Challenges and Opportunities for Management of Crop and Tree Diseases in Northern Ireland
Abstract
Phytopathogens have beleaguered the island of Ireland since the 19th century great famine caused by the potato blight oomycete, Phytophthora infestans and in recent times by cohorts of serious bacterial wilt diseases such as Erwinia, Dickeya, Ralstonia, and fungal wilt diseases predominantly caused by saprophyte fungi (e.g. Fusarium oxysporum, Pythium, Rhizoctonia) have all shown their growing resistance to various conventional chemical control agents, and create enormous impact on sustainable agriculture, further exacerbated by global climate change pressures. The plant health outlook in Northern Ireland has further been tested by emergent Europe wide agro-forestry related diseases including the recent broad host insect vector-borne Xanthamonas bacterial phytopathogen Xylella fastidiosa that can affect a wide array of ornamental, arable crops and tree species alike. This mini-review focuses on potential alternative plant extracts and microbial sources as agents broadly known as biostimulants for not only their growth promotion via plant and soil nutrient management but also controlling phytopathogens in Northern Ireland. The multi-actor approach comprising stake holders, plant health policy makers, farmers, plant health advisors, researchers, knowledge transfer centres will be the key drivers for an effective data input and sustainable plant health. Integration of precision farming with latest information and communications technology (ICT), advanced automation for need based/site-specific use of biostimulants to combat biotic and abiotic stress, on-field plant pathogen remote sensors and their extended new tool applications for soil and phytosanitory inspections at port of entry points are some of the overarching comprehensive strategies planned for the future.
Keywords
Biological control, Crops, Trees, Diseases, Alternative biopesticides
Introduction
History has already shown human populations to be vulnerable to episodes of starvation, particularly when dependent on a single crop (or monoculture) which is unexpectedly affected by a pathogen. Potato (Solanum tuberosum) crops in Ireland were ravaged by a disease known as potato blight, caused by the oomycete Phytophthora infestans, between 1845 and 1852. The Great Famine caused approximately one million deaths in Ireland, and the emigration of another million people further heightening how much humanity needs antimicrobials to control plant/crop pathogens, in addition to antimicrobials of therapeutic use in human and veterinary medicine. The Food and Agriculture Organization [1] estimates that plant pests and diseases are responsible for about 25% of global post-harvest crop losses and thus continuing advances in the science of phytopathology are essential to improve disease control. Major crops all play host to microbial pathogens mainly fungi and viruses, but also bacteria. Notable fungal pathogens include Fusarium (causal agents of wilt diseases) and Armillaria species, (also known as honey fungus) which are virulent pathogens of trees. Typical virus infections include tobacco mosaic virus and bacterial infections include soft rots caused by Erwinia species. As well as potato blight caused by P. infestans, this oomycete genus has come to note globally recently as the causative organism of Sudden Oak Death, (P. ramorum).
Northern Ireland has many exotic invasive plant pathogens and pests registered as risk pathogens within the registry of the European Plant Protection Organization (EPPO) and Department of Agriculture, Food and Rural Affairs (DEFRA, UK) pertaining to plant diseases occurrence, prevalence and spread within the monoculture crops and tree plant host species. The global trends in exponential emergence of plant diseases, pathogens, pesticide usage, plant susceptibility/pathogen resistances meant that exploring for alternative disease control measures in Northern Ireland, has also concomitantly become multi-variant and complex. For instance, often the local farmers, and plant health inspectorate deal from disease to disease with disparate causal agents ranging from bacteria, oomycete or fungal pathogens and pests (e.g. nematodes) attacking variant monoculture crops or forest tree plants. To this end, the 'fire-fighting' exercise of plant pathogen surveillance, monitoring, diagnostics, containment tasks within the local Department of Agriculture, Environment and Rural affairs, Northern Ireland (DAERA, NI) also highlights the recent outbreaks of new variant plant diseases [e.g. Chalarafraxinea, oomycete P. ramorum, P.lateralis, (trees), Ralstonia spp, E. amylovora, Dickeyasolani (potatoes), Fusarium fungal wilt of arable and horticulture crops].
In this review we have therefore outlined diverse causal agent challenges exemplified above amongst others faced by Northern Ireland for disease management via non-chemical alternative sources of antimicrobials as biological control agents for plant diseases for conserving biodiversity and environmental protection. We have overviewed generically on the locally relevant plant versus the disease, their impact on agriculture, environmental and economic costs, and the upturn in worldwide trends developments in seeking natural biological resources themselves as alternative sustainable antimicrobial agents for plant disease management.
Environmental Significance of Antimicrobial Resistance to Agriculture, Food and Human Clinical Pathogens
It will be prudent to review the environmental costs of using antimicrobials (which refer to veterinary and human use antibiotics) for animal agriculture (e.g. livestock, pigs, poultry etc.), for agri-food operations as well as for human clinical pathogen control. The use of antimicrobials against plant pathogens has been a practice since the 1950s. The more recent example reported by Vidaver [2] of using streptomycin and tetracycline in orchards against bacterial phytopathogens showed widespread resistance to streptomycin amongst bacterial phytopathogens such as E. amylovora, the causative agent of Fire blight on fruit trees, leading to the use of oxytetracycline as a replacement. In Latin America the antibiotic gentamicin was introduced into arable farming in ploughed agriculture, again to control Erwinia, which led to the USA banning imports of fruit treated with gentamicin in 1999, due to its significance in clinical medicine. European Union (EU) has banned the use of prophytlactic antibiotics altogether from 2022 on animal agriculture farming (livestock, poultry) and to our knowledge EU countries have not known to have administered either human or veterinary antibiotics on plant crops. The use of antibiotics in the environment raises health issues and additionally issues concerning intra- and inter-specific transfer of antibiotic resistance genes, as well as creating selective pressures on microbial populations [3,4]. The issue of selection in non-clinical environments, where antibiotic concentrations are up to several hundred-fold below the minimal inhibitory concentration (MIC) of susceptible bacteria [5] also brings to the fore the experimental effects observed for minimal selective concentration (MSC) for ciprofloxacin and tetracycline which were 100 pg/ml and 15 ng/ml, respectively. The selection of resistance bacteria does not have to occur at high levels of antibiotics. Low level of antimicrobial drugs (i.e., minimal selective concentrations, MSC) can also select for a given resistance mutation within a bacterial population. Antibiotics found in minimal levels (i.e., at concentrations several hundred-fold below the minimal inhibitory concentration (MIC)) in aquatic and soil environments [6] influence the selection and long-term persistence of resistance factors in soil-dwelling or aquatic bacterial pathogens (e.g. Pseudomonas aeruginosa, Escherichia coli). Therapies developed from medicinal plants and fungi as viable treatment options to attain human pathogen control, and prevent antimicrobial resistance, offer a framework to finding alternatives to plant pathogenic disease management.
Exploring Plants and Fungi as a Reservoir for Antimicrobial Alternatives in Clinical Pathogens in Northern Ireland
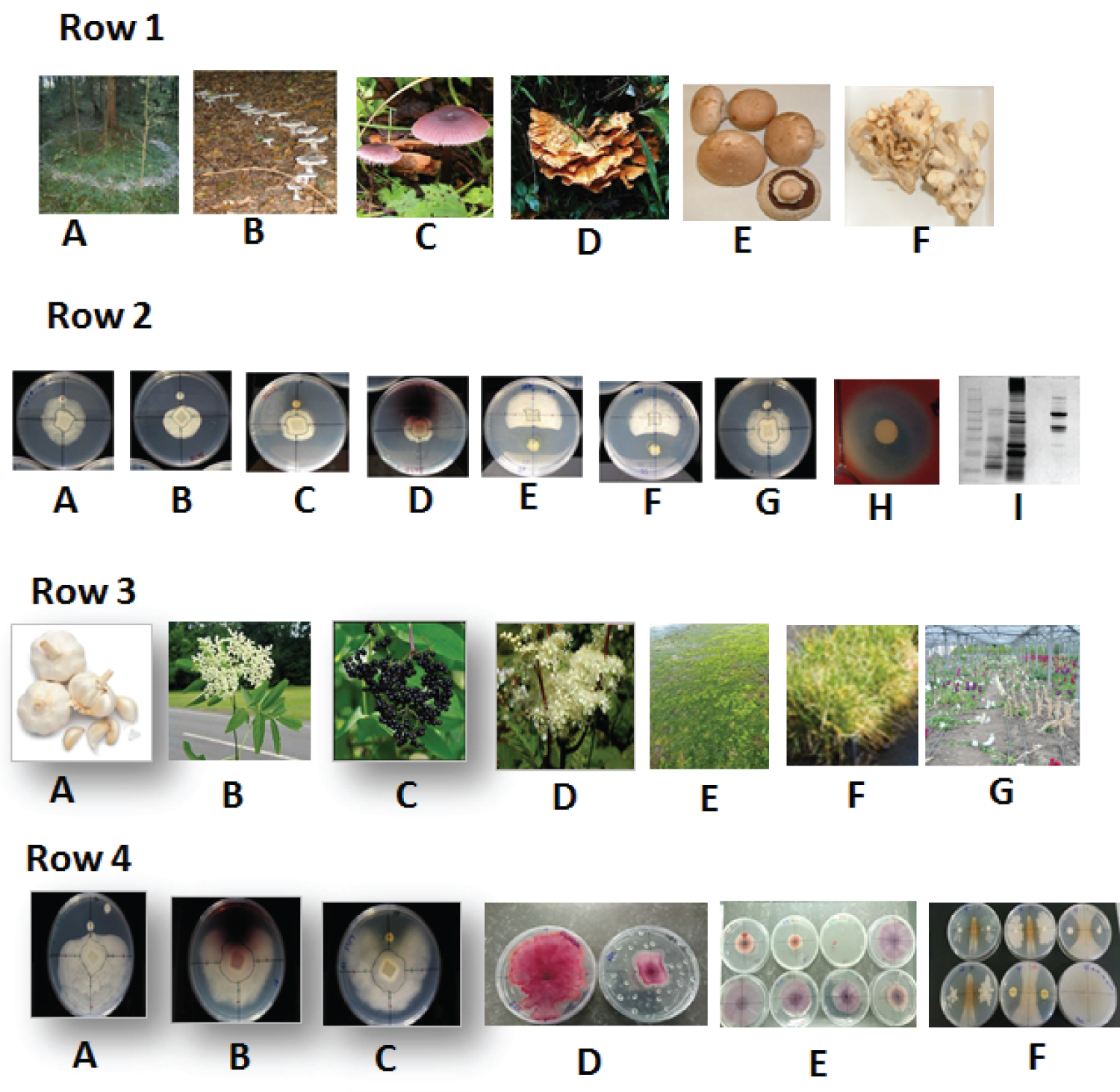
Pathogenic microbes and their growing resistance to various control agents including conventional antibiotics create enormous environmental and human health impact. In terms of both biomedical and agri-food and veterinary applications the presence of high level antibiotic resistance in clinical and environmental isolates leads to a treatment dilemma, driven through ever-decreasing availability of efficacious antibiotics [7,8]. Therefore, there is an urgent clinical requirement to develop new effective antibiotics, or to repurpose existing pharmaceuticals through novel fungal and plant species [9]. Not only is this an ancient practice, but still today, medical practice in Japan, China, Korea, and other Asian countries continues to use fungal-derived antibiotics. In search of novel therapeutic alternatives, many fungal based studies have found compounds with various clinical properties, including anti-parasitic [10] and antimicrobial potentials in locally sourced Shiitake mushrooms (Figure 1, Row 1) and in our own research expedition [11]. Members of the macrofungal genera Phellinus and Inonotus have been shown [12] to be composed of yellow polyphenol pigments, principally a styrylpyrone class of compounds, with anti-viral effects. Styrylpyrone pigments in mushrooms are thought to have a role similar to that of flavonoids in plants, whereby the unique carbon skeleton of fused styrylpyrone might be an attractive molecular scaffold for pharmacological applications.
Plants have been well documented for being a rich resource for searching new antibiotics for clinical applications, and a number of locally sourced plant species Yarrow - Achillea millefolium), Meadow sweet (Filipendula ulmaria), Comfrey leaf (Symphytum officinale), Ragwort (Senecio jacobaea), Dandelion leaf and roots (Taraxacum officinale) garlic (Allium sativum) and onion (A. cepa) were examined against thirty four microorganisms (24 bacteria and 10 fungi) in one of our early studies [13]. Other plant extracts included in our early trials were Elder, Sambucus nigra flower or berry [14]. Local seaweed (extracts of) brown algae species Ascophyllum nodosum, Laminaria hyperborea, Fucus spp. and the cyanobacteria Spirulina sp., (illustrated in (Figure 1 Row 2) showed - promising inhibitory effect of fucoidan on Phytophthora spp. The local forage grass, Loliumperenne varieties that possess defense eliciting capabilities [15] exhibited potential as alternative antimicrobial agents to combat phytopathogens. Recently, the antimicrobial activity of 23 native macrofungal (mushrooms/toadstools) taxa, collected from woodlands in Northern Ireland against six clinical (CF) isolates of Mycobacterium abscessus, as well as M. abscessus NCTC Reference strain (NCTC 13031) showed [16] that macrofungi represent a source of novel antimicrobials against M. abscessus, and may require intensive clinical tests. Such evidence offered by a wide range of aromatic plant and medicinal macrofungi prompted us in part, to address biotic stresses surmounted by climate change vis-à-vis "replacement non-chemical" biological alternatives, with minimal long-term environmental impacts of residues and resistances imposed on agriculture systems for sustainable crop disease management options.
Biocontrol and Plant Pathogens
Pathogenic microorganisms affecting plant health (phytopathogens) are a major and chronic threat to food production and the stability of ecosystems worldwide. As agricultural production has intensified worldwide over recent decades, agri-food producers became increasingly dependent on the use of agrochemicals, as a relatively reliable means of crop protection to assist with the economic stability of their operations [17]. However, the ever-increasing use of chemicals causes a number of undesirable effects, such as pathogen resistance to the applied agents. Additionally, collateral damage to non-target ecosystems and the environment, such as the carriage of the pesticide dichlorodiphenyltrichloroethane (DDT) to water courses by surface runoff has been reported since the 1930s [18]. The increasing cost of agrochemicals and a rising demand from consumers for pesticide-free food has necessitated a search for alternative control methods.
"Biological control refers to the purposeful utilization of introduced or resident living organisms, other than disease resistant host plants, to suppress the activities and populations of one or more plant pathogens [19]." This could involve the use of microbial inoculants to suppress a single type or class of plant diseases, but most narrowly, biological control or biocontrol refers to the suppression of a single pathogen by a single antagonist, or biological control agent (BCA), in a single cropping system. A recent review [20] highlighted the versatility of Bacillus species due to their production of lipopeptides, antibiotics and enzymes, and topromote plant growth and systemic induced resistance as combatants against plant pathogens including Fusarium, Rhizoctonia, Pythium, Erwinia and Phytophthora for biocontrol.For instance, the antibiotic mycosubtilin produced by Bacillus subtilis BBG100, is used to control Pythium aphanidermatum, a causal agent of 'damping off' of seedlings [21]. By contrast, another control mechanism is known as direct antagonism. An example of this is the hyperparasitism or predation of the fungus Trichoderma atroviride (formerly T. harzianum), which is an EU approved "active substance" [22] on other fungal species Sclerotium rolfsii and R. solani, where one organism is parasitic on another [23].
Recent Advances in Searching for Plant and Microbial Resources as Biocontrol Agents
Over the past century improvements in agricultural productivity have mainly been driven by the use of new crop varieties, increased use of mineral fertilizers and extensive use of agrochemicals [24]. Until recently, recycling of nutrients and the importance of biological control processes has been given much less attention in agricultural practice. Over recent decades there has been a growing awareness of the need facilitate sustainable plant production via broad term "biostimulants" and the recent "bio-effectors" [BEs] (www.biofector.info) [25]. Biostimulants and Bes comprise bacteria and fungi also known as plant growth promoting rhizobacteria (PGPR). These together with bio-active natural compounds (extracts from seaweed, plants and composts) with the ability to improve plant growth, nutrient acquisition and enhance stress tolerance of crops, are utilized for either crop production or protection. Recent reviews [26,27] highlight the increasing trends of utilizing biostimulants for their functional benefits, in the backdrop of climate change and the concomitant pressures of biotic and abiotic stress. The concept of bio-stimulants, also called bio-effectors (BEs) covers a diverse group of natural products. This includes either viable microorganisms (bacteria and fungi) or extracts from bacteria, fungi, algae or plants containing bioactive compounds. Humic substances typically extracted from composted plant material or manure has shown to be effective plant growth promoters [28]. Seaweed extracts obtained from marine macroalgae contain plant growth-stimulating compounds [29] and the most commonly used are brown seaweeds (Phaeophyta) of such genera as Ascophyllum, Fucus and Laminaria. Their physico-chemical evaluations [30] revealed their wide range of sustainable crop growth/protection utilities.
The inoculation of plants with viable microorganisms, broadly referred to as Plant Growth Promoting Microorganisms (PGPM) has been inundated with much research focused on plant growth promoting rhizobacteria (PGPR) of the genera Bacillus [31] and Pseudomonas (e.g. [32]). Notable others included Paenibacillus polymyxa [33] and Stenotrophomonas maltophilia [34] The use of sustainable biological alternatives [35,36] and natural remedial solutions [37] can be found within the complex soil habitat itself [38] and researchers discovered biocontrol organisms (Clonostachys and Trichoderma spp.) antagonistic to the banana wilt (Fusarium sp.) on the banana fruits (Musa spp.) surface. This was a key pointer in the evidence and innovation considered for Northern Ireland phytosanitory measures. Native bacteria themselves have been demonstrated to have rescued sudden wilt disease that emerged during the continuous cropping [39].
Naturally sourced biocontrol agents are usually administered as either soil drenches, root dips, or spent compost dressings. A number of commercial biological formulations were evaluated [40], comprising five lab-isolated Bacillus subtilis strains and five commercial biocontrol agents [Actinovate (Stretomyces lydicus strain WYEC108), Serenade Max (B. subtilis strain QST713), Mycostop (Streptomyces griseoviridis strain K61), Root Shield (T. harzianum strain T-22), and Prestop WP (Gliocladium catenulatum 26 strain J1446) and mycoparasitic fungi such as Clonostachys rosea. These were evaluated both in vitro in petri dishes and in the greenhouse for their efficacy as control agents of Fusarium wilt in Northern Ireland and UK floriculture [41]. PGPM generally have a low degree of host specificity meaning that plants may profit from inoculation with an organism isoated from the rhizosphere of a distantly related plant species [42,43] which increases the potential of PGPM as commercial products. There are several examples of cases where a synergistic effect of combining two or more biostimulants which has resulted in a synergistic effect such as those demonstrated on sugarcane yield [44] and the inoculation with more than one microorganism such as the endophytic bacteria used in combination with humic substances increase the yield in maize crops [45]. Bioeffectors represent non-chemical alternatives to improve plant protection (e.g. pathogen avoidance) and productivity (efficient nutrient managers) without acting as direct agents [46,47].
With in the EU the national legislation on these products varies considerably between the EU member states [48]. The European Commission is in the process of regulating a number of plant and microbial products in the broad category of biological control agents (BCA), biofertilisers, and recent biofectors (www.biofector.info) for their type, rate and modes of applications, toxicity, persistence, costs and benefits, that act as either crop protection or crop production non-chemical and biological agents. For example, Fusarium wilt of ornamental plants is the most frequently encountered glasshouse/polytunnel raised crop disease, followed by othersoil-borne phytopathogens Pythium and Rhizoctaniain Northern Ireland [49]. Wilt pathogen F. oxysporum was first reported in Europe [50] [50] and is regarded as a major pathogen [51] in the UK polytunnel raised horticulture crops [52] and ornamentals. Fusarium oxysporum is distinguished into formae speciales (f. sp.) on the basis of their host specificity. Fusarium is implicated in causing crown and root rots as well as vascular wilts on a very diverse range of crop hosts worldwide, including onion, leek, lettuce, tomato, brassicas, asparagus, cucurbits, peppers, coriander, spinach, basil, beans, peas, strawberry, watermelon and banana [53]. Fusarium also affects economically important ornamental crops, such as Brompton Stock (Matthiola incana), Carnation (Dianthus) and Daffodil (Narcissus), all of which are important in the cut-flower market. Its wide host range, as well as its economic and scientific impact, means that F. oxysporum was recently identified as the fifth most important plant pathogenic fungus. Thus Fusarium wilt disease control measures pose a formidable challenge to the glasshouse crops industry [54] due to stringent Europe-wide pesticide usage regulations, high costs, environmental, human and soil health impact.
Challenges and Opportunities for Biological Control Measures to Improve Phytosanitation Strategies Including Those That Impact Soil Health in Northern Ireland
Climate change induced emergent biotic and abiotic stress challenges
Northern Ireland serves an ideal microcosm of geographical region, land linked with the Republic of Ireland but almost 2/3rds of the province is surrounded by the Atlantic ocean and the Irish sea, presenting a unique opportunity to overview the crop and disease incidence, surveillance, and management strategies [55]. Climate change and burgeoning global trade across the continents are major contributors to pests and pathogen outbreaks across the British Isles. For example, over 50 organisms of threat to forestry and horticulture industry and as many to grass and cereals have been further recently enlisted in the DEFRA Plant Health Risk Registry [56]. Thus, biosecurity [57] has become a priority for all four regional governments within the UK [58]. In the context of plant pathogen surveillance, monitoring and diagnostics, recent projects within the local Department of Agriculture, Environment and Rural affairs, Northern Ireland (DAERA, NI) were nevertheless focused on outbreaks of new plant diseases [59,60] such as C. fraxinea, P, ramorum, P. lateralis, (trees), and D. solani (potatoes). In addition, the recent notifications of leaf spot/blight diseases such as Microdochium spp., and Drechslera spp. on forage grass varieties of Lolium perenne [61] has been highlighted in a DAERA Animal and Plant Health reports (www.daerani.gov.uk).
Local meteorology factors as a microcosm of environmental impact on plant diseases
In Northern Ireland, summer temperatures [62] are highest in southern lowland areas (13.6-15.4 ℃) whereas in contrast in northern upland regions, they are lower (9.6 ℃ - 13.6 ℃). Winter temperatures are highest in coastal areas (5.0 ℃ - 6.5 ℃), and cooler in central and upland areas (1.5 ℃ - 5.0 ℃). Summer rainfall is lowest in southern lowland regions (140-240 mm), and highest in northern upland regions (240-460 mm). Winter rainfall is again higher in northern upland regions (300-400 mm), with lowest rainfall in central and eastern regions (200-300 mm). Predicted changes in the Northern Ireland climate in the 2020s, 2050s and 2080s are typically that an average year in the 2050s will have 13% less rainfall in the summer, but 9% more in the winter as a result of more intense rainfall events. Mean summer and winter temperatures are expected to increase by 2.2 ℃ and 1.7 ℃ respectively. Agricultural productivity increases are expectedly concomitant to elevated CO2 concentrations and increased winter rainfall associated with climate change, provided variant plant-diseases (e.g. Xylella fastidiosa) are adequately controlled [63]. In Northern Ireland not treating pests and diseases is a likely to cause a net reduction of between 1-10% of crop value ca. £995M, in the next 30-years [64].
Chemical Pesticides Replacement with Biopesticide Alternatives: Cost-Benefit Considerations
The development, regulation and use of biopesticides for integrated pest management has been comprehensively reviewed by [65] and further followed up in AMBER project at Warwick Crop Research Centre, UK [66] and in tandem with AHDB (Agriculture and Horticulture Development Board), UK [67]. In a nutshell, Biopesticides are broadly derived from natural material such as animal, plants, bacteria, and certain minerals (e.g. seaweeds). As of April 2016, there were ca. 299 registered biopesticide active ingredients and ca. 1401 active biopesticide product registration - US Environmental Protection Agency EPA [68] and regulated by EU [69]. Biopesticide offers a more sustainable solution to pest control than synthetic alternative and are heavily favoured in pan Asia and pacific agriculture [70]. A recent review [71] on pesticides, biopesticides and their environmental implications, it is estimated that in the last decade, pesticide sales have been roughly stable worldwide with an overall budget of $40 billion, with the US market accounting for 31.6% of the total. Currently, biopesticides comprise a small share of the total crop protection market globally, with a value of about $3 billion worldwide, accounting for just 5% of the total crop protection market. The reviewer also has outlined the most important characteristics of biopesticides, which are low-risk to environmental impacts (e.g. persistence and residues, generally safer to plants, quicker to market at lower overall cost (e.g. estimated costs within 3-years amounting to $5 million to develop vs. 10-years and $200 million of chemical pesticide and complex modes of action.
The UK climate change predictions of milder, wetter winters, and hotter, drier summers can be expected to add abiotic stress besides biotic (pathogen) impact on cereals [72,73] and tree pathogens [74]. At present, if the tree surveillance measures detect a notifiable tree disease, there is a policy of cut and burn of the affected forest belt area whilst in the case of crops, it is audited pesticide usage [75,76]. Given the above cost margins of vegetation with and without chemical pesticide usage to combat pests and diseases in the medium-longer term, the key challenge lies in searching for cheaper plant and microbial sources as alternative biopesticides to counter the phytopathogens. In the absence of cost of chemical pesticide replacement with biopesticide alternatives to control causal agents of major diseases that threaten the Northern Ireland agri-food and forestry industry, it is nevertheless apparent that such options underpin the bulk of the local gross domestic products. The concept of biostimulants including biopesticides are in its embryonic state in the island of Ireland and at present the major biological crop improvement products are mainly produced by seaweed extracts suppliers [e.g. OileannGlasTeoranta (OGT), http://www.ogt.ie, Bioatlantis http://www.bioatlantis.com/] while our own research upon soil Bacillus species and plant extracts that exhibited in vitro antagonism on dieback oomycete Phytophthora species and fungi Fusarium crop wilt [9] need bulk volume production, commercialization and further protracted field testing.
Economics and cost benefits far outweigh the costs of utilising locally available natural products for meeting plant health requirements. In this mini-review we focus mainly on the above challenges posed for adoption of biological means of achieving phytosanitation, of either tree or crop phytopathogens as an alternative environmentally sustainable strategy in Northern Ireland, as a replacement for the current dependence on chemical means of suppressing the disease causal agent which devastates the limited tree populations and the arable crops and horticulture products. For instance a recent Northern Ireland Forestry Commission Report [77] estimates which amounts to ca. 22K hectares of the woodland that is roughly 6-10% of the land area of Northern Ireland that has a large inland water body (Lough Neagh). This compares fairly lower than that of the UK (13%) within EU whose average woodland covers 37%) [78]. Thus UK is one of the least forested parts of the EU, and in turn Northern Ireland is the least forested part of the UK which further emphasises the need for protection of such limited woodland ecosystems within EU [79] conservation of forest resources and natural protection of bioversity.
Biological Control of Phytophthora ramorum (Sudden Oak Death)
The challenges and opportunities for tree disease protection in Northern Ireland
The greatest benefit is an eco-friendly disease control strategy that avoids chemical fungicides in favour of extracts or antimicrobials from native plants, fungi or soil-sourced bacteria. The total value of forestry and associated wood processing for the island of Ireland is of the order of €2.2 billion. In 2002, a new species of Phytophthora, P. ramorum, had been identified as the causative agent of the death of Oak (Quercus) and Tanoak (Lithocarpus) species in California [80]. Due to its association with Quercus, the popular press had billed it as 'Sudden Oak Death' and it reached epidemic proportions in coastal California. The same species was found in Germany in 2000, associated with nursery-grown Rhododendron, (since then known as an 'alternative host' [81]. P. ramorum reached the British Isles by 2003, infecting amongst others, Beech trees (Fagus sylvatica L.), in the same family, Fagaceae, as Quercus and Lithocarpus (Department for Environment, Food and Rural Affairs (DEFRA), [82]. In Northern Ireland P. ramorum has been associated with commercial plantations of Japanese Larch (Larix kaempferi) and clear-felling of affected local forests has been undertaken as an act of containment, once P. ramorum was confirmed. Thus, the possibility of using native bacteria and plant/fungal extracts were tested under laboratory conditions to control P. ramorum in situ would be greatly beneficial for the environment. The generic ethos and agroecology principles and practice for this objective was that the importance of a locally sourced bacterium, plant or fungal extract being that no further 'exotic' or alien introductions would be made to affected forests in Northern Ireland, albeit biocontrol agents.
The cultures of emerging tree pathogen P. ramorum and related species, were exposed to extracts from a range of local plants and fungi [83]. These, together with inhibitory bacteria isolated from the soil of infected forests, were incorporated into a modified Kirby-Bauer assay, to determine whether it would be possible to find a locally-sourced biocontrol agent for use against P. ramorum. Explants or 6mm diameter agar plugs of Phytophthora cultures were set onto fresh plates of Potato Dextrose Agar (PDA), at specified distances between two 6 mm mast discs soaked in plant or fungal extracts, or else discs impregnated with a broth culture of potentially inhibitory bacteria (dual-culture in vitro assays). Inhibition was measured as outlined previously in [84], using the UVP Bioimaging system and software. Extracts of a native soil-dwelling fungus (Clitocybe nebularis) and two bacteria isolated from local forest soil (Figure 1 Row 1 and Row 2) associated with Japanese Larch (B. licheniformis and B. pumilis) were found to display anti-phytophthoral effects, and encourages future in vivo studies on utilising natural forest soil dwelling antifungal biological agents themselves for sustainable forest disease management. To this end, it is noteworthy that earlier researchers [85] have also isolated a range of microorganisms comprising rod shaped four endospore forming unidentified Bacillus species from composted animal manure and amongst others that were able to significantly inhibit the growth of Phytophthora cinnamomi using dual-culture in vitro assays. However, compared to field trialsof crops with a plethora of biological products for testing, tree phytopathogen biocontrol manipulation is understandably more complex and problematic.
Recent study [86] overviewed the complex web of fungal and oomycete diseases of tropical tree fruit crops to have co-evolved across the agri-food, horticulture and forestry ecosystems. For instancethese reviewers have articulated as to the reality of many tree crops in tropical agriculture (avocado, bananas, citrus, mangoes, cashews, coconut and palm) being grown as perennial monocultures over large areas to meet market demands for nuts, oils or fresh produce. They have further highlighted as to how this agro-forestry lead to a loss of local biodiversity and a double threat of co-evolution of pathogens to follow their hosts to their new environments, and concomitantly render themselves vulnerable to new emerging infectious plant diseases. Undoubtedly plant and pathogen migrations via trade have heightened in the last few decades across agri-food, horticulture including home gardens and in natural forest ecosystems. Notable worldwide examples of devastating diseases emerging from human activities include chestnut blight, Dutch elm disease, sudden oak death, Phytophthora dieback, and ash dieback. It is therefore prudent to have a shared objective of seeking biological solutions for disease management to suppress phytopathogens (e.g. oomycete Phytophthora) in agro-forestry and fungi (e.g. Fusarium banana wilt) in horticulture.
Biological Control of Fusarium oxysporum Crop Wilt Disease
The challenges faced by the Northern Ireland based horticulture industry
On account of the limited geographical arable land area, the land-use and ecology in Northern Ireland has a delicate balance between biodiversity preservation and utilization of land for arable crops such as winter wheat, barley, potato and the remainder for horticulture produce.
In Northern Ireland a consortium of cut-flower growers reported [87] the problems with the soil dwelling phytopathogen F. oxysporum f. sp. matthiola, specific to crops of Brompton Stock (Matthiola incana), whose damages at times > 80% (Figure 1 Row 3G) causing significant economic losses reported in similar stock crops [88] in England. The potato crops encounter mainly bacterial wilt pathogens viz., Erwinia, Ralstonia and Dickeya species, whilst leaf spot/blight diseases such as Microdochium spp., and Drechslera spp [89] are increasingly reported on forage grass varieties of Lolium perenne).
Plausible biological control options and solutions at hand for local horticulturists adoption
The generic global outlook of the vexatious cycles of crop harvest enhancement, pesticides usage moderation and the host/pathogen resistance to chemical control agents over the last three decades, in part have prompted plant scientists to developing an eco-friendly biocontrol strategy for both pre- and post-harvest disease containment situations [90]. To this end such biocontrol measures would also largely benefit the local agriculture and horticulture farmers, and eventually for their sustainability in market demands and trade by moving away from the use of fungicides altogether. (Figure 1, Row 4) illustrates an example of the isolation of a number of Fusarium species from the Northern Ireland growers' soil, [91] and F. oxysporum affected plants. During the isolation of Fusarium, plates carrying mixed populations of fungi and bacteria gave rise to bacteria exhibiting antagonism towards the Fusarium species upon the in vitro assays carried out using plant extracts, the species of bacteria, and inhibitory compounds commercially available to the flower growers. One of these contained another fungus, (Trichoderma viride) as a hyperparasitic biocontrol agent, as reported by [92]. Based on the genome information, the feasibility of using a DNA barcoding approach based on amplicon sequencing to analyse Fusarium species within entire microbial communities was also examined [93]. Including the investigations in Northern Ireland [91], bacterial species viz., B. amyloliquefaciens, B. subtilis and Paenibacillus polymyxa isolated soil infested with Fusarium from proved to be effective suppressors of the crop wilt phytopathogen. Such observations are similar to those of [94] for cereal crops who found a strain of B. subtilis which was able to reduce rhizoplane colonisation of F. verticilloides on Maize roots (Zea mays). Another study [95] reported that B. amyloliquefaciens produced volatile organic compounds (VOCs) that inhibit growth and spore germination of F. oxysporum f. sp. cubense. This gave hope that perhaps a cocktail of the three bacteria could be returned to soil infested with F. oxysporum f.sp. matthioli for an effective biocontrol activity.
Scanning electron microscopy, cultural and 16S rRNA PCR analyses revealed potent native antifungal bacteria Pseudomonas fluorescens, Stenotrophomonas maltophila and P. polymyxa attached to the hyphal surfaces of F. oxysporum as ectosymbionts. These bacterial species are known for prompting fungal virulence regulatory interactions and have concomitant implications for host plants' wilt disease control, via possibly promoted "virulence silencing mechanisms" in F. oxysporum [96]. Furthermore, fungal-fungal, fungal-bacteria and bacteria-bacteria themselves have a molecular dialogue in their host-microbe interactions [97] in plant, animal and human pathogenesis. Some of these interesting aspects in plant pathology have been previously reviewed by [98]. In a recent review, [99] reported that bacterial BCAs that are in use to counter bacterial wilt diseases caused by R. solanacearum and cited a number of studies conducted using the rhizobacterium B. amyloliquefaciens, which showed in vitro antibiosis of R. solanacearum and in studies of biocontrol for tomato bacterial wilt. Amongst the newest reported BCAs was P. polymyxa, [100] for suppression of crown and root rot of field grown wheat.
Conclusions and Future Horizon Scanning for Plant Health and Disease Management Strategy
The promise and the potential of plant- and macrofungi- derived antimicrobials in controlling two phytopathogens viz., a tree pathogen oomycete P. ramorum and a soil saprophytic crop fungal pathogen F. oxysporum is illustrated in (Figure1). The current review is not an exhaustive know-all survey on plant health issues. Nonetheless it is a simplistic approach to portray a local knowledge-based narrative relating to some of the complex phytosanitory matters that challenge the crop and tree scientists' in Northern Ireland. The importance of alternative biological control options for crop and tree diseases are paramount to environmental sustainability of farming, and agroforestry sectors given the backdrop of climate change exacerbated emergent plant pathogens and rising resistance of crops and pathogens to agrochemicals, concomitant toxic residues and farming costs is highlighted in this mini review. Future investigations will focus on evaluation of precision agriculture (PA) involving farming information technology (IT) led tools including evidence based applications (apps) for multi-actor disease management linking local plant health advisory agents with on-farm data loggers (farmers) integrated with remote sensing (i.e. satellite and terrestrial) platforms, geographical information system (GIS) and global position system (GPS) with pluggable add-on sensors primed to offer early detection, monitoring and surveillance of invasive pathogens of risk to Northern Ireland. The precision farm information technology developed can also be utilized in future for automated need based/site-specific use of biostimulants to prime plant for combating biotic (e.g. pathogens, pests) and abiotic (climate change) stress and for implementing point of entry plant trade phytosanitory inspections via platform mounted or hand-held devices.
Acknowledgment
The authors gratefully acknowledge the Department of Agriculture, Environment and Rural Affairs (DAERA), Northern Ireland for facilitating this literature review process through an Evidence and Innovation project 16/3/11, (activity 48125) and the European Commission funding via EU FP7-BIOFECTOR Grant (Agreement No. 312117), administered at Agri-Food & Biosciences Institute (AFBI), (www.afbini.gov.uk) to support plant health research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- (2004) Food agriculture organization of the United Nations. Registration of Biopesticides in Europe and OECD Countries FAO.
- Vidaver AK (2002) Uses of antimicrobials in plant agriculture. Clinical Infectious Diseases 34: S107-S110.
- McManus PS, Stockwell VO, Sundin GW, et al. (2002) Antibiotic use in plant agriculture. Annual Review of Phytopathology 40: 443-465.
- McManus PS, Stockwell VO (2001) Antibiotic use for plant disease management in the United States. Plant Health Progress 2: 14.
- Gullberg E, Cao S, Berg OG, et al. (2011) Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathogens 7: e1002158.
- Bengtsson Palme J, Kristiansson E, Larsson DG J (2017) Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiology Reviews 42: 25.
- Nelson D, Moore JE, Millar BC, et al. (2019) Antimicrobial properties of native macrofungi (mushrooms and toadstools) to clinical pathogens. The Ulster Medical Journal 88: 130-132.
- Nelson DW, Moore JE, Rao JR (2019) Antimicrobial resistance (AMR): Significance to food quality and safety. Food Quality and Safety 3: 15-22.
- Nelson DWVA (2017) Antimicrobials: Novel insights into plant health and biomedical applications. Thesis, PhD, University of Ulster, Northern Ireland, UK.
- Lenzi J, Costa TM, Alberton MD, et al. (2018) Medicinal fungi: A source of antiparasitic secondary metabolites. Applied microbiology and biotechnology 102: 5791-5810.
- Hearst R, Nelson D, McCollum G, et al. (2009) An examination of antibacterial and antifungal properties of constituents of shiitake (Lentinula edodes) and oyster (Pleurotus ostreatus) mushrooms. Complementary Therapies in Clinical Practice 15: 5-7.
- Lee IK, Yun BS (2011) Styrylpyrone-class compounds from medicinal fungi phellinus and inonotus spp., and their medicinal importance. The Journal of Antibiotics 64: 349-359.
- Woods Panzaru S, Nelson D, McCollum G, et al. (2009) An examination of antibacterial and antifungal properties of constituents described in traditional Ulster cures and remedies. The Ulster Medical Journal 78: 13-15.
- Hearst C, McCollum G, Nelson D, et al. (2010) Antibacterial activity of elder (Sambucus nigra) flower or berry against hospital pathogens. Journal of Medicinal Plants Research 4: 1805-1809.
- Selby C, Carmichael E, Sharma HSS (2016) Bio-refining of perennial ryegrass (Lolium perenne): Evaluation of aqueous extracts for plant defence elicitor activity using French bean cell suspension cultures. Chemical and Biological Technologies in Agriculture 3: 253.
- Millar BC, Nelson D, Moore RE, et al. (2019) Antimicrobial properties of basidiomycota macrofungi to Mycobacterium abscessus isolated from patients with cystic fibrosis. International Journal of Mycobacteriology 8: 93-97.
- Compant S, Duffy B, Nowak J, et al. (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology 71: 4951-4959.
- Rattner BA (2009) History of wildlife toxicology. Ecotoxicology 18: 773-783.
- Pal KK, McSpadden Gardener B (2006) Biological control of plant pathogens. The Plant Health Instructor.
- Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: A review. Biotechnology & Biotechnological Equipment 31: 446-459.
- Leclere V, Bechet M, Adam A, et al. (2005) Mycosubtilin overproduction by bacillus subtilis BBG100 enhances the organism's antagonistic and biocontrol activities. Applied and Environmental Microbiology 71: 4577-4584.
- www.pan-europe.info/old/Campaigns/pesticides/documents/loopholes/Directive%2091-414.pdf
- Kotasthane A, Agrawal T, Kushwah R, et al. (2015) In-vitro antagonism of Trichoderma spp. against Sclerotium rolfsii and Rhizoctonia solani and their response towards growth of cucumber, bottle gourd and bitter gourd. European Journal of Plant Pathology 141: 523-543.
- Fuglie KO, Wang SL, Ball VE (2012) Productivity growth in agriculture: An international perspective; CABI: Cambridge.
- Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant and Soil 383: 3-41.
- Sharma HSS, Fleming C, Selby C, Rao, et al. (2014) Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. Journal of Applied Phycology 26: 465-490.
- Saa S, Olivos Del Rio A, Castro S, et al. (2015) Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Frontiers in Plant Science 6: 87.
- Canellas LP, Olivares FL (2014) Physiological responses to humic substances as plant growth promoter. Chemical and Biological Technologies in Agriculture 1: 3.
- Khan W, Rayirath UP, Subramanian S, et al. (2009) Seaweed Extracts as Biostimulants of Plant Growth and Development. Journal of Plant Growth Regulation 28: 386-399.
- Sharma HSS, Selby C, Carmichael E, et al. (2016) Physicochemical analyses of plant biostimulant formulations and characterisation of commercial products by instrumental techniques. Chemical and Biological Technologies in Agriculture 3: 3.
- Maheshwari DK (2011) Bacteria in agrobiology: Plant growth responses. Springer: Heidelberg, New York. 370.
- Mosimann C, Oberhänsli T, Ziegler D, et al. (2017) Tracing of two pseudomonas strains in the root and rhizoplane of maize, as related to their plant growth-promoting effect in contrasting soils. Frontiers in Microbiology 7: 2150.
- Grady EN, MacDonald J, Liu L, et al. (2016) Current knowledge and perspectives of Paenibacillus: A review. Microbial Cell Factories 15: 203.
- Singh RP, Jha PN (2017) The PGPR stenotrophomonas maltophilia SPB-9 augments resistance against biotic and abiotic stress in wheat plants. Frontiers in Microbiology 8: 1985.
- Alabouvette C, Olivain C, Migheli Q, et al. (2009) Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. The New phytologist 184: 529-544.
- Eilenberg J, Hokkanen HMT (2006) An Ecological and Societal Approach to Biological Control, Springer Netherlands: Dordrecht.
- Ndiaye M, J, TA, van Bruggen AHC (2010) Effects of compost amendment and the biological control agent Chlonostachys rosea on the development of charcoal rot (Macrophomina phaseolina) on cowpea. Journal of Plant Pathology 92: 173-180.
- Alvindia DG, Hirooka Y (2011) Identification of clonostachys and trichoderma spp. from banana fruit surfaces by cultural, morphological and molecular methods. Mycology 2: 109-115.
- Santhanam R, van Luu T, Weinhold A, et al. (2015) Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proceedings of the National Academy of Sciences of the United States of America 112: E5013-E5020.
- Tian X, Zheng Y (2013) Evaluation of biological control agents for fusarium wilt in Hiemalis begonia. Canadian Journal of Plant Pathology 35: 363-370.
- Nelson D, Beattie K, McCollum G, et al. (2014) Performance of natural antagonists and commercial microbiocides towards in vitro suppression of flower bed soil-borne Fusarium oxysporum. Advances in Microbiology 4: 151-159.
- Marasco R, Rolli E, Vigani G et al. (2013) Are drought-resistance promoting bacteria cross-compatible with different plant models?. Plant Signaling & Behavior 8: e26741.
- Timmusk S, Abd El Daim IA, Copolovici L, et al. (2014) Drought-Tolerance of Wheat Improved by Rhizosphere Bacteria from Harsh Environments: Enhanced Biomass Production and Reduced Emissions of Stress Volatiles. PLoS One 9: e96086.
- Da Silva SF, Olivares FL, Canellas LP (2017) The biostimulant manufactured using diazotrophic endophytic bacteria and humates is effective to increase sugarcane yield. Chemical and Biological Technologies in Agriculture 4: 24.
- Canellas LP, Balmori DM, Médici LO, et al. (2013) A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant and Soil 366: 119-132.
- Du Jardin P (2015) Plant biostimulants: Definition, concept, main categories and regulation. Scientia Horticulturae 196: 3-14.
- Halpern M, Bar Tal A, Ofek M, et al. (2015) The Use of Biostimulants for Enhancing Nutrient Uptake. Elsevier 130: 141-174.
- La Torre A, Battaglia V, Caradonia F (2016) An overview of the current plant biostimulant legislations in different European Member States. Journal of the Science of Food and Agriculture 96: 727-734.
- Anon (2012) Personal communications; College of Agriculture Food Rural Enterprise www.cafre.ac.uk: Greenmount Co. Antrim Northern Ireland.
- Garibaldi A, Gilardi G, Gullino ML (2002) First report of fusarium oxysporum on lettuce in europe. Plant Disease 86: 1052.
- Vágány V (2012) Characterisation of fusarium pathogens in the UK; University of Warwick: Coventry, 278.
- Taylor A (2018) Technical review on lettuce Fusarium wilt, caused by Fusarium oxysporum f. sp. lactucae. Final Report: SCEPTREplus Project, February 2018. Agriculture and Horticulture Development Board (AHDB), UK.
- Anon (2019) Tropical Race 4 (TR4) of Fusarium wilt (Fusarium oxysporum f.sp. cubense).
- Belanger RR (2006) Controlling disease without fungicides: A new chemical warfare. Canadian Journal of Pathology 28: 233-238.
- Anon (2011) UK National Ecosystem Assessment: Technical report; United Nations environment programme world conservation monitoring centre: Cambridge.
- Anon (2015) Agriculture in the United Kingdom: Produced by department for environment, food and rural affairs (DEFRA, UK), Department for Agriculture and Rural Development (Northern Ireland), Welsh Assembly Government, The Department for Rural affairs and Heritage, The Scottish Government, Rural and Environment Research and Analysis Directorate.
- (2014) The handbook of plant biosecurity: Principles and Practices for the Identification, Containment and Control of Organisms that Threaten Agriculture and the Environment Globally. Gordh G, McKirdy S, Eds. Springer Netherlands: Dordrecht.
- Anon (1994) EPPO Standard PP 2/1(1) Guideline on good plant protection practice: principles of good plant protection practice. Bulletin OEPP/EPPO Bulletin 24: 233-240.
- Cooke LR, Fleming CC, Mc Cracken AR (2013) Efficacy of biocides, disinfectants and other treatments to limit the spread of ash dieback caused by Chalara fraxinea: Review for DARD E&I Project 12.3.S7.
- Quinn L, O'Neill PA, Harrison J, et al. (2013) Genome-wide sequencing of Phytophthora lateralis reveals genetic variation among isolates from Lawson cypress (Chamaecyparis lawsoniana) in Northern Ireland. FEMS Microbiology Letters 344: 179-185.
- Fleming CC, Entwistle K, Fleming T, et al. (2014) Biosecurity and emerging plant health problems in turf production and maintenance. European Journal of Horticultural Science 79: 108-115.
- www.gov.uk/government/organisations/department-for-environment-food-rural-affair.
- https://www.gov.uk/government/publications/uk-climate-change-risk-assessment-2017.
- http://ukclimateprojections.metoffice.gov.uk.
- Chandler D, Bailey AS, Tatchell GM, et al. (2011) The development, regulation and use of biopesticides for integrated pest management. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 366: 1987-1998.
- https://warwick.ac.uk.
- https://horticulture.ahdb.org.uk/benefits-and-drawbacks-biopesticides
- Seiber JN, Coats J, Duke SO, et al. (2014) Biopesticides: State of the art and future opportunities. Journal of Agricultural and Food Chemistry 62: 11613-11619.
- Villaverde JJ, Sevilla Morán B, Sandín España P, et al. (2014) Biopesticides in the framework of the European Pesticide Regulation (EC) No. 1107/2009. Pest Management Science 70: 2-5.
- Watts M, Williamson S (2015) Replacing chemicals with biology: Phasing out highly hazardous pesticides with agroecology. Pesticide Action Network Asia and the Pacific: [George Town], Penang, Malaysia 210.
- AL Ahmadi MS (2019) Pesticides, anthropogenic activities, and the health of our environment safety. In Pesticides, Anthropogenic Activities and the Health of our Environment, Intech Open.
- Doohan F, Brennan JM, Cooke BM (2003) Influence of climatic factors on Fusarium species pathogenic to cereals. European Journal of Plant Pathology 109: 755-768.
- Newton AC, Johnson SN, Lyons GD, et al. (2008) Impacts of climate change on arable crops-adaptation challenges. Proceedings Crop Protection in Northern Britain.
- Englander L, Browning M, Tooley PW (2006) Growth and sporulation of Phytophthora ramorum in vitro in response to temperature and light. Mycologia 98: 365-373.
- Barker I, Mace A, Parrish G, et al. (2018) Pesticide usage report.
- Garthwaite D, Hudson S, Barker I, et al. (2017) Grassland and fodder crops in the United Kingdom. Pesticide Usage Survey Report 255.
- Allen M (2013) Overview of plant health and biosecurity in Northern Ireland. Northern Ireland Assembly, Research and Information Service Briefing paper NIAR 53-13.
- Woodland Trust (2019) The State of the UK's Forests, Woods and Trees.
- Anon (2012) Interim Report, Tree Health & Plant Biosecurity Expert Taskforce.
- Rizzo DM, Garbelotto M, Davidson JM, et al. (2002) Phytophthora ramorum as the Cause of Extensive Mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Disease 86: 205-214.
- Werres S, Marwitz R, Man In't veld WA, et al. (2001) Phytophthora ramorum sp. nov., a new pathogen on Rhododendron and Viburnum. Mycological Research 105: 1155-1165.
- Anon (2006) Phytophthora ramorum. EPPO Bulletin 36: 145-155.
- Hearst C, Nelson D, McCollum G, et al. (2013) Forest fairy ring fungi (Clitocybe nebularis), soil Bacillus species and plant extracts exhibit in vitro antagonism on dieback Phytophthora species. Natural Resources 4: 189-194.
- Moore JE, McCollum G, Murphy A, et al. (2010) Assessment of inhibition/growth-promoting properties of new agents on moulds: Description of a simple bio-imaging technique. British Journal of Biomedical Science 67: 145-146.
- Aryantha NP, Guest DI (2006) Mycoparasitic and antagonistic inhibition on phytophthora cinnamomi rands by microbial agents isolated from manure composts. Plant Pathology Journal 5: 291-298.
- Drenth A, Guest DI (2016) Fungal and oomycete diseases of tropical tree fruit crops. Annual Review of Phytopathology 54: 373-395.
- www.afbini.gov.uk.
- O'Neill TM, Shepherd A, Inman AJ, Lane CR (2004) Wilt of stock (Matthiola incana) caused by Fusarium oxysporum in the United Kingdom. Plant Pathology 53: 262.
- Archer J (2014) The assessment of Lolium perenne L F2 clones for Drechslera siccans disease resistance and susceptibility using rapid analytical techniques. PhD Thesis, Queen's University, Belfast, UK.
- Wisniewski M, Droby S, Norelli J, et al. (2016) Alternative management technologies for postharvest disease control: The journey from simplicity to complexity. Postharvest Biology and Technology 122: 3-10.
- (2018) Alternative pesticide active microbiological sources for pest and disease control DAERA Evidence and Innovation project Report.
- Dubey SC, Suresh M, Singh B (2007) Evaluation of Trichoderma species against Fusarium oxysporum f. sp. ciceris for integrated management of chickpea wilt. Biological Control 40: 118-127.
- https://horticulture.ahdb.org.uk/sites/default/files/research_papers/POBOF%20452_Report_Annual_2018.pdf.
- Cavaglieri L, Orlando J, Rodríguez MI, et al. (2005) Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Research in Microbiology 156: 748-754.
- Yuan J, Raza W, Shen Q, et al. (2012) Antifungal activity of Bacillus amyloliquefaciens NJN-6 volatile compounds against Fusarium oxysporum f. sp. cubense. Applied and Environmental Microbiology 78: 5942-5944.
- Minerdi D, Moretti M, Gilardi G, et al. (2008) Bacterial ectosymbionts and virulence silencing in a Fusarium oxysporum strain. Environmental Microbiology 10: 1725-1741.
- Frey Klett P, Burlinson P, Deveau A, et al. (2011) Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiology and Molecular Biology Reviews 75: 583-609.
- (2009) Disease control in crops: Biological and environmentally friendly approaches. Walters D, (Edn) Wiley-Blackwell: Oxford, UK.
- Nion Y, AsiY, Toyota K (2015) Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes and Environments 30: 1-11.
- Lounaci L, Guemouri Athmani S, Boureghdea H, et al. (2016) Suppression of crown and root rot of wheat by the rhizobacterium Paenibacillus polymyxa. Phytopathologia Mediterranea 55: 355-365.
Corresponding Author
Prof. Juluri R Rao, Sustainable Agri-Food Sciences Division, Agri-Food & Biosciences Institute, Newforge Lane, Belfast BT9 5PX, UK, Tel: 028-90-255258, Fax: 028-90-255007.
Copyright
© 2020 Rao JR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.