The Protective Role of Melatonin in Alleviation of Copper Toxicity of a Greek Grapevine (Vitis Vinifera L.) Variety
Abstract
Grapevine is a major fruit crop with high economic importance, especially in the Mediterranean region. During the last few decades, increased heavy metal contamination has become one of the most important abiotic stress affecting grapevine production. Melatonin is a plant hormone that plays an important role related to antioxidant activity and also enhances plant growth and abiotic stress tolerance. Another role of melatonin is as a biostimulator and alleviator of toxicity from heavy metal and other chemicals. The effect of melatonin in copper contamined soils, on growth and biochemical characteristics were studied in a grapevine variety (Vitis vinifera L. cvs 'Pamidi'. The grapevine variety (Pamidi) was grown in the field. The factors examined in the experiments were three copper rates (0, 100, 150 µΜ) and also exogenous melatonin treatment (0.1 mM). Morphological characteristics such as height showed notable decreases due to photosynthetic disturbances especially at high levels (150 µM CuSO4). In addition CuSO4 treatment at high levels (100 mM CuSO4) caused significant increased, in lipid peroxidation (MDA), proline and hydrogen peroxide (H2O2) content, while the antioxidant activity was decreased. Moreover injuries caused by copper toxicity were alleviated by melatonin application especially in the higher concentration. In general, melatonin treatment increases Cu tolerance, plant growth, accumulation of carotenoids, and flavonoid contents as compared with non-treated plants. Furthermore, water use efficiency and assimilation rate of CO2 were improved in melatonin-treated plants. The apparent lower oxidative stress in Pamidi variety after melatonin application is explained by a more efficient activation of antioxidant system since plants under copper exposure and melatonin application induced a higher accumulation of antioxidant compounds.
These results indicate differences in antioxidant responses to oxidative stress caused by copper stress which could be possibly associated with melatonin foliar application and the different adaptation strategies to abiotic conditions. Melatonin application elevated the antioxidants to protect the membrane functions from reactive oxygen species (ROS) injury in grapevine Of course further investigation is needed in order to understand better the mechanisms of tolerance.
Keywords
Heavy metal stress, Malondialdehyde (MDA), Photosynthetic disturbances, Antioxidant compounds, Oxidative stress, Melatonin
Introduction
Grapevines are widely cultivated and are of great economic importance. In recent years, many studies have been carried out to identify the potential of grape growing regions in order to produce quality wines based on the concept of terroir [1]. Many abiotic factors such as drought, salinity and heavy metals are very important factors, which not only reduce but also affect the quality of vine production in the Mediterranean [2].
In Greece, Vitis vinifera L. has been cultivated since ancient times and ampelographic collection accounts for up to 660 cultivars [3]. Although grapevine varieties are extensively cultivated in Greek fields, the impact of climatic changes upon wine production limited the number vineyards. Furthermore at the beginning of 20th century, copper salts (CuSO4) has been widely applied into vineyard soils in order to control downy mildew [4]. The repeated use of Cu fungicides for over 100-years has resulted in high levels of Cu accumulation all over the world. Heavy metals like Cu are essential for normal plant growth, but their excess is toxic [5]. A metal ion binds to a sulfhydryl group in enzymes and other proteins by inhibiting their activity or disrupting their structure [6]. Additionally, heavy metals cause oxidative damage to biomolecules by triggering free radical-mediated chain reactions resulting in lipid peroxidation, protein and nucleic acid oxidation [7]. Copper (Cu) salts have also been used to study the protective and biostimulatory effect of melatonin. Melatonin has been defined as a biostimulator of plant growth, especially under environmental stress conditions, whether abiotic (water deficit and waterlogging, extreme temperature, UV radiation, salinity, alkalinity, specific mineral deficit/excess, metals and other toxic compounds, etc.) or biotic (bacteria, fungi, and viruses) [8]. Exogenous melatonin treated plants have been seen to have a high tolerance to stressors, minimizing possible harmful effects through the control of reactive oxygen species (ROS) levels and activating antioxidative responses.
Melatonin induces metabolic activity, especially glycolysis and the pentose phosphate pathway, to generate more ATP. Melatonin treatment broadly altered gene expression under Cu stress, increasing the levels of GSH and phytochelatin to chelate excess Cu and promoting cell wall trapping, retaining more Cu in the cell wall and vacuole. In addition to its known effect on the molecular mechanism in the presence of Cu, which was one of the first roles of melatonin, low levels of melatonin provide greater tolerance of the presence of Cu in cucumber plants [9]. Generally melatonin contributes in a more efficient phytoremediation [10].
In response to heavy metal stress, plant tissues accumulate compatible solutes, mainly proline, in order to achieve ionic balance in the vacuoles [6]. Proline has been previously associated with different functions, such as being a free radical scavenger, a cell redox balancer, a cytosolic pH buffer and a stabilizer for subcellular structures, especially during osmotic and salt stresses [11]. However heavy metal stress conditions have also shown that lead to higher levels of proline and also limitation of photosynthetic rate due to stomatal or non stomatal limitations [12].
The effect of melatonin on grape variety that was imposed to increased heavy metal stress has been previously studied however the actual role of melatonin as well as its physiological importance still remains unclear. The objective of the present study was (a) To evaluate the effects of copper stress, on growth, physiology and antioxidant activity of a Greek grapevine variety, that is threatened under extinction in the Greek-Bulgarian cross-border area (b) To investigate whether different copper concentrations can induce differential proline accumulation (c) To evaluate the level of lipid peroxidation in the grapevine variety under differing copper concentrations, and (d) To reveal any tolerance mechanisms that may have been established after the foliar spray of melatonin.
Materials and Methods
Experimental design
Pamidi is one of the local Greek grape varieties that is utilized for making red wine in Thrace region and threatened under extinction in the Greek-Bulgarian cross-border area.
Field experiment was conducted twice during the 2019 and 2020 from April till September (this period is related to the growth period) at the agricultural farm of the International Hellenic University (IHU, Sindos-Thessaloniki). The site is located at 22° 55 N, 40° 38 E. Experiment was established. Electrical conductivity (EC) of the saturated soil extract in 0-60 cm depth was 2.4 dS m-1 and the soil moisture was 17%. Nitrogen and phosphorus (P2O5) at 80 and 40 kg ha-1, respectively, were incorporated as diammonium phosphate. Additionally, 50 kg N ha-1 as ammonium nitrate. The factors examined in the experiment were the one melatonin rate (0.1 mM) and three copper rates (0, 100, 150 µM CuSO4). The vineyard was located on a loamy soil (45% sand, 30% silt, and 25% clay). The plants from each plot were foliar-sprayed (to run-off) with a low pressure hand-wand sprayer. And was applied 5 times at 10 days intervals. First application of copper and melatonin was made when plants had five to six leaves.
Gas exchange measurements
Gas exchange was measured on the youngest fully expanded leaf with a Li-6400 portable photosynthesis meter (LiCor, Inc. Lincoln, NE) supplied with IRGA (Li-6400). Calculations of net photosynthetic rate (A), transpiration rate (E), and water use efficiency (WUE = A/E) from gas exchange measurements were according to [13].
Determination of proline
Young leaves were cut into small pieces weighed and placed separately in glass vials containing 10 mL of 80% (v/v) ethanol, and heated at 60 ℃ for 30 min. The extract was then filtered and diluted with 80% (v/v) ethanol up to 20 mL. The shoot-leaf concentrations of free proline were determined in this extract following the acid ninhydrin reagent method [14].
Determination of antioxidants
Total phenolics: Total phenols content was determined by the Folin-Ciocalteu method, as was described by [15], with some modifications. The powdered sample (500 mg) was extracted with 50 mL of 80% methanol for 30 min in a hot plate. The solution of the reaction consists of 2400 µl Folin-Ciocalteu (1:10 v/v), 80% (v/v) methanolic extract (100 µl) and nanopure water (500 µl). The mixture was allowed to react for 3 min and then 2 mL of Na2CO3 (7.5% w/v) solution was added and mixed well. The solution was incubated at 37 ℃ for 5 minutes. The phenolic compounds in the sample are oxidised using the Folin-Ciocalteu reagent which is proportional to the total phenolic concentration. The tubes were left to be cooled at room temperature (23 ℃). The absorbance was measured at 760 nm using a spectrophotometer (Prim, SECOMAM, France) and the results were expressed in gallic acid equivalents (GAE; mg/100g fresh mass) using a gallic acid standard curve.
DPPH: The ability of the leaves to act as hydrogen or electron donors in the transformation of DPPH into its reduced form DPPH-H was investigated. Total antioxidant activity was determined following the method of [16]. Leave extract (50 µL) added to 0.1 mM DPPH solution (2.95 mL). After 1h the absorbance of the reaction mixture was measured in triplicate at 517 nm on a spectrophotometer. The control solution was prepared by adding absolute ethanol (50 µL) to the DPPH solution. Measurements were expressed as scavenging activity %. The antioxidant activity was determined by the following formula:
Scavenging Activity (%) = {(Abs control-Abs sample)/Abs control} × 100 (1)
where Abs is the absorbance at 517 nm.
Determination of lipid peroxidation
At the end of the experiment, the level of lipid peroxidation in the grapevine leaves was measured as malondialdehyde (MDA) content determined by reaction with 2-thiobarbituric acid (TBA) reactive substances according to [17]. The tissue was homogenized in 0.3% TBA in 10% trichloracetic acid (TCA) at 4 ℃. The concentration of MDA was calculated from the difference of the absorbance at 532 nm and 600 nm using the extinction coefficient of 155 mmol-1 cm-1 and expressed as nmol (MDA) g-1 of fresh weight.
H2O2 assay
Hydrogen peroxide concentrations in leaves were determined according to [17]. The reaction mixture consisted of 0.5 mL leaf extract with 0.1% trichloroacetic acid (TCA), 0.5 mL of 100 mM K-phosphate buffer and 2 mL reagent (1 M KI (w/v) in fresh double distilled H2O). The blank probe consisted of 0.1% TCA in the absence of leaf extract. The reaction was developed for 1h in darkness. H2O2 content was calculated using the absorbance of the supernatant at 410 nm.
Chlorophyll and carotenoids estimation
For estimation of photosynthetic pigments, fresh leaf blade material (0.1 g) was placed in 25 mL glass test tubes and 15 mL of 96% (v/v) ethanol was added to each tube. The tubes with the plant material were incubated in a water bath at a temperature of 79.8 ℃ until complete discoloration of samples, after about three to four hours. The absorbance of chlorophyll a was measured at 665 and 649 nm. Total chlorophyll was determined following the method described by [17].
Statistical analysis
Twelve experimental plots (six treatments each plot) were set up randomly using a split-plot design. Each plot contained six single plants in 2 rows with 3 plants per row spaced 0.80 m apart with-in each row. Distance between rows was 1.0 m and between plots 1.0 m. Data was subjected to analysis of variance (ANOVA) using the SPSS 18.0.1 for Windows statistical package (SPSS, Chicago, USA). For comparison of the means, the Duncan's multiple range tests (P ≤ 0.05) were employed.
Results
Copper in low concentration had no significant negative effects on grapevine growth (Table 1). However, plant height significantly decreased by increased copper toxicity (150 µM CuSO4). A decline of 22% in plant height under 150 µM CuSO4 was recorded, whereas at copper and melatonin combination the suppression was 15% compared to control plants.
Effect of Copper on chlorophyll fluorescence measurements
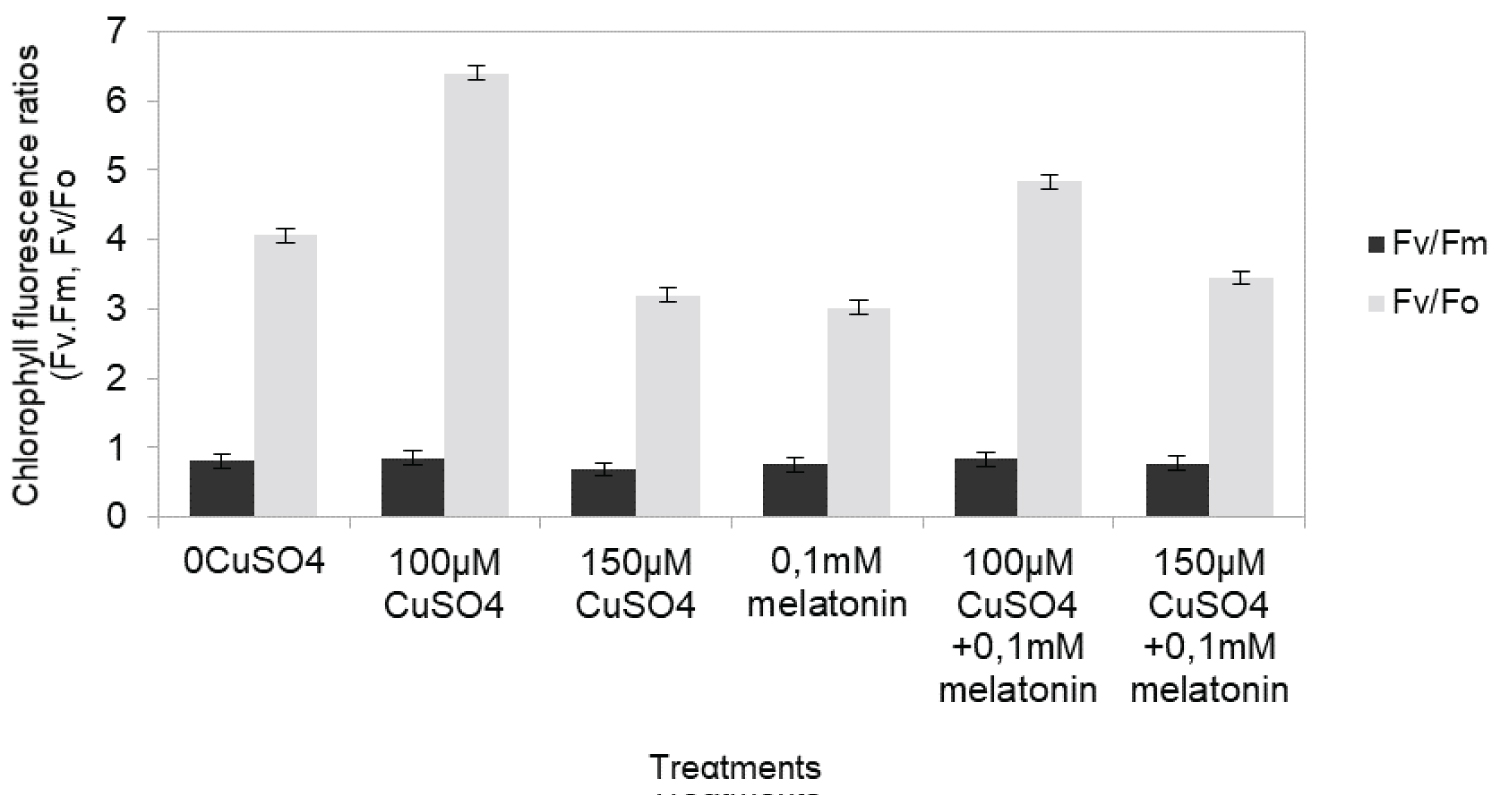
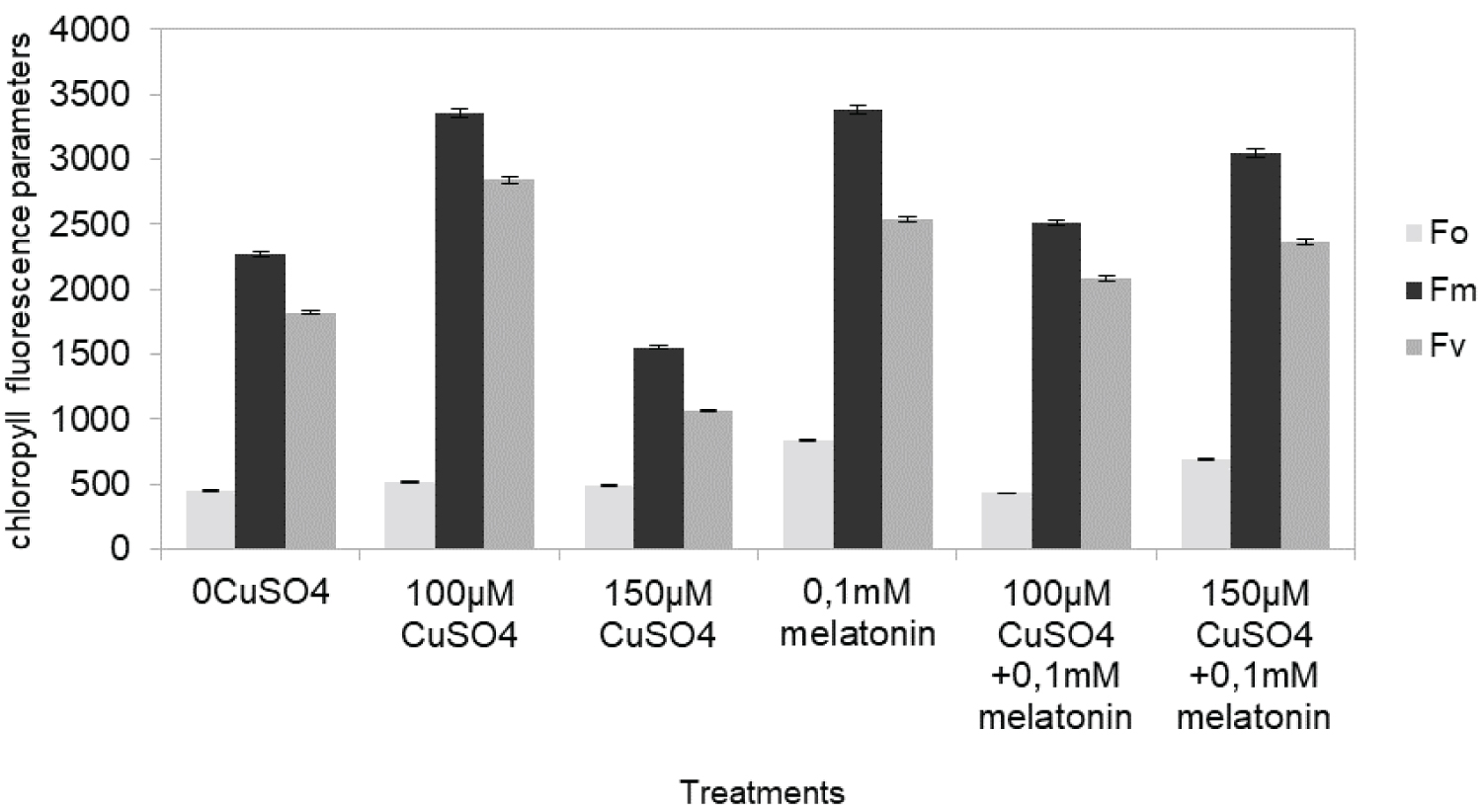
The maximum quantum efficiency of PSII photochemistry (Fv/Fm), as well as the efficiency of the water-splitting complex on the donor side of PSII (Fv/Fo), remarkably decreased depending on Cu concentration (Figure 1). The treatments 100 μM Cu gave the same Fv/Fm and Fv/Fo values compared to control, while applications of 150 Cu, decreased significantly Fv/Fm and Fv/Fo values (Figure 1). The maximum quantum yield of charge separation in the PSII as measured by Fv/Fm reflected the changes of Fo or Fv (Figure 2) The Fm, Fv were significantly (p ≤ 0.05) affected by Cu treatment. The treatments with 100 μM Cu, showed increased values compared to control by 30% and 20% respectively, while the values of 150 μM Cu, were decreased by 28%, 34%, respectively compared to control. Treatment with melatonin simultaneously with 150 μM Cu) showed no significant alterations compared to control.
Effect of Copper on gas exchange measurements
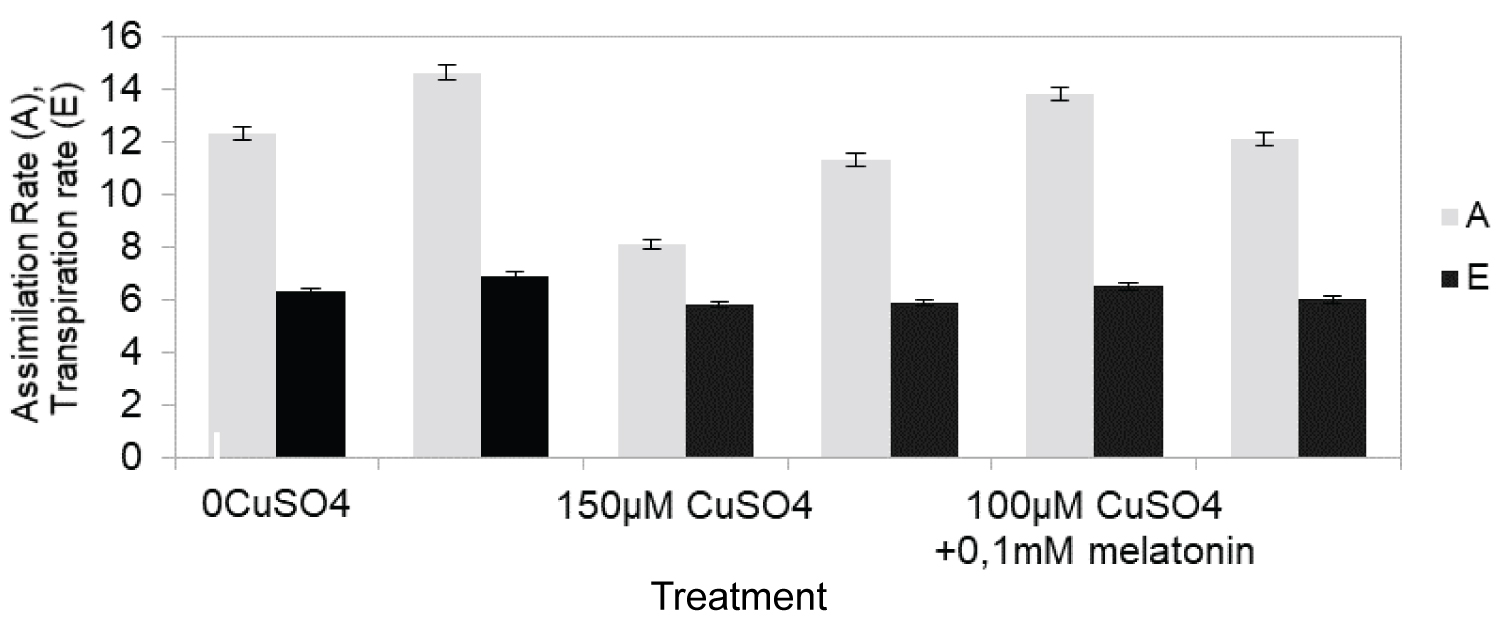
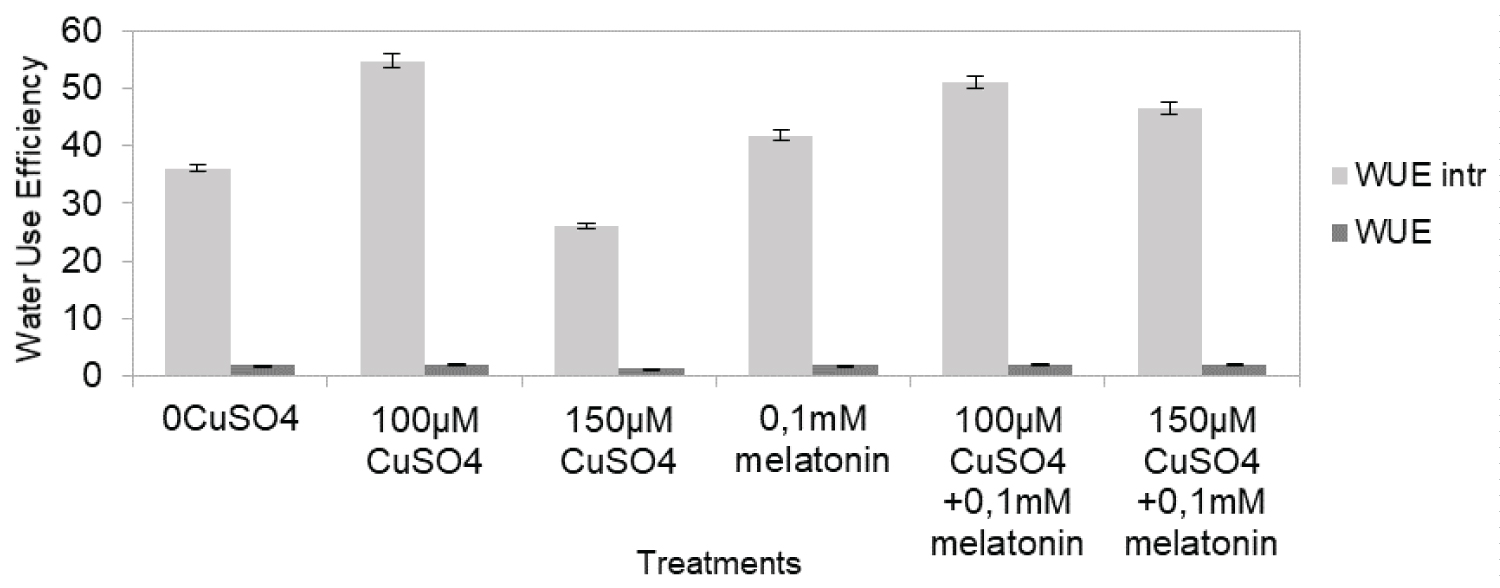
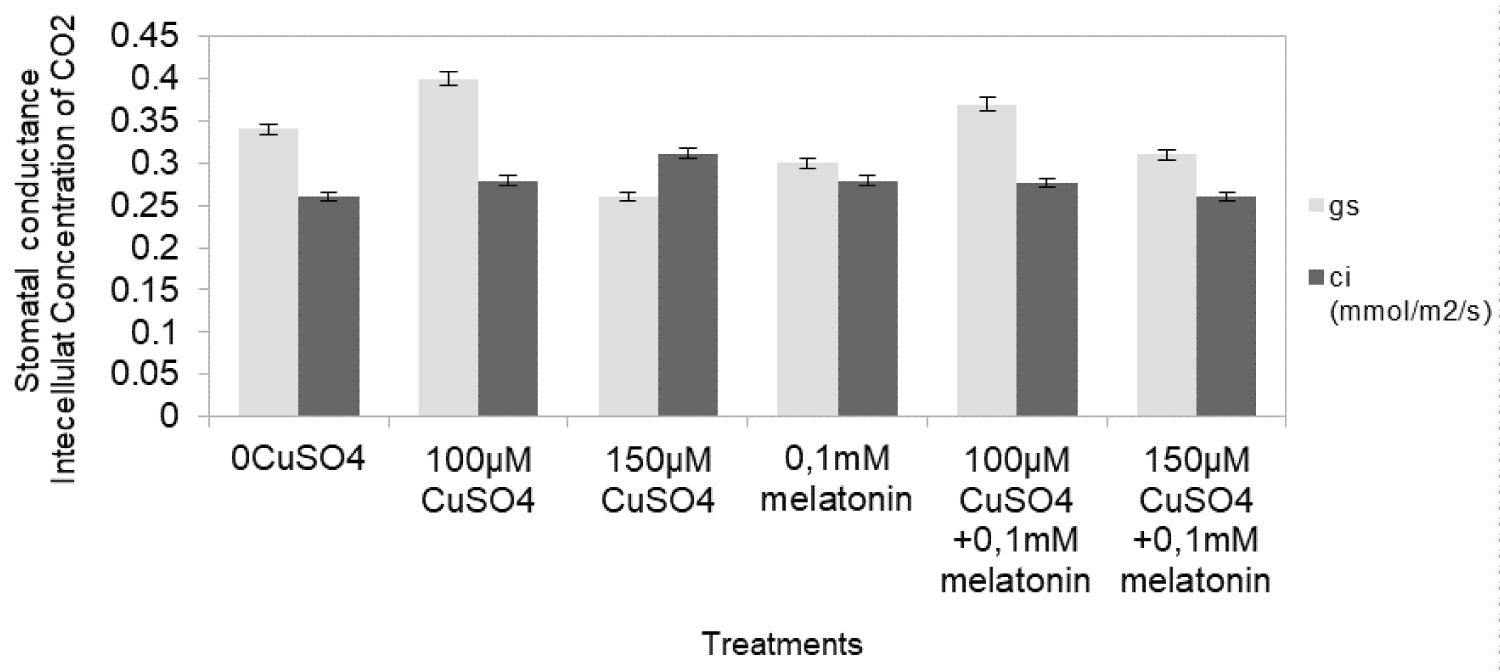
In order to estimate the effect of high content of CuSO4 on the photosynthetic apparatus, we measured photosynthetic parameters. Assimilation rate of CO2 when compared to the control treatment (0 µM CuSO4), was markedly reduced by 69% (Figure 3) under 100 µM CuSO4, whereas under simultaneous treatment with copper salts and melatonin slight depression in the photosynthesis of grapevine plants was observed (9%). Low CuSO4 content stress caused less photosynthetic changes than high CuSO4 content did. Similar pattern was displayed by the transpiration rate. The WUE also decreased more under high level of CuSO4 exposure compared to control plants (by 36% of the control) than, under simultaneous treatment with elevated CuSO4 and melatonin (by 20% of the control) (Figure 4). Additionally stomatal conductance when compared to the control treatment (0 µM CuSO4), was markedly reduced by 19% (Figure 5).
MDA content
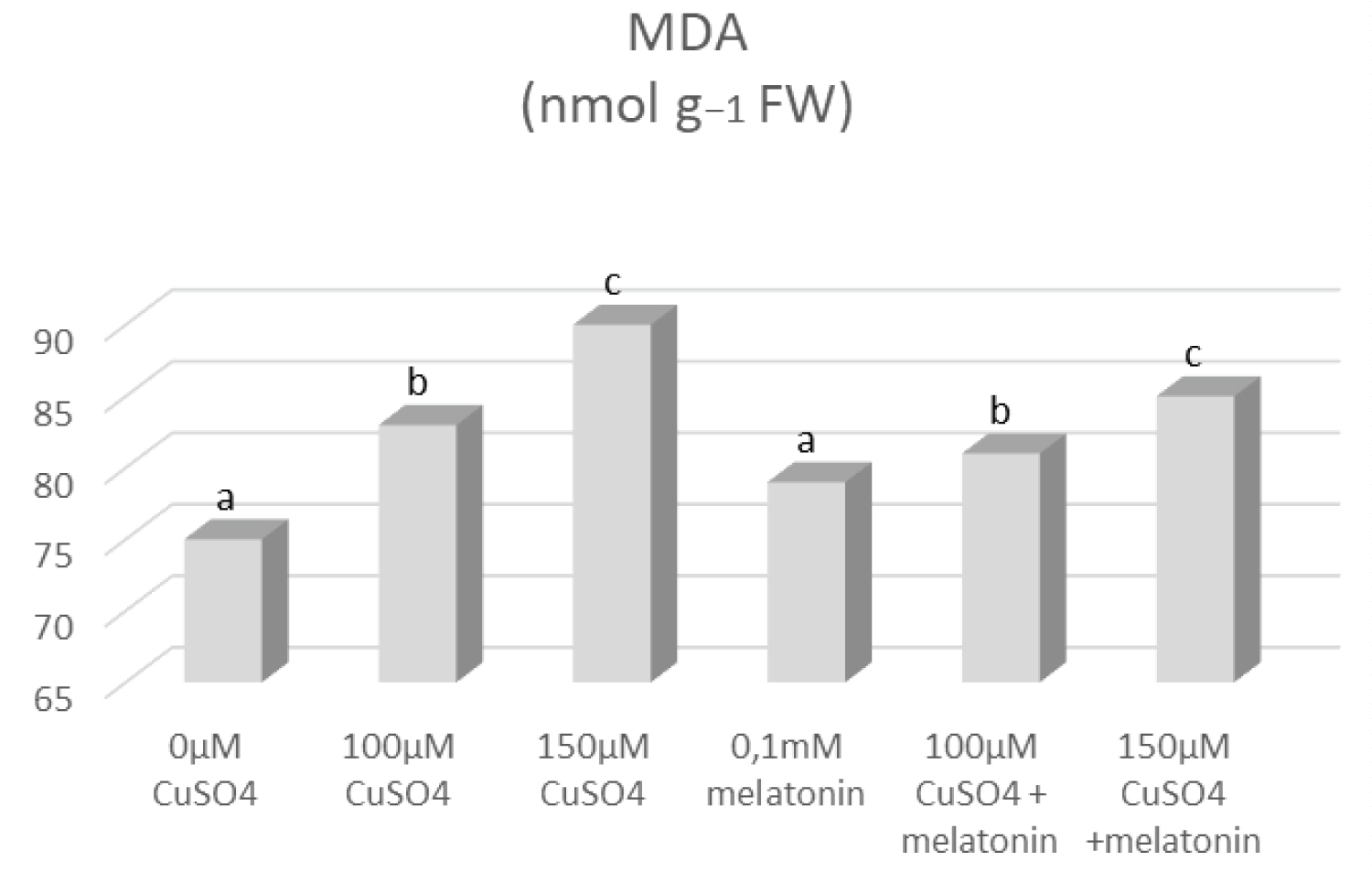
The increase of MDA content is displayed in plants under high-level of oxidative stress [18]. In our experiment, significant increases in MDA concentration were observed, especially under high rates of copper toxicity (Figure 6). Its concentration displayed a linear relationship with CuSO4 levels. MDA values were 2-times higher under high rates of copper compared to control plants, while under copper stress and melatonin application the increase was only 1.2-times of the control. A similar trend for H2O2 was observed with its highest concentration measured under 150 µM CuSO4 (Table 2).
Effect of copper toxicity on proline
Proline increased gradually under copper toxicity levels. The maximum proline accumulation was recorded at 150 µM CuSO4 (increased by 60% of the control) (Figure 7). Induction of such substances in the leaves of grapevine during heavy metal stress indicates their involvement in stress response. The level of their induction is greater under copper alone than under simultaneous exposure to melatonin and copper toxicity.
Effect of copper toxicity on antioxidants
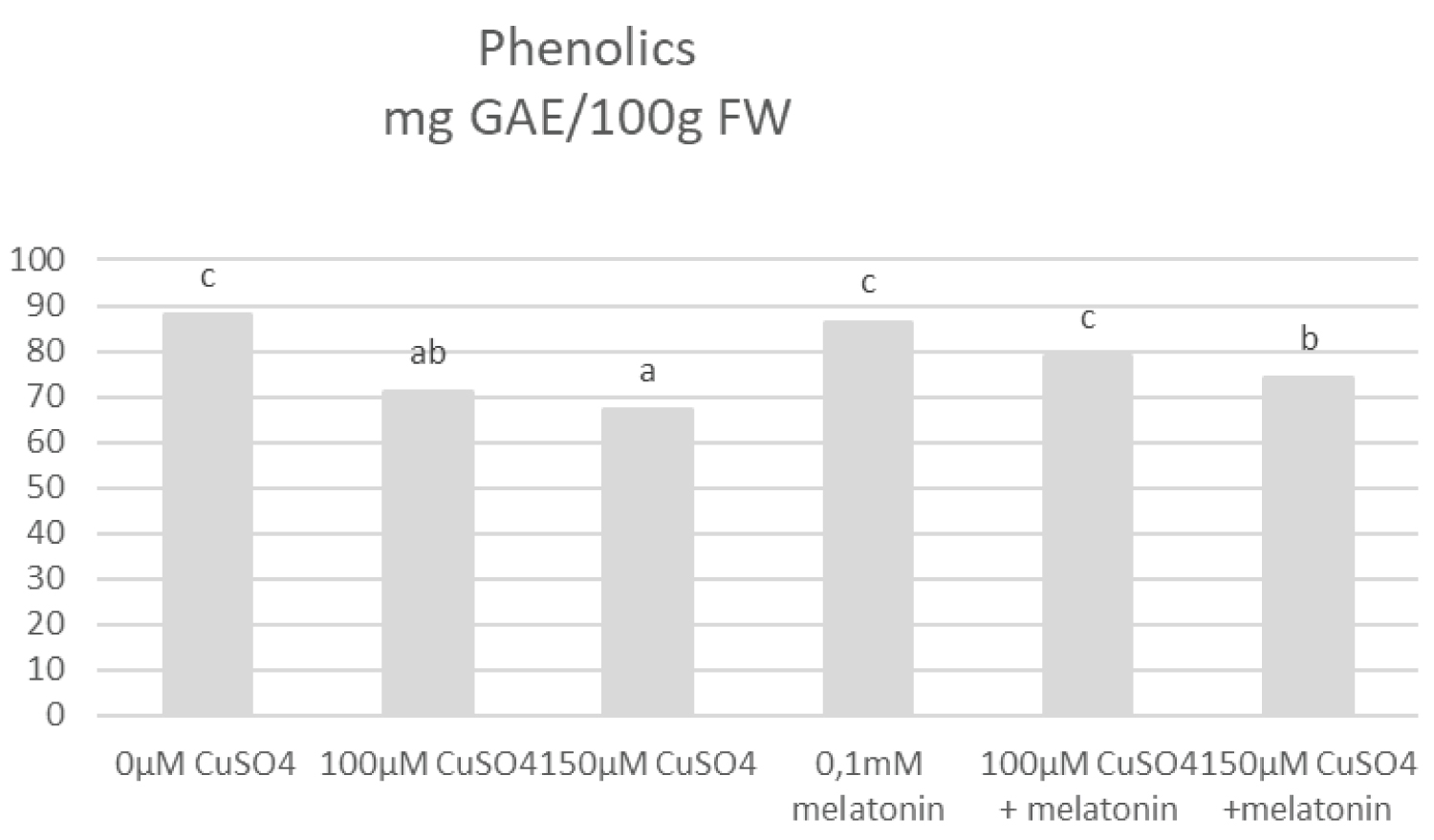
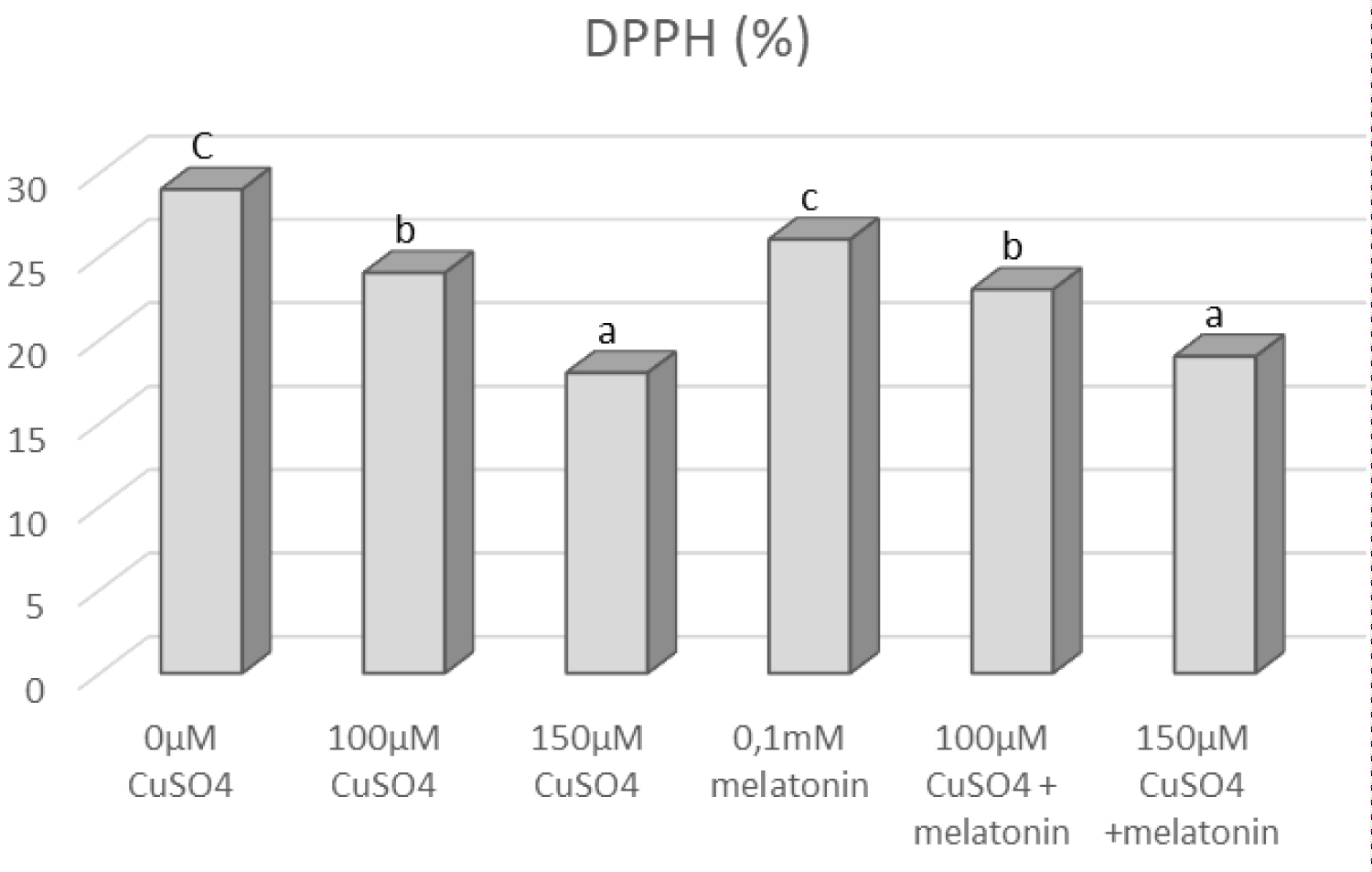
The lowest level of total Phenols concentration (21%) compared to control was measured at 150 µM CuSO4 (Figure 8). Additionally, our data indicated that salt concentration influenced the DPPH concentration (Figure 9). The lowest DPPH concentration was recorded at 150 µM CuSO4 compared to control. However after the treatment with melatonin, DPPH concentration (%) and total Phenols increased significantly.
Discussion
Copper is an essential metal for normal plant growth, but when there is excess supply of this micro-element is toxic. The toxic effects of excess copper can cause disorders by disturbing the biochemical and physiological processes such as photosynthesis, enzyme activity, pigment and protein synthesis, and cell division [17,19]. Effect of CuSO4 on growth and yield performance CuSO4 toxicity symptoms first appeared as marginal chlorosis of grapevine leaves and gradually changed to necrosis. The symptoms were more pronounced in high concentrations of CuSO4. Copper had negative effects on grapevine growth at high concentrations. The highest growth reduction was revealed in the higher copper exposure (150 µM) because greater amounts of copper were taken by the leaf system causing higher injuries to the plant tissues.
Recently reports [20,21] observed that melatonin had an ameriolative effect on heavy metal stress conditions. It seems that melatonin alleviates copper toxicity via improvement of antioxidant activity. Similar results for morphological characteristics in wild grapevine variety grown under copper toxicity and other abiotic stress were found by [19]. Melatonin is a plant growth regulator that plays a vital role in regulating plant growth and has been demonstrated as an effective plant biostimulant against biotic and abiotic stress (drought, salinity, heavy metals, etc.) [22]. Melatonin triggers the accumulation of compatible solutes such as total soluble sugars and proline content [9]. Many studies reported that exogenous melatonin could enhance plant tolerance to many kinds of stress [18,23]. For example, melatonin alleviates oxidative damage during drought stress by directly scavenging ROS and MDA content and by enhancing antioxidant activity [24-26]. In addition, melatonin regulates the transcription of various essential genes involved in antioxidative defense mechanisms [9]. The effect of melatonin on the improvement of plant growth was also reported in kiwifruit seedlings [27] and also photosynthetic machinery function in maize [28].
However similar mechanisms that melatonin seems to has in order to protect grapevine plants against Cu toxicity have been also developed to Pinus sylvestris from some mycorrhizal species [29], although the amount of Cu retained by different fungi vary considerably. The mechanisms employed by the fungi are probably through binding to extracellular materials. Organic acids excreted by plants can facilitate metal uptake, but these molecules can also inhibit metal acquisition by forming a complex with it outside the root so that it is not taken up. In the same sense, citrate appears to be responsible for Cu tolerance in Arabidopsis thaliana [30].
Photosynthesis is highly sensitive to temperature, drought, salt, and heavy metal, and usually suppressed when exposed to these stresses. A concentration dependent response of copper stress was observed on photosynthetic apparatus. As copper stress progressed, biochemical constraints might limit photosynthetic CO2 fixation more directly. The observed diminution of C3 photosynthetic machinery generates an excess of reducing power and finally leads to the formation of reactive oxygen species (ROS) which finally causes photoinhibitory and photooxidative damage [31,32]. In our study, the low CO2 availability measured by gs affects negatively net photosynthesis under copper stress. We also propose that the reduction in chlorophyll and carotenoid concentration in grapevine leaves under Cu stress can be regarded as a specific plant response to metal stress, which resulted in chl degradation and inhibition of photosynthesis. Changes of gas exchange indices under abiotic stress have been referred to many grapevine varieties [33]. According to other authors [22] stomatal and non-stomatal limitation of photosynthesis has been also reported under osmotic stress. However, melatonin can enhance chlorophyll contention, electron transport, and stomatal conductance to alleviate photosynthetic inhibition that is caused by stress [11,23]. It seems that melatonin mitigated the negative impact of CuSO4 on photosynthetic efficiency. Melatonin application also regulated electron transport system, and further increased the maximal quantum yield of PSII photochemistry (Fv/Fm) [34,35].
A common observation of previous Cu stress studies with Cu ecologically concentrations was that Cu usually does not affect the maximum dark-adapted quantum yield of PSII, measured as Fv/Fm [36]. In the present investigation, the increase in Fo and decrease in Fm under high Cu concentrations occurred related to a decrease in Fv/Fm. Additionally previous researchers mentioned, two mechanisms at least, are involved in producing the changes in the fluorescence parameters under abiotic stress. One mechanism results in an increase in Fo, possibly due to the reduced plastoquinone acceptor (QA), being unable to be oxidized completely and the other is responsible for the decrease in Fm that indicates processes related to a decrease in the activity of the water-splitting enzyme complex [36]. Chlorophyll fluorescence parameters with the increased peroxidation under high heavy metal exposure, representing the beginning of inhibition in growth. Overall, the toxic effects of copper on maximum quantum efficiency of PSII (Fv/Fm) and in the efficiency of the water-splitting complex on the donor side of PSII (Fv/Fo) is altered. Similar results are also shown in the green microalga Scenedesmus exposed to heavy metal stress [11]. These authors suggested that the heavy metals replace manganese (Mn) from the water-splitting apparatus of the oxidizing side, and that water-splitting apparatus of PSII is the primary site of action of heavy metals.
Furthermore, H2O2 accumulation is another ROS that is implicated in enhanced lipid peroxidation and membrane damage-causing cell death. Treatment with increased Cu concentrations (150 μM) initially resulted in a significant increase in MDA concentration. These observations are in conformity with earlier reports [17]. Thus, the enhanced MDA and H2O2 indicate the prevalence of oxidative stress and this may be one of the possible mechanisms by which toxicity due to copper salts could be reduced because of melatonin treatment [21,37].
Changes of antioxidants indices under abiotic stress such as salinity and heavy metals have been examined to different plant species [18,23] and have shown similar results to our investigation. It seems likely that under the melatonin foliar application a possible protective strategy against metal toxicity can be developed. Plants that were treated with melatonin had stronger tolerance to heavy metal stress according to recent reports [21,24]. Pea plants (Pisum sativum) pretreated with melatonin survived after 100 µM copper treatment, but control plants died. This result suggested that melatonin could enhance the copper tolerance of plants. Since melatonin is safe to many organisms, as well as inexpensive, it may be an eco-effective approach to clean environmental contaminations. In algae, the dosage of heavy metals (Cu, Cd, Pb and Zn), had a positive effect on melatonin levels, and metal stress tolerance of algae was increased by exogenous melatonin [26]. Additionally, growth under copper stress was improved after melatonin treatment (1 and 10 µM). Melatonin also enhanced the tolerance of Cd stress in Solanum lycopersicum by improving the plant growth, photosynthesis and antioxidant enzymes [34]. In addition, the oxidative damage that was caused from copper toxicity was alleviated after melatonin application [28].
Finally we observed an enhanced content of proline in the leaves of grapevine plants growing under copper toxicity. Our data suggest that these strategies are involved with protection against oxidative damage. Proline is an important osmolyte to adjust the plant under stress conditions and its accumulation in response to abiotic stress was described in several plants [33]. Previous papers reported that melatonin application, particularly at grapevine leaves that treated with concentrations no more than 150 µM, increased proline accumulation when the plants were under various abiotic stresses [11,33]. The effect of melatonin on improving proline accumulation has also been reported in Zea mays L. [34] and Coffea arabica L. [35,37]. In a previous study [38], the authors proposed that melatonin increases the expression of a gene for proline biosynthesis [39,40]. Similar results in seedlings of watermelon and wheat suggested that melatonin could enhance heavy metal tolerance via activation of the plant's antioxidant system [41-45]. However, additional experiments are in progress to ascertain all these defense strategies to copper toxicity.
Conclusions
Heavy metal contamination in soil is a serious environmental problem especially to plants. Copper toxicity was examined in 'Pamidi' a red Greek grapevine variety. More specific, copper stress induced modifications in proline and antioxidant activity especially in high concentration (150 mM). It is obvious that these changes could be considered as important adaptation mechanisms of grapevines to copper toxicity conditions. Moreover the foliar spray of melatonin alleviated the oxidative damage and induced accumulation of antioxidant compounds and proline. However more studies are needed to confirm these results, in order to clarify the tolerance strategies in grapevines treated with melatonin and under copper toxicity conditions.
Funding
This research was funded by the European Regional Development Fund (ERDF) and national funds of Greece and Bulgaria through the Cooperation Programme INTERREG V-A "Greece-Bulgaria 2014-2020", Research Project: "SOS for endangered traditional vine varieties-VineSOS" (Project Number MIS: 5016071).
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization, A.G. and S.A.; methodology, A.G., software, A.G. and S.A..; validation, A.G. and S.A.; formal analysis, A.G., investigation, A.G.,; resources, A.G. and S.A.; writing-original draft preparation, A.G.; writing-review and editing, A.G. and S.A.; supervision and project administration, A.G. and S.A.; All authors have read and agreed to the published version of the manuscript.
References
- Albuquerque dos Santos E, Florisbal LM, Loss A, et al. (2018) Geology and wine 15. producing wine at altitude: The terroir of são Joaquim, Brazil. Geoscience Canada 45: 137-149.
- Flexas J, Bota J, Cifre J, et al. (2004) Understanding down-regulation of photosynthesis under water stress: Future prospects and searching for physiological tools for irrigation management. Ann Appl Biol 144: 273-283.
- Papapetrou M, Loukovitis D, Papadopoulos O, et al. (2020) Genetic diversity of local greek and bulgarian grapevine (Vitis vinifera L.) varieties. Diversity 12: 273.
- Chaignon V, Sanche Neira I, Hermann P, et al. (2003) Copper bioavailability and extractability as related to chemical properties of contaminated soils from a vine-growing area. Environ Pollut 123: 229-238.
- Pätsikkä E, Kairavuo M, Sersen F, et al. (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129: 1359-1367.
- Prasad MNV, Strzalka K (1999) Impact of heavy metals on photosynthesis. In: Prasad MNV, Hagemeyer J, Heavy Metal Stress in Plants, Springer Publishers, Berlin, 117-138.
- Puig S, Thiele DJ (2002) Molecular mechanisms of copper uptake and distribution. Curr Opin Chem Biol 6: 171-180.
- Arnao MB, Hernández Ruiz J (2021) Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. Journal of the Science of Food and Agriculture 101: 5297-5304.
- Cao Y, Qi D, Li S, et al. (2018) Melatonin alleviates copper toxicity via improving copper sequestration and ROS scavenging in Cucumber. Plant Cell Physiol 60: 562-574.
- Sharma A, Wang J, Xu D, et al. (2020) Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Carya cathayensis plants. Sci Total Environ 713: 136675.
- Askri H, Gharbi F, Rejeb S, et al. (2018) Differential physiological responses of Tunisian wild grapevines (Vitis vinifera L. subsp. sylvestris) to NaCl salt stress. Brazilian Journal of Botany 41: 795-804.
- Sadmann G, Böger P (1980) Copper-mediated lipid peroxidation processes in photosynthetic membranes. Plant Physiol 66: 797-800.
- Von Caemmer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and gas exchange of leaves. Planta 153: 376-387.
- Khan AA, Mcneilly T, Collins C (2000) Accumulation of amino acids, proline, and carbohydrates in response to aluminum and manganese stress in maize. J Plant Nutr 23: 1303-1314.
- Scalbert A, Monties B, Janin G (1989) Tannins in wood: Comparison of different estimation methods. J Agri Food Chem 37: 1324-1329.
- Su M, Silva JL (2006) Antioxidant activity, anthocyanins and phenolics of rabbit eye blueberry (Vaccinium ashei) by products as affected by fermentation. Food Chem 97: 447.
- Giannakoula A, Therios I, Chatzissavvidis C (2021) Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. the role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 10: 155.
- Camrolle J, García JL, Figueroa M, et al. (2015) Evaluating wild grapevine tolerance to copper toxicity. Chemosphere 120: 171-178.
- Wang LY, Liu JL, Wang WX, et al. (2016) Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 54: 19-27.
- Posmyk MM, Kuran H, Marciniak K, et al. (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45: 24-32.
- Silalert P, Pattanagul W (2021) Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings Notulae Botanicae Horti Agrobotanici Cluj-Napoca 49: 67-75.
- Wei W, Li QT, Chu YN, et al. (2019) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J Exp Bot 66: 695-707.
- Biao Z, Qing M, Song LJ, et al. (2015) Roles of melatonin in abiotic stress resistance in plants. J Exp Bot 66: 647-656.
- Arnao M, Hernández Ruiz J (2019) Role of melatonin to enhance phytoremediation capacity. Appl Sci 9: 5293.
- Tan D, Manchester LC, Helton P, et al. (2007) Phytoremediative capacity of plants enriched with melatonin. Plant Signal Behav 2: 514-516.
- Ding F, Wang G, Zhang S (2018) Exogenous melatonin mitigates methyl viologen-triggered oxidative stress in poplar leaf. Molecules 23: 2852.
- Liang D, Ni Z, Xia H, et al. (2019) Exogenous melatonin promotes biomass accumulation and photosynthesis of kiwifruit seedlings under drought stress. Scientia Horticulturae 246: 34-43.
- Ahmad S, Kamran M, Ding R, et al. (2019) Exogenous melatonin confers drought stress by promoting plant growth, photosynthetic capacity and antioxidant defense system of maize seedlings. Peer J 7: e7793.
- Van Tichelen KK, Colpaert JV, Vangronsveld J (2001) Ectomycorrhizal protection of Pinus sylvestris against copper toxicity. New Phytol 150: 203-213.
- Murphy A, Taiz L (1995) A new vertical mesh transfer technique for metal-tolerance studies in Arabidopsis (Ecotypic Variation and Copper-Sensitive Mutants). Plant Physiol 108: 29-38.
- Yruela I, Gatzen G, Picorel R, et al. (1996) Cu (II)-inhibitory effect on photosystem II from higher plants. A picosecond time-resolved fluorescence study. Biochemistry 35: 9469-9474.
- Yruela I, Pueyo JJ, Alonso PJ, et al. (1996) Photoinhibition of photosystem II from higher plants: Effect of copper inhibition. J Biol Chem 271: 27408-27415.
- Hernández Ruiz J, Arnao M (2018) Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 8: 33.
- Tan DX, Manchester L, Esteban Zubero E, et al. (2015) Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 20: 18886-18906.
- Mallick N, Mohn FH (2003) Use of chlorophyll fluorescence in metal-stress research: A case study with the green microalga Scenedesmus. Ecotoxicol Environ Saf 55: 64-69.
- Ozden M, Demirel U, Kahraman A (2009) Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Scientia Horticulturae 119: 163-168.
- Hasan M, Ahammed GJ, Yin L, et al. (2015) Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front Plant Sci 6: 601.
- Campos CN, Ávila RG, Souza de KRD, et al. (2019) Melatonin reduces oxidative stress and promotes drought tolerance in young Coffea arabica L. plants. Agricultural Water Management 211: 37-47.
- Ouzounidou G, Moustakas M, Strasser RJ (1997) Sites of action of copper in the photosynthetic apparatus of maize leaves: Kinetic analysis of chlorophyll fluorescence, oxygen evolution, absorption changes and thermal dissipation as monitored by photoacoustic signals. Funct Plant Biol 24: 81-90.
- Cherono S, Ntini C, Wassie M, et al. (2021) Exogenous application of melatonin improves drought tolerance in coffee by regulating photosynthetic efficiency and oxidative damage. Journal of the American Society for Horticultural Science 146: 24-32.
- Shi H, Chen K, Wei Y, et al. (2016) Fundamental issues of melatonin-mediated stress signaling in plants. Frontiers in Plant Science 7: 1124.
- Zaidi RA, Fuad AA, Ahmed B, et al. (2020) Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv 10: 38379-38403.
- Romero A, Ramos E, Los de Ríos C, et al. (2014) A review of metal-catalyzed molecular damage: Protection by melatonin. J Pineal Res 56: 343-370.
- Nawaz MA, Jiao Y, Chen C, et al. (2018) Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J Plant Physiol 220: 115-127.
- Zuo Z, Sun L, Wang T, et al. (2017) Melatonin improves the photosynthetic carbon assimilation and antioxidant capacity in wheat exposed to Nano-ZnO stress. Molecules 22: 1727.
Corresponding Author
Dr. Giannakoula Anastasia, Assistant. Professor, Department of Agriculture (Crop Production), International Hellenic University, Sindos 57400, Greece.
Copyright
© 2022 Giannakoula A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.