salinity and stress significantly reduced transpiration rate (27%) in the cultivar de barao chornyy, the application of NaCl + Si alleviated the stress and increased its value by 29% above the NaCl treatment. NaCl treatment led to a decrease in stomatal conductance in both cultivars by 29% and 61% respectively, above the control rate.
However, NaCl + Si (5.0 mM) treatment positively influenced stomatal conductance by stabilizing conductance value at the same value with the control in both varieties (Figure 1c).
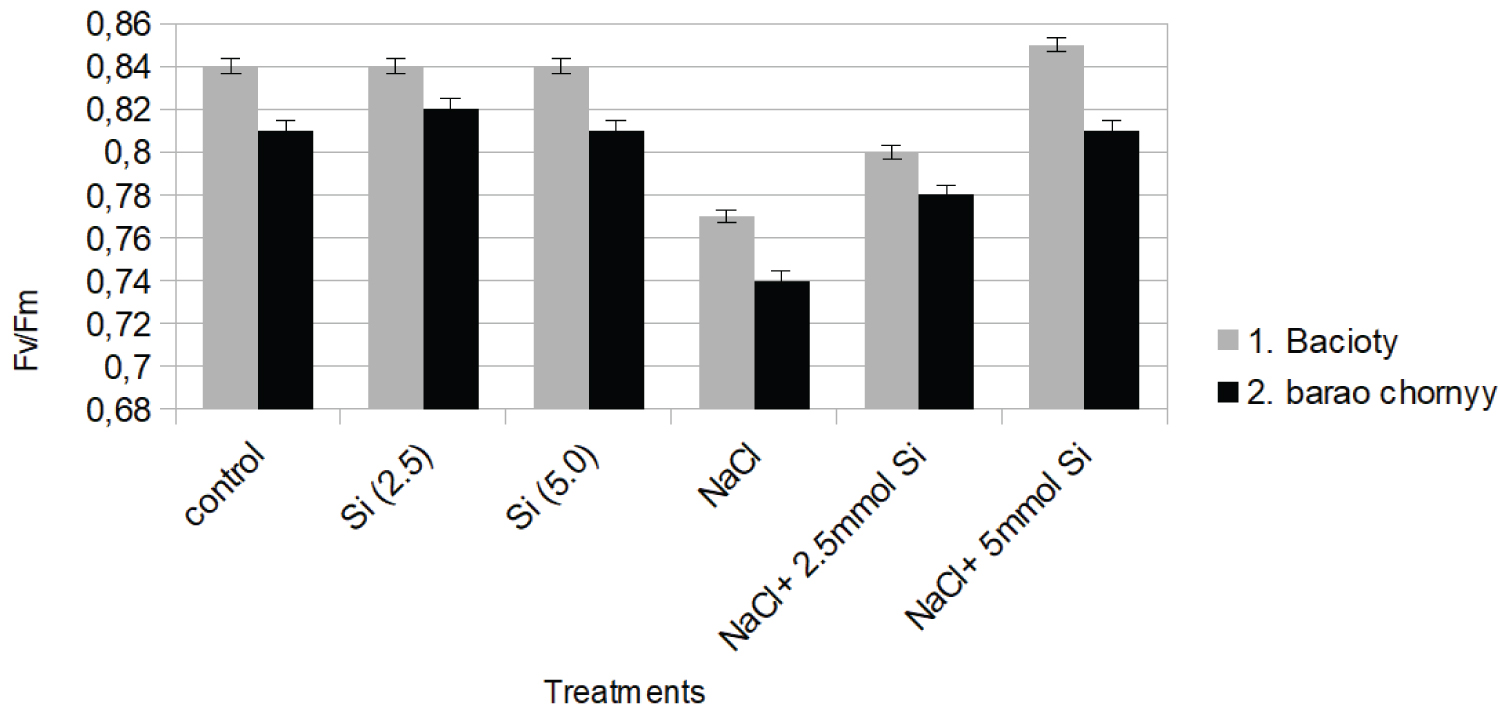
The maximum quantum efficiency of PSII photochemistry (Fv/Fm) was significantly reduced by salinity stress. The values dropped by 20% and 17% in comparison to control in both cultivars respectively (Figure 1e).
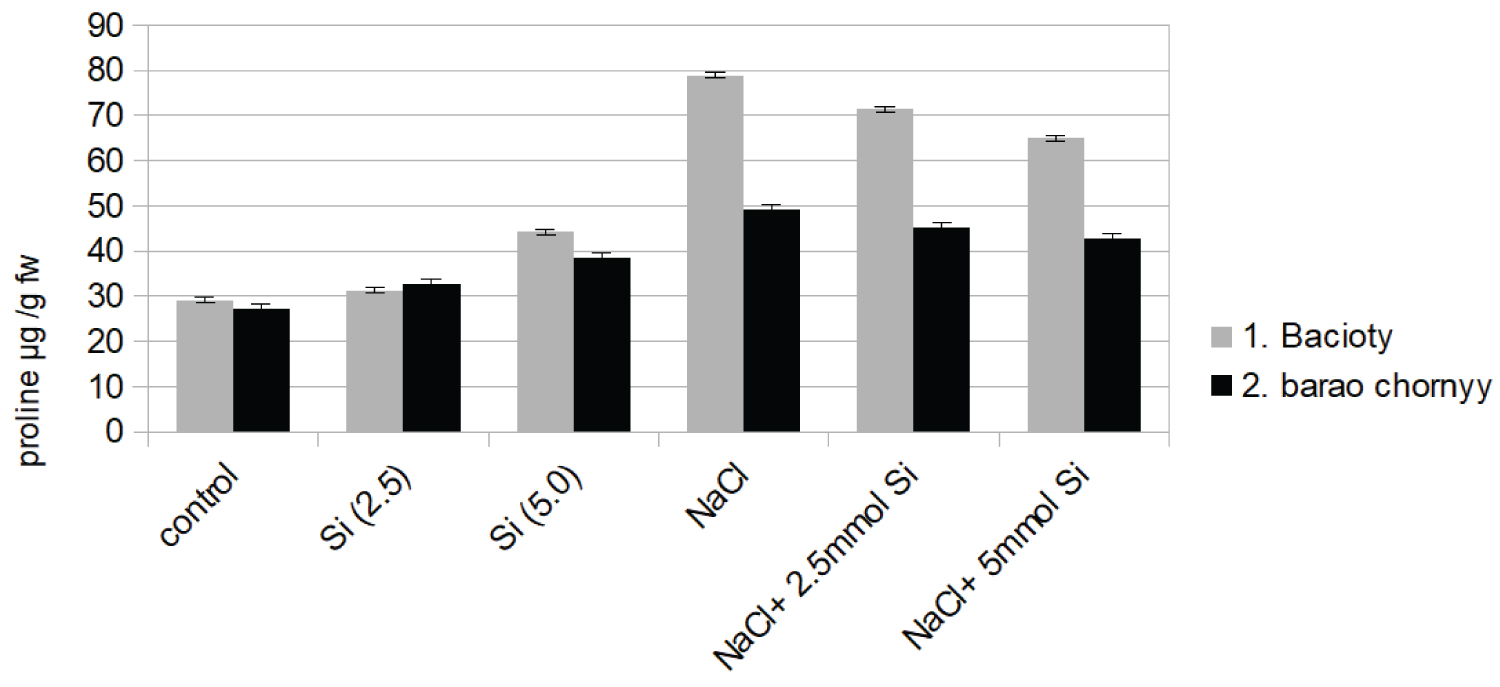
A significant increase in Fv/Fm value was revealed under NaCl + Si (5.0 mM) in both varieties. Moreover, significant changes in proline concentration under salinity stress are shown (Figure 1f).
It seems that proline over-accumulated under salinity stress relatively to control. The increase was about 2.5 times higher than the control, especially in bacioty cultivated variety. Similarly, significant increases in MDA concentration were observed under salinity (Table 1).
In Table 2 and Table 3 are shown the loss of the photosynthetic pigments and secondary metabolites respectively, after the treatment with NaCl in comparison to control. Si addition had ameliorative effects on total chlorophyll carotenoids and lycopene content in both varieties. The increased salinity concentration caused a significant reduction in total phenolic content in both varieties.
Additionally, salinity-induced oxidative stress occurred in both tomato varieties cultivated, as it was indicated by the increased levels of H2O2 and lipid peroxidation (50% in both varieties) after one month of salinization (Table 1). Additionally the organoleptic characteristics (fruit color, firmness) show fluctuations when treated with salt. There was a significantly dropped of the color (L) by 12% and 14% respectively when exposed to salinity stress. Moreover the firmness decreased by 20% and 17% in comparison to control in both cultivars respectively (Table 3 and Table 4).
Furthermore, the drop of the antioxidant values with the addition of silicium. Were all significant different compared to control and were accompanied by even more decreased values when treated with salt (NaCl) (Table 3).
Discussion
This study aimed to investigate the protective effects of Si on two tomato varieties exposed to the NaCl (salt) stress. Silicium is known as an essential element that has been shown to give increased yield and quality characteristics under salt stress conditions in tomato [20,36], wheat [37], and cucumber [38]. Plants respond to many stresses by accumulating significant metabolites, as amino acids for example, and particularly proline.
Proline is another compatible solute which accumulates under salt stress in plants. In the present study, an increase in proline accumulation occurred in both cultivars under salinity. Moreover, proline, while moderately enhancing growth under saline stress did not significantly improve plant water relations. Proline also decreased after Si treatment. Although the role of proline accumulation is still disputed, proline is known as a compatible solute involved in osmotic adjustment [39]. The accumulation of proline may be through an increase in its synthesis constantly with inhibition of its catabolism [40] and may be a mechanism for stress tolerance. However, its role in imparting stress resistance under saline conditions is controversial. Anyway, the activity of biosynthesis, transport and role of proline under stress and the mechanism that regulate stress-induced accumulation is significant for stress tolerance in plants [41]. During osmotic stress, availability of atmospheric CO2 is reduced due to increased stomatal closure and consumption of NADPH by the Calvin Cycle is decreased [42]. Activity of the chloroplasts can recycle NADP+, the last acceptor of the photosynthetic electron transfer chain, which may reduce ROS production at the photosystem I. The conclusion is that without Si treatment, the plant height and photosynthetic efficiency of tomato were significantly affected under NaCl stress. However, Si application in tomato plants significantly enhanced the height and the photosynthetic machinery.
Antioxidants are essential in keeping higher photosynthetic rates as well as the detoxification of toxic free radicals of oxygenic photosynthesis particularly reactive oxygen species (ROS). Radical oxygen species (ROS) and their scavengers, including lycopene, have also been directly involved in stress protection. The antioxidants are activated under NaCl stress conditions in order to maintain the concentration of ROS at low rates. It is known that the application of Si not only improves the photosynthetic efficiency of plants by increasing light interception due to more erectness in leaves and higher Rubisco activity [43,44], but also improves the efficiency of the antioxidant system, which is required for the detoxification of ROS [45].
In addition, the variation of phenolic compounds in different plants under increasing salinity has been discussed [46,47]. Also reported an increase in total phenolic content in red pepper plants under moderate salinity levels.
In this study the experiments that took place indicated that an enhanced Si supply to tomato decreases markedly total phenolics, carotenoids and lycopene contents, when treated with NaCl. Carotenoids are antioxidant compounds whose nutritional value is highly respected [48] and increases their internal quality. However, it is not clear how Si can influence the carotenoid content of the tomato fruit.
Oxidative stress occurring after the effect of salinity and silicium treatments on tomato fruits were measured by changes in the content of H2O2 and lipid peroxidation.
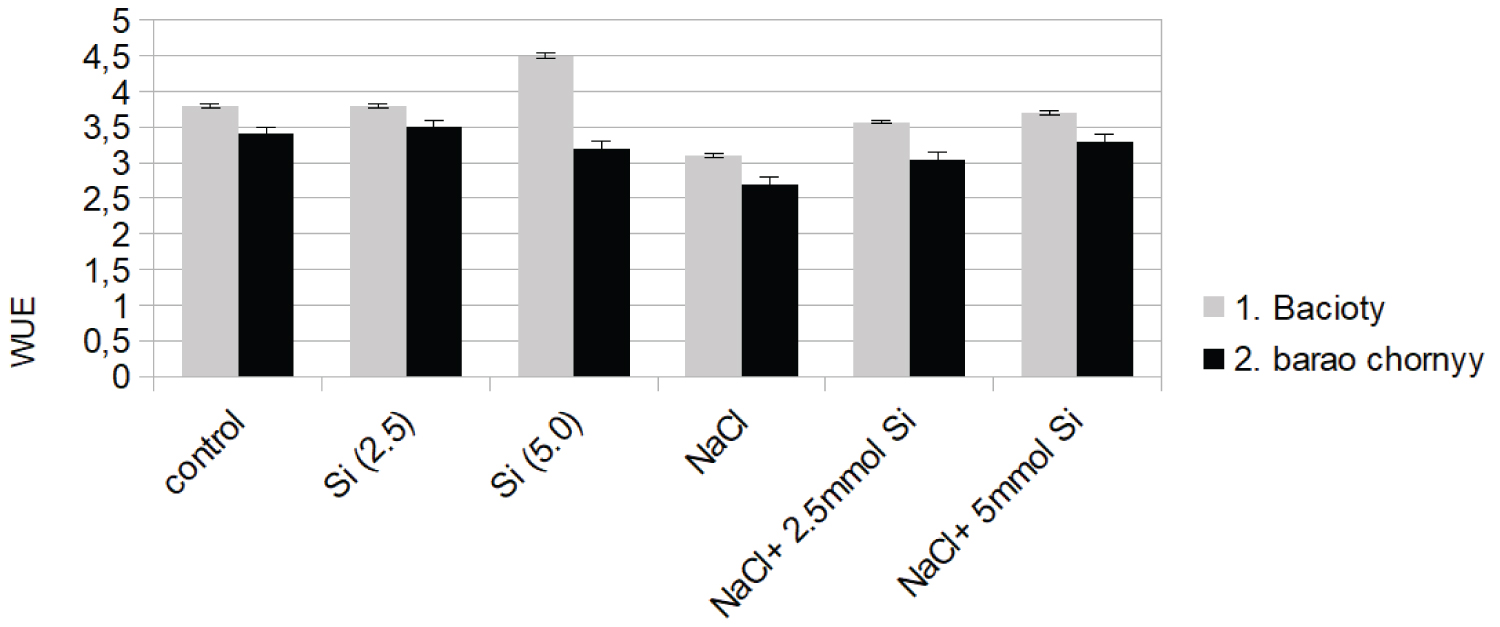
Production of H2O2 in our study and its localization in leaf tissue contributed in elucidating the photoprotective mechanisms to salinity-induced oxidative stress conditions, confirming the conclusion by [49]. Salinity-stressed tomato plants with the addition of Si showed reduced MDA and H2O2 values, suggesting the protective role of Si against oxidative damage. Similar results have been found by [24,38] and [50] in cucumber and tomato plants. Salt stress had a significant effect on gas exchange parameters, but no significant difference was observed between the cultivars in this relation. Salinity caused the loss of integrity of PSII response centers, resulting in reduced photosynthetic rate and water use efficiency (WUE). The reason why stomatal carbon dioxide was decreased in both cultivars is because leaf stomatal conductivity decreases with increasing salinity stress to prevent water loss, but this in turn also prevents carbon dioxide to diffuse into the stomata.
In the case of transpiration reduction, stomatal closure is more effective than carbon dioxide uptake [51,52]. In this study, stomatal closure in barao chornyy caused more transpiration reduction resulting in this cultivated variety being more tolerant to salinity stress.
Salt stress causes reduction in total chlorophyll contents in tomato plants, whereas Si application alleviated the negative impacts of salts stress by enhancing the production of these photosynthetic pigments. This increase in chlorophyll contents by the foliar application of Si might be due to increased photosynthetic efficiency [53,54]. Moreover, the loss of qualitative and organoleptic characteristics of fruits with a significant reduction in total antioxidant capacity and total phenolic concentration was accompanied by a degradation of total soluble solids (brix) and a significant increase in the acidity of tomato juice to a greater extent in the de barao chornyy variety compared to the bacioty variety.
Conclusion
The results of this experiment show that salinity stress caused substantial damage to plant growth and indicate a protective role of Si in regulating oxidative stress response. From the results of the present study, we suggest that Si could be used as a potential candidate to improve stress. Without Si supplementation, the plant biomass and photosynthetic efficiency of the two cultivars of tomato were significantly affected under stress. The findings of this study suggest enhanced tolerance of tomato against salinity by reduced oxidative damage due to improved activities of antioxidants however, further investigations are needed particularly under greenhouse conditions.
Author Contributions
AG, GD and GO designed the study. AG, performed the experiments. AG and GO wrote the manuscript with support of GD. AG, GO and GD critically reviewed the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
References
- Slimestad R, Verheul M (2009) Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill) cultivars. J Sci Food Agric 89: 1255-1270.
- Stahl W, Sies H (1996) Perspectives in biochemistry and biophysics Lycopene: A biologically Important carotenoid for humans? Arch Biochem Biophysics 336: 1-9.
- Hare PD, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21: 79-102.
- Saradhi P, Alia P, Arora S, et al. (1995) Proline accumulates in plants exposed to UV radiation and protects them against UV induced peroxidation. Biochem Biophys Res Commun 209: 1-5.
- Siripornadulsil S, Train S, Verma DPS, et al. (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14: 2837-2847.
- Matysik J, Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82: 525-532.
- Dix PJ, Pearce RS (1981) Proline accumulation in NaCl resistant and sensitive cell lines of Nicotana sylvestris. Zeitschrift fur Pflanzenschuts Physiologie 102: 243-248.
- Moftah A, Michel B (1987) The effect of sodium chloride on solute potential and proline accumulation in soybean leaves. Plant Physiol 83: 238-240.
- Kumar SG, Reddy A?, Sudhakar C (2003) NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Science 165: 1245-1251.
- Ramanjulu S, Sudhakar C (2001) Alleviation of NaCl salinity stress by calcium is partly related to the increased proline accumulation in mulberry (Morus alba L.) callus. J Plant Biol 28: 203-206.
- Rengasamy P (2010) Soil processes affecting crop production in salt-affected soils. Funct Plant Biol 37: 613-620.
- Liang Y, Zhang W, Chen O, et al. (2006) Effect of exogenous silicon (Si) on H+ -AT Pase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ Exp Bot 57: 212-219.
- Tuna AL, Kaya C, Higgs D, et al. (2008) Silicon improves salinity tolerance in wheat plants. Environ Exp Bot 62: 10-16.
- Shafaqat A, Farooq MA, Yasmeen T, et al. (2013) The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol Environ Saf 89: 66-72.
- Bibordy A (2016) Influence of zeolite, selenium and silicon upon some agronomic and physiologic characteristics of canola grown under salinity. Commun Soil Sci Plant Anal 47: 832-850.
- Gong HJ, Randall DP, Flowers TJ (2006) Silicon deposition in roots reduces sodium uptake in rice (Oryza sativa L.) seedling by reducing bypass flow. Plant Cell Environ 29: 1970-1979.
- Mahdieh M, Habibollahi N, Amirjani MR, et al. (2015) Exogenous silicon nutrition ameliorates salt-induced stress by improving growth and efficiency of PSII in Oryza saliva L. cultivars. J Soil Sci Plant Nutr 15: 1050-1060.
- Lee SK, Sohn EY, Hamayun M, et al. (2010) Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor Syst 80: 333-340.
- Ashraf M, Rahmatullah K, Afzal M, et al. (2010) Alleviation of detrimental effects of NaCl by silicon nutrition in salt-sensitive and salt-tolerance genotypes of sugarcane (Saccharum officinarum L.). Plant Soil 326: 381-391.
- Romero Aranda MR, Jurado O, Cuartero J (2006) Silicon alleviates the deleterious salt effecon tomato plant growth by improving plant water status. J Plant Physiol 163: 847-855.
- Muneer S, Park YG, Manivannan A, et al. (2014) Physiological and proteomic analysis in chloroplast of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int J Mol Sci 15: 21803-21824.
- Li H, Zhu Y, Hu Y, et al. (2015) Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol Plant 37: 71-80.
- Khoshgoftarmanesh AS, Haghighi M (2014) Effect of silicon nutrition on lipid peroxidation and antioxidant response of cucumber plants exposed to salinity stress. Arch Agron Soil Sci 60: 639-653.
- Ouzounidou G, Giannakoula A, Ilias I, et al. (2016) Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Brazilian Journal of Botany 39: 531-539.
- Wintermans JF, De Mots A (1965) Spectrophotometric characteristics of chlorophyll 'a' and 'b' and their pheophytins in ethanol. Biochimica et Biophysica Acta (BBA)-Biophysics including Photosynthesis 109: 448-453.
- Lichtenthaler HK (1987) Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol 148: 350-382.
- Yang CM, Chang KM, Yin MH, et al. (1998) Methods for the determination of the chlorophylls and their derivatives. Taiwania 43: 116-122.
- Von Caemmer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta 153: 376-387.
- Fish WW, Perkins Veazie P, Collins J (2002) A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J Food Compost Anal 15: 309-317.
- Perkins Veazie P, Collins JK (2004) Flesh quality and lycopene stability of fresh-cut watermelon. Postharvest Biol Technol 31: 159-166.
- Giannakoula A, Ilias IF, Dragišic Maksimovic JJ, et al. (2012) Does overhead irrigation with salt affect growth, yield, and phenolic content of lentil plants? Arch Biol Sci Belgrade 64: 539-547.
- Su MS, Silva J (2006) Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chemistry 97: 447-451.
- Dubois M, Gilles KA, Hamilton JK, et al. (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 26: 350-356.
- Scalbert A, Monties B, Janin G (1989) Tannins in wood: Comparison of different estimation methods. J Agri Food Chem 37: 1324-1329.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of the freeproline in water stress studies. Plant Soil 39: 205-207.
- Al Aghabary K, Zhu Z, Shi QH (2004) Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J Plant Nutr 27: 2101-2115.
- Tahir MA, Aziz T, Farooq M (2011) Silicon-induced changes in growth, ionic composition, water relations, chlorophyll contents and membrane permeability in two salt-stressed wheat genotypes. Archives of Agronomy and Soil Science 58: 247-256.
- Zhu Z, Wei G, Li J, et al. (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt stressed cucumber (Cucumis sativus L.). Plant Sci 167: 527-533.
- Azooz MM, Shaddad MA, Abdel Latef AA (2004) Leaf growth and K +/Na + ratio as an indication of the salt tolerance of three sorghum cultivars grown under salinity stress and IAA treatment. Acta Agronomica Hungarica 52: 287-296.
- Yoshiba Y, Kiyosue T, Nakashima K, et al. (1997) Regulation of levels of proline as an osmolyte in plants under water stress. Plant Cell Physiol 38: 1095-1102.
- Kavikishore PB, Sangam S, Amrutha RN, et al. (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci 88: 424-438.
- Paranychianakis NV, Chartzoulakis KS (2005) Irrigation of Mediterranean crops with saline water: From physiology to management practices. Agric Ecosyst Environ 106: 171-187.
- Gunes A, Inal A, Bagci EG, et al. (2007) Silicon mediates changes to some physiological and enzymatic parameters symptomatic for oxidative stress in spinach (Spinacia oleracea L.) grown under B toxicity. Sci Horti 51: 113-119.
- Epstein E (2009) Silicon: Its manifold roles in plants. Ann Appl Biol 155: 155-160.
- Farooq MA, Saqib ZA, Akhtar J, et al. (2015) Protective role of silicon (Si) against combined stress of salinity and boron (B) toxicity by improving antioxidant enzymes activity in rice. Turkish Journal of Agriculture and Forestry 39: 718-729.
- Muthukumarasamy M, Gupta SD, Panneerselvam R (2000) Enhancement of peroxidase, polyphenol oxidase and superoxide dismutase activities by triadimefon in nacl stressed raphanus sativus L. Biologia Plantarum 43: 317-320.
- Navarro JM, Flores P, Garrido C, et al. (2006) Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity. Food Chem 96: 66-73.
- Khachik F, Goli MB, Beecher GR, et al. (1992) Effect of food preparation on qualitative and quantitative distribution of major carotenoid constituents of tomatoes and several green vegetables. J Agric Food Chem 40: 390-398.
- Jubany Marí T, Munné Bosch S, Alegre L (2010) Redox regulation of water stress responses in field-grown plants. Role of hydrogen peroxide and ascorbate. Plant Physiol Biochem 48: 351-358.
- Shalata A, Tal M (1998) The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii. Physiol Plant 104: 167-174.
- Taiz L, Zeiger E (2006) Plant Physiology. (4th edn), Sinaur Associates Inc, Sunderland, Massachusetts.
- Tardieu F (2005) Plant tolerance to water deficit: Physical limits and possibilities for progress. Geo Sci 337: 57-67.
- Sattar MA, Cheema T, Abbas A, et al. (2017) Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Russian Plant Physiology 64: 341-348.
- Giannakoula A, Ilias I (2013) The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon esculentum Mill). Arch Biol Sci 65: 611-620.
Corresponding Author
Giannakoula A, Department of Agriculture, International Hellenic University, 57400 Sindos, Greece.
Copyright
© 2022 Giannakoula A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.