A Novel Nutrient Mixture Induces Apoptosis in Human Ovarian and Cervical Cancer Cells
Abstract
Cervical cancer and ovarian cancer are the deadliest gynecological malignancies and are the fourth and fifth leading causes of death in women respectively. The 5-year survival rate of patients has dropped to 15-20% when these cancers metastasize to distant organs. A unique formulation of nutrients containing green tea extract, ascorbic acid, lysine and proline, has exhibited anticancer effects in various cancers. In our earlier in vivo studies, the nutrient mixture significantly, reduced tumor weight and tumor burden of ovarian and cervical cancers. Based on those results, we studied weather, the antitumor effects are due to induction of apoptosis in these cancers. Cervical cancer cell like (HeLa) and ovarian cancer cells line (SK-OV-3) were cultured in DME media and cells were treated with the nutrient mixture at 0-1000 µg/ml doses. Cell proliferation was measured by MTT assay, morphology was studied by H&E and apoptosis was studied by Live Green Caspase analysis. At 100 µg/ml, the nutrient mixture showed some toxicity to HeLa cells, which considerably increased at 500 µg/ml. However, the nutrient mixture did not affect SK-OV-3 cell growth. H&E staining showed a very similar apoptotic pattern for both the cell lines. HeLa cells and SK-OV-3 cells treated with the nutrient mixture exhibited several characteristics of apoptosis such as shrinkage of cytoplasm, and densely staining nuclei. Both early and late apoptosis were detected moderately at 100 µg/ml, and significant at 1000 µg/ml. These results suggest that the nutrient mixture has therapeutic potential in the treatment of cervical and ovarian cancer by inducing apoptosis of cells.
Keywords
Cervical cancer, Ovarian cancer, HeLa, SK-OV-3, Apoptosis, Caspase
Introduction
The American Cancer Society estimated approximately 12,990 new cases of invasive cervical cancer will be diagnosed in 2016 in the US, and it is predicted that 4,120 women will die from cervical cancer. Some of the risk factors for cervical cancer are infection with Human Papilloma Virus (HPV), teenage pregnancy, multiple pregnancies, and extended use of hormonal contraceptive pills. The availability and reliability of PAP smears has considerably reduced the incidence and mortality rate from cervical cancer; nevertheless, cervical cancer is the leading cause of death from cancer in women [1].
On the other hand, ovarian cancer is the deadliest gynecological cancer and the fifth leading cause of death of women in the USA. Ovarian cancer is also termed as silent killer because there are no obvious signs or symptoms for early diagnosis. Majority of patients are diagnosed at advanced stages of the disease with cancer already metastasized in the intraperitoneal region. According to the American Cancer Society's 2016 estimates, approximately 21,000 women will be diagnosed with ovarian cancer in the US. Women over 60, those who are obese, and have taken hormone treatments for fertility or menopause are at a higher risk of developing ovarian cancer. A woman's risk of developing ovarian cancer increases if a biological relative has or had ovarian or breast cancer, as it is likely that they would share an inherited genetic mutation associated with the BRCA gene. There are different types of ovarian cancers depending on the cell of origin, and the most common type of ovarian cancer arises from the cells lining the ovaries (epithelial) [2].
Despite advances, the standard treatment methods of surgery, radiation, and chemotherapy have failed to effectively treat cervical and ovarian cancer that has already metastasized. The 5-year survival rate of metastasized cervical and ovarian cancer is merely 15-20% [2]. Due to the limitation of current treatment modalities, there is an urgent need for a safe and effective therapeutic approach. Due to their antitumor activity, which includes induction of apoptosis, a number of plant-based phytochemicals are increasingly being used as important treatment of cancers.
Consumption of plant-based materials have shown to decrease the cancer development and progression. We have developed strategies to inhibit cancer development and its spread using a unique combination of naturally occurring nutrients. The antitumor nutrient mixture (NM) combination containing lysine, proline, ascorbic acid, and green tea extract has demonstrated anti-carcinogenic activity against a number of cancer cell lines. In our earlier in vivo and in vitro studies, the NM has significantly inhibited the tumor weight and tumor burden in cervical and ovarian cancer [3-5]. In this study, we investigated whether the anticancer effects of the NM are due to induction of apoptosis in these cancers using HeLa and SK-OV-3 cell lines.
Materials and Methods
Composition of the nutrient mixture
The stock solution of the NM (total weight 4.4 g) consists of: Vitamin C (as ascorbic acid and as Magnesium ascorbate, Calcium ascorbate and Palmitate ascorbate) 700 mg, L-lysine 1000 mg, L-proline 750 mg, L-arginine 500 mg, N-acetyl cysteine 200 mg, Standardized green tea extract 1000 mg (from green tea leaves obtained from US Pharma Lab with total polyphenol 80%, Catechins 60%, Epigallocatechin gallate [EGCG] 35%, and Caffeine 1%), Selenium 30 µg, Copper 2 mg, Manganese 1 mg.
Cancer cell lines and culture
The Cervical cancer cell line (HeLa), and Ovarian cancer cell line (SK-OV-3) were obtained from ATTCC (American Type Culture Collection, Rockville, MD, USA). The cells were cultured in complete media, consisting of 10% FBS, and antibiotics. When the cells reached confluency, they were treated with NM in triplicate, at concentrations of 0, 100, 500, and 1000 µg/ml.
MTT assay
MTT assay was carried out as described earlier [6]. Briefly, cell suspensions were plated in 24-well tissue culture plates (Nunc, Denmark) at the density of 3 × 104 cells/well. When the cells were near confluence, they were treated with NM for 24 hours at 37 ℃ in a humidified incubator, the cells were treated with the NM at concentrations of 0, 100, 500 and 1000 μg/ml for 24 hours and the viability was determined with the MTT assay reagent (Sigma No. M-2128-0.5 mg/ml in media) was added to each well followed by 2-hour incubation at 37 ℃. Following incubation, the solution was carefully aspirated from the wells, the formazan product was dissolved in 1 ml DMSO, and the absorbance (OD) was measured on a microplate reader at a wavelength of 570 nm in a BioSpec 1601 Shimadzu spectrometer. The OD 570 of the DMSO solution in each well was considered to be proportional to the number of cells.
Apoptosis and live green caspase assay
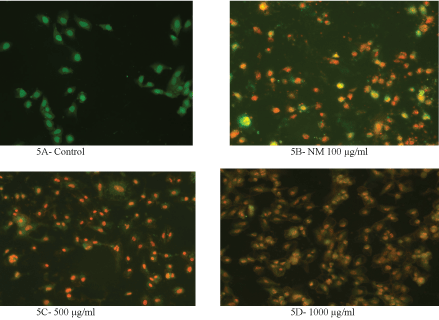
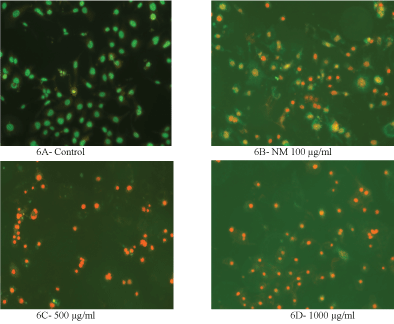
The cells were grown to near confluence and treated with the NM dissolved in media at 0, 100, 500 and 1000 μg/ml for 24 hours. The media was removed and was washed with PBS and treated with the caspase reagent as specified in the manufacturer's instructions (Molecular Probes Image-IT Live Green Poly Caspases Detection Kit 135104, Invitrogen). The cells were photographed under the fluorescence microscope and counted. Green colored cells represent viable cells, orange and red colors represent early and late apoptotic cells, respectively.
H&E staining
The cells were cultured in 24-well plates and were treated with NM in test concentrations of 0, 100, 500 and 1000 μg/ml. After 24-hour incubation, the cells were washed with PBS, fixed with methanol, and then stained with haematoxylin and eosin (H&E), and images were captured by microscope.
Statistical analysis
The results were expressed as mean ± standard deviation (SD) for the groups. Data was analyzed by the independent t-test.
Results
Morphology and viability study
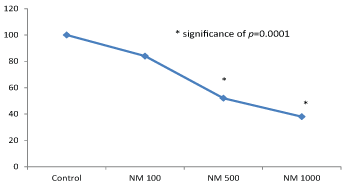
NM exhibited slight toxicity to HeLa cells at 100 µg/ml, and considerable at 500 and 1000 µg/ml. As can be seen in (Figure 1), the results showed a significant dose dependent decrease with 84% viable cells at 100 μg/ml NM, 52% at 500 μg/ml NM, and 38% at 1000 μg/ml, respectively, as compared to the untreated control group.
In contrast, NM showed slight insignificant effect on ovarian cancer cell growth as shown in (Figure 2).
H&E staining
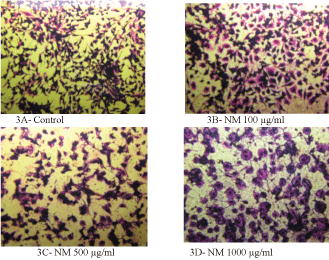
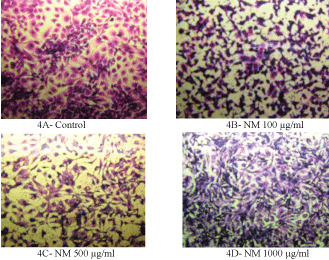
H&E staining revealed a similar apoptotic pattern for both HeLa cells and SK-OV-3 cell lines in dose dependent manner at 0, 100, 500, and 1000 µg/ml of NM. HeLa cells treated with NM showed characteristic morphological changes such as the shrinkage of the cytoplasm, and darkly stained nuclei with intensely acidophilic cytoplasm. These changes were dose dependent, increasing in intensity with increasing NM concentrations as shown in (Figure 3) and (Figure 4) (A-D).
Apoptosis
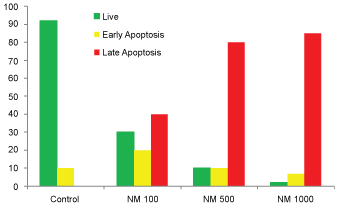
Analysis using the Live Green Caspase exhibited a dose dependent changes characteristic of apoptosis in both HeLa and SK-OV-3 cells, with a slight apoptosis found at 100 μg/ml, moderate apoptosis at 500 μg/ml, and severe apoptosis at 1000 μg/ml. As seen in (Figure 5) (A-E), quantitative analysis of the data revealed the percentage of apoptotic HeLa cells to be 20% at 100 μg/ml (in early phase apoptosis), 10% at 500 μg/ml (in late phase apoptosis), and 95% for 1000 μg/ml (10% in early phase apoptosis and 85% in late phase). It was observed that there was a corresponding decrease in percentage of live cells and an increase in percentage of cells in late apoptosis with increasing NM concentrations.
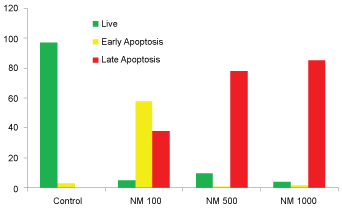
Similarly, the quantitative analysis of apoptotic data of SK-OV-3 cells showed 58% cells in early phase apoptosis, and 30% in late phase apoptosis. The percentage of apoptotic cells at 500 µg/ml of NM was 82% (2% in early phase apoptosis, and 80% in late phase apoptosis), and at 1000 µg/ml of NM 90% (5% in early phase apoptosis, and 85% in late phase of apoptosis) were noted, as shown in (Figure 6A, Figure 6B, Figure 6C, Figure 6D, and Figure 6E).
Discussion
While cervical cancer can be detected early using PAP smear screening, there is no effective screening method available for ovarian cancer. The signs and symptoms of ovarian cancer are very vague in the beginning. In more than 75% of all diagnosed ovarian cancer cases, metastasis is already present at the time of diagnosis. Therefore, ovarian cancer tends to be diagnosed at advanced stages of the disease, when it remains incurable. These cancers are usually treated by surgery, chemotherapy, and radiation; yet, there is no significant benefit with regards to survival in cases of metastatic disease.
We have developed therapeutic strategies to inhibit cancer growth and its progression by inhibiting invasion, metastasis, and inducing apoptosis by using an innovative combination of synergistic nutrients that include natural substances like ascorbic acid, lysine, proline and green tea extract. It was postulated that this nutrient combination exerts potential synergistic anticancer activity rather than using a single nutrient.
The nutrient mixture (NM) used in the study was specifically developed to combine the individual anti-tumorigenic and pro-apoptotic properties of the component micronutrients. The inhibitory effects of the individual nutrients comprising the novel nutrient formulation have been reported in both experimental and clinical studies.
Recently, more attention has been paid to anticancer activity by inducing apoptosis and a number of plant-based materials and phytochemicals have shown anticancer activity by inhibiting proliferation, invasion, metastasis, and by inducing apoptosis. Ascorbic acid is well known for its antioxidant and free radical scavenging functions and detoxification of xenobiotics [7]. Previous studies have proven that the action of ascorbic acid in cancer prevention, [8-11] includes its role in collagen synthesis, basement membrane integrity and hyaluronidase inhibition [12,13] which may be important in inhibiting tumor spread and micrometastases. Ascorbic acid has also been linked to the induction of apoptosis in cervical cancer and increasing sensitivity of HeLa cells to chemotherapy drugs by stabilizing the P53 gene [14]. Green tea catechins, including epigallocatechin gallate (EGCG), have been proven to be chemo preventive agents in vitro and in many in vivo animal models of induced carcinogenesis [15,16]. EGCG alone is also a potent anti-cancer agent and has been reported to have a growth inhibitory effect against human cancer cell lines [17-19]. Moreover, EGCG also down regulated the expression of an integral membrane protein (aquaporin), and inhibited cell proliferation and induced apoptosis in ovarian and cervical cancer cells by modifying the expression of MMP-9 enzymes and their tissue inhibitors, TIMP-1 [20,21].
However, it has been observed in earlier studies that a specific combination of substances such as EGCG, ascorbic acid, and proline and lysine, show anticancer effect which is much more pronounced in combination than any of the individual nutrients alone. [22] NM has also been substantiated by our other cancer studies that demonstrate its efficacy in inhibiting MMP expression and invasion and migration of HeLa and SK-OV-3 cells [4,5]. We have shown that NM can significantly inhibit ovarian cancer cell (SK-OV-3) invasion by blocking expression of cancer biomarkers-matrix metalloproteinase (MMP) enzymes and enhancing the structure and integrity of the extracellular matrix. We also tested the effects of NM in vivo and in vitro on cancer cell progression, tumor growth, and MMP expression on ovarian cancer cell line ES-2. NM was shown to inhibit tumor weight and tumor burden of ES-2 tumors by 59.2% (p < 0.0001) and 59.7% (p < 0.0001), respectively. In vitro, NM also inhibited expression of MMP-2 enzymes [3].
Although fully treatable in the early stages, patient outcome is poor once cervical cancer has metastasized. Our previous in vivo and in vitro studies have indicated that NM was able to inhibit growth of HeLa cells in athymic nude mice. In those studies, the tumor growth was evaluated by measuring cancer markers such as Ki67 (proliferation); matrix metalloproteinase (MMP) -2, -9 (invasion/metastasis); vascular endothelial growth factor (VEGF) (angiogenesis) and others, was seen to be significantly reduced [21].
Our current studies show that the NM has inhibited growth of cervical cancer (HeLa) cells in dose dependent manner with more efficacy observed at higher concentrations. NM had no significant effect on proliferation of ovarian cancer (SK-OV-3) cells. Both HeLa and SK-OV-3 cells showed similar morphological changes pertaining to apoptosis such as shrinkage of cytoplasm, acidophilic cytoplasm, and condensation of nuclei in dose dependent manner.
Activation of caspase enzymes is a characteristic feature of the early stages of apoptosis, which participates in a series of reactions in response to pro-apoptotic signals and result in the cleavage of protein substrates and in the subsequent disassembly of the cell. Increased caspase activity was detected upon treatment with 100, 500 and 1000 μg/ml of NM in a dose dependent fashion, which progressed from moderate changes of apoptosis at 100 μg/ml, to significant (85-90%) apoptosis at 500 and 1000 μg/ml NM concentration.
Furthermore, NM has also proven to be a safe therapeutic agent in vivo as well. Our other studies have shown that MN was ineffective in inducing enzymes in vital organs such as heart, kidney and liver, even at high concentrations. This shows that NM is non-toxic [23]. Thus, treatment with NM can serve as a multipronged approach to target female gynecological cancers.
In conclusion, these results suggest that the NM has therapeutic potential in the treatment of cervical and ovarian cancer by inducing apoptosis.
Acknowledgements
The research study was funded by Dr. Rath Foundation (Santa Clara, CA, USA) a non-profit organization.
References
- https://www.cancer.org/cancer/cervical-cancer.html.
- https://www.cancer.org/cancer/ovarian-cancer.html.
- Roomi MW, Kalinovsky T, Niedzwiecki A, et al. (2016) A nutrient mixture modulates ovarian ES-2 cancer progression by inhibiting xenograft tumor growth and cellular MMP secretion, migration and invasion. Int J Clin Exp Med 9: 814-822.
- Roomi MW, Ivanov V, Kalinovsky T, et al. (2006) Inhibition of matrix metalloproteianse-2 secretion and invasion by human ovarian cancer cell line SK-OV-3 with lysine, proline, arginine, ascorbic acid, and green tea extract. J Obstet Gynaecol Res 32: 148-154.
- Roomi MW, Monterrey JC, Kalinovsky T, et al. (2010) In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers, and inhibitors. Oncol Rep 23: 605-614.
- Roomi MW, Shanker N, Niedzwiecki A, et al. (2015) Induction of apoptosis in the human prostate cancer cell line DU-145 by a novel micronutrient formulation. Open Journal of Apoptosis 4.
- Hodges RE (1980) Ascorbic acid. In: Goodhart RS, Shils ME, Modern nutrition in health and disease. (6th edn), PA: Lea and Febiger, Philadelphia, 259-273.
- Levine M (1986) New concepts in the biology and biochemistry of ascorbic acid. N Engl J Med 314: 892-902.
- Block G, Menkes M (1989) Ascorbic acid in cancer prevention. In: Moon TE, Micozzi MS, Diet and cancer prevention. Investigating the role of micronutrients. Marcel Dekker, Inc, New York, 341-388.
- Block G (1991) Vitamin C and cancer prevention: the epidemiologic evidence. Am J Clin Nutr 53: 270S-282S.
- Hanck AB (1988) Vitamin C and cancer. Progress in Clinical and Biological Research 259: 307-320.
- Cameron E, Pauling L (1973) Ascorbic acid and the glycosaminoglycans: An orthomolecular approach to cancer and other diseases. Oncology 27: 181-192.
- Cameron E, Pauling L (1974) The orthomolecular treatment of cancer I. The role of ascorbic acid in host resistance. Chemico-Biological Interactions 9: 273-283.
- Zhang Z, Liu X, Wu T, et al. (2011) Selective suppression of cervical cancer Hela cells by 2-O-β-D-glucopyranosyl-L-ascorbic acid isolated from the fruit of Lycium barbarum L. Cell Biol Toxicol 27: 107-121.
- Liao S, Kao YH, Hiipakka RA (2001) Green tea: biochemical and biological basis for health benefits. Vitam Horm 62: 1-94.
- Liao S, Umekita Y, Guo J, et al. (1995) Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett 96: 239-243.
- Valcic S, Timmerman BN, Alberts DS, et al. (1996) Inhibitory effects of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticancer Drugs 7: 461-468.
- Mukhtar H, Ahmed N (2000) Tea polyphenols: Prevention of cancer and optimizing health. Am J Clin Nutr 71: 1698S-1702S.
- Yang GY, Liao J, Kim K, et al. (1998) Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 19: 611-616.
- Yan C, Yang J, Shen L, et al. (2012) Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch Gynecol Obstet 285: 459-467.
- Roomi MW, Kalinovsky T, Cha J, et al. (2015) Effects of a nutrient mixture on immunohistochemical localization of cancer markers in human cervical cancer HeLa cell tumor xenografts in female nude mice. Exp Ther Med 9: 294-302.
- Netke SP, Roomi MW, Ivanov V, et al. (2003) A specific combination of ascorbic acid, lysine, proline and epigallocatechin gallate inhibits proliferation of extracellular matrix invasion of various human cancer cell lines. Research communications in Pharmacology and Toxicology: Emerging Drugs 2: 37-50.
- Roomi MW, Ivanov V, Netke S, et al. (2003) Serum markers of the liver, heart, and kidney and lipid profile and histopathology in ODS rats treated with nutrient synergy. Journal of the American College of Nutrition 22: 477.
Corresponding Author
Aleksandra Niedzwiecki, Oncology Department, Dr. Rath Research Institute, USA, 1260 Memorex Drive, Santa Clara, CA-95050, USA, Tel: 408-807-5564.
Copyright
© 2018 Roomi MW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.