Neoadjuvant Chemotherapy with Paclitaxel and Nedaplatin plus Radical Surgery in Cervical Cancer during Pregnancy: A Case Report and Review of the Literature
Abstract
Introduction
Cervical cancer in the 3rd trimester of pregnancy is rare and the management is thorny. The disease control and fetal outcome are cautiously considered by the clinicians.
Case presentation
A 36-year-old Han Chinese female with cervical cancer staged as IB1 at the gestational age of 30 + 3 weeks was referred to our department. The patient and the family refused to term pregnancy and wanted pregnancy-preserving management, she was treated by neoadjuvant chemotherapy with paclitaxel 230 mg plus nedaplatin 120 mg first, and caesarean section; radical hysterectomy and pelvic lymph node dissection were performed three weeks later. Based on the pathology report, the patient underwent further radiotherapy, and the neonate was healthy.
Conclusions
The patient with cervical cancer diagnosed in the 3rd trimester of pregnancy can be performed with neoadjuvant chemotherapy (Paclitaxel/nedaplatin) before operation, while the proper chemotherapy doses must be carefully studied considering the pharmacokinetic alteration during pregnancy and the adverse effects for the pregnant patient and the fetus. Thus, further investigation is required regarding the safety and outcome.
Keywords
Cervical cancer, Pregnancy, Neoadjuvant chemotherapy
Abbreviations
NACT: Neoadjuvant Chemotherapy; CIN: Cervical Intraepithelial Neoplasia; CL: Clearance; RSE: Relative Standard Error; BDP: Biparietal Diameter; FL: Femoral Length; IMRT: Intensity-Modulated Radiation Therapy
Introduction
Cervical cancer is one of the most common tumors during pregnancy with the incidence from 1.6 to 10.6 per 10,000 pregnancies [1,2]. Owing to the scarcity of cases reported in the literature, most of gynecological oncologists are still confused about the standard management of the pregnant patients with cervical cancer [1,2]. Here, we reported a case of cervical cancer staged at IB1 diagnosed in the 3rd trimester of pregnancy treated by neoadjuvant chemotherapy (NACT) once, the regimen was nedaplatin (80 mg/m2) and paclitaxel (150 mg/m2), and then performed a caesarean section with radical hysterectomy plus bilateral pelvic lymphadenectomy 3 weeks later. After that, the patient received concurrent chemo radiation therapy (CCRT) for the lymph-vascular space invasion (LVSI). By now, the patient shows no evidence of recurrence, and the neonate was healthy.
Case Report
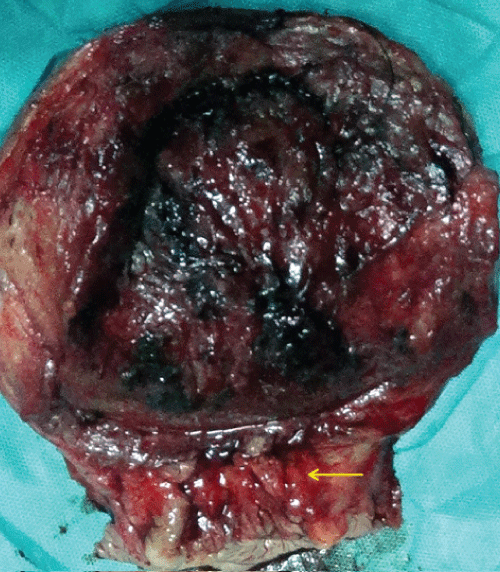
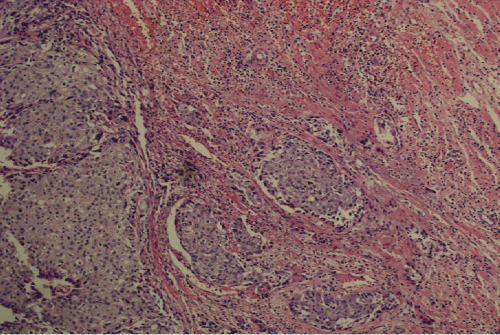
A 36-year-old female of Chinese Han nationality at the gestational age of 30 + 3 weeks, gravid 4, para 0, without the history of family hereditary, smoking and drinking, presented an invasive squamous cervical carcinoma. The patient complained intermittent vaginal spotting 2 years ago without any physical examination and treatment, and has a history of vaginal bleeding one month ago. She was sent to our hospital and diagnosed by colposcopy biopsy. Pelvic examination revealed a 3.5 cm cervical mass without parametrial or vaginal involvement. An ultrasound scan revealed a live intrauterine pregnancy of size consistent with gestational age, and normal fetal anatomy (Biparietal diameter 76 mm and femoral length 56 mm). The patient was staged by two gynecological oncology specialists as cervical cancer IB1 (FIGO 2014). After a complete consultation, the patient and her family refused to term pregnancy and wanted pregnancy-preserving management, they agreed with neoadjuvant chemotherapy with paclitaxel 230 mg plus nedaplatin 120 mg (body surface area = 1.58 m2). Three weeks later she came back for operation and found tumor decrease (3 cm mass) and liver dysfunction such as higher ALT, AST, TBIL, and DBIL as listed in table 1. The BDP was 84 mm, and the FL was 64 mm according to the ultrasonic examination. Glucocorticoid therapy (Dxm 10 mg, 5 times before operation) was administered for fetal lung maturation. We also administered 20 mg vitamin K1 and the pharmacies for liver function protection before operation. The patient had a history of vaginal delivery 9 years before, and wanted no more children. Thus, caesarean section, radical hysterectomy and pelvic lymph node dissection were performed at 34 + 3 weeks' gestation. A female infant weighing 2,030 g with Apgar score at 1 and 5 min of 9' and 9' was delivered. The infant was healthy and transferred to the preterm birth ward. The final pathological report on the hysterectomy specimen (Figure 1) indicated that non-keratinizing squamous cervical cancer (Figure 2), neither infiltration of parametrium and nor the resection margin of the vaginal involvement was found, the 16 left and 12 right pelvic lymph nodes were free of disease, both lymph-vascular and the serous layer of the cervix were involved. The patient underwent further radiotherapy with Intensity-Modulated Radiation Therapy (IMRT), the target included total pelvic lymph nodes, vaginal cuff and parametria tissue, 95% PTV 5040cGy/28f/5.5w.
Discussion
The currently recommended neoadjuvant chemotherapy (NACT) regimen for cervical cancer is platinum-based chemotherapy (cisplatin 75 mg/m2), preferably with paclitaxel (175 mg/m2) at a 3-week interval [2-4]. However, the evaluation of the efficacy and safety of NACT in pregnancy is difficult for the rarity of reports and less clinical trial; we have considered all of the data and publications available about NACT during pregnancy in the Pubmed (Table 2). We chose nedaplatin (80 mg/m2) and paclitaxel (150 mg/m2) for chemotherapy in view of the heart overload during pregnancy and the anemia for this patient (Table 1). It has not been reported about the chemotherapy with nedaplatin during pregnancy before, which has the benefit of without need of hydration, less nausea, vomiting and nephrotoxicity than other platinum-containing drugs. The fetus did not show intrauterine growth restriction after chemotherapy. Unfortunately, the patient showed damage to liver function three weeks after chemotherapy (Table 1).
The physiologic alterations during pregnancy have an effect on the most important pharmacokinetic processes including absorption, distribution, metabolism, and excretion. Alterations in drug metabolism begin at 4 weeks of gestation, progressively increase, and are more pronounced in the third trimester of pregnancy [22]. Pharmacokinetic analysis has shown that no gestational effect could be estimated on clearance (CL) for doxorubicin; for epirubicin, docetaxel and paclitaxel, a fold-change of 1.1 (relative standard error, RSE 9%), 1.19 (RSE 7%) and 1.92 (RSE 21%) were respectively, estimated on CL. Calculated dose modification requirements for doxorubicin, epirubicin, docetaxel and paclitaxel were + 5.5%, + 8.0%, + 16.9% and + 37.8%, respectively [23]. It has not been reported about the CL for nedaplatin, and we chose the common doses for the patient without pregnancy. However, the adverse effects of chemotherapy with the adjustment doses should also be considered, and it needs further study and more clinical data.
This patient was diagnosed as cervical cancer at gestation of 30 + 3 weeks. At this 3rd trimester of pregnancy, a complete pelvic and paraaotic lymphadenectomy is difficult to perform; therefore, the management cannot rely on the nodal status. Although the neoplasm diameter was 3.5 cm, the patient refused to term pregnancy and wanted to pregnancy-preserving management. We performed NACT and find the tumor decrease before operation.
A good number of the pregnant patients diagnosed as cervical cancer were in the early stages [24]. Management with conization or trachelectomy may be indicated for microinvasive or invasive disease [25]; while management is dependent on the clinical stages and gestation weeks for invasive cervical cancer. For the patients at gestational age more than 22 weeks, early-stage (Ia2-Ib) with negative lymph node status, delay of treatment until fetal maturity may be appropriate; Ib > 2 cm with negative lymph node status NACT is recommended until fetal maturity. The patient with positive lymph node status is suggested to term the pregnancy [22,24,26,27]. Laparoscopic lymphadenectomy during pregnancy is difficult for the enlargement of uterus. According to the reports, either laparoscopic or laparotomic lymphadenectomy can safely be performed between the 13th and 25th weeks of pregnancy. Lymphadenectomy in minimal number of 10 lymph nodes were suggested by International Federation of Gynecology and Obstetrics [22,28-31].
In summary, NACT for the treatment of cervical cancer during pregnancy seems to be a sensible choice for the patients who refuse to give up their pregnancies. Nevertheless, the reasonable chemotherapy doses, drugs and regimens should be carefully selected, the corresponding side effects should also be considered.
Conclusion
The patient with cervical cancer staged at IB1 diagnosed in the 3rd trimester of pregnancy can be performed with NACT before operation, while the proper chemotherapy doses must be cautiously considered in view of the pharmacokinetic alteration during pregnancy and the adverse effects for the pregnant patient and the fetus.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
ZY and WDB designed the report; ZY, CG, ZXF, CL, CY, SKL and WDB were attending doctors for the patient; WHY performed the pathology diagnosis; XF and ZY organized and wrote the report; WDB gave the final approval. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81001168, 81272881).
References
- Nguyen C, Montz FJ, Bristow RE (2000) Management of stage I cervical cancer in pregnancy. Obstet Gynecol Surv 55: 633-643.
- Li J, Wang LJ, Zhang BZ, et al. (2011) Neoadjuvant chemotherapy with paclitaxel plus platinum for invasive cervical cancer in pregnancy: two case report and literature review. Arch Gynecol Obstet 284: 779-783.
- Zagouri F, Sergentanis TN, Chrysikos D, et al. (2013) Platinum derivatives during pregnancy in cervical cancer: a systematic review and meta-analysis. Obstet Gynecol 121: 337-343.
- Chun KC, Kim DY, Kim JH, et al. (2010) Neoadjuvant chemotherapy with paclitaxel plus platinum followed by radical surgery in early cervical cancer during pregnancy: three case reports. Jpn J Clin Oncol 40: 694-698.
- Giacalone PL, Laffargue F, Benos P, et al. (1996) Cis-platinum neoadjuvant chemotherapy in a pregnant woman with invasive carcinoma of the uterine cervix. Br J Obstet Gynaecol 103: 932-934.
- Caluwaerts S, K VANC, Mertens L, et al. (2006) Neoadjuvant chemotherapy followed by radical hysterectomy for invasive cervical cancer diagnosed during pregnancy: report of a case and review of the literature. Int J Gynecol Cancer 16: 905-908.
- Tewari K, Cappuccini F, Gambino A, et al. (1998) Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: a report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer 82: 1529-1534.
- Marana HR, de Andrade JM, da Silva Mathes AC, et al. (2001) Chemotherapy in the treatment of locally advanced cervical cancer and pregnancy. Gynecol Oncol 80: 272-274.
- Bader AA, Petru E, Winter R (2007) Long-term follow-up after neoadjuvant chemotherapy for high-risk cervical cancer during pregnancy. Gynecol Oncol 105: 269-272.
- Benhaim Y, Pautier P, Bensaid C, et al. (2008) Neoadjuvant chemotherapy for advanced stage cervical cancer in a pregnant patient: report of one case with rapid tumor progression. Eur J Obstet Gynecol Reprod Biol 136: 267-268.
- Palaia I, Pernice M, Graziano M, et al. (2007) Neoadjuvant chemotherapy plus radical surgery in locally advanced cervical cancer during pregnancy: a case report. Am J Obstet Gynecol 197: e5-6.
- De Lima CA, Barcelos AC, Paschoini Mde C, et al. (2013) Conservative treatment of uterine cervical adenocarcinoma in pregnancy. Case Rep Obstet Gynecol.
- Peculis LD, Ius Y, Campion M, et al. (2015) Stage IB2 adenosquamous cervical cancer diagnosed at 19-weeks' gestation. Aust N Z J Obstet Gynaecol 55: 94-97.
- Fruscio R, Villa A, Chiari S, et al. (2012) Delivery delay with neoadjuvant chemotherapy for cervical cancer patients during pregnancy: a series of nine cases and literature review. Gynecol Oncol 126: 192-197.
- Kong TW, Lee EJ, Lee Y, et al. (2014) Neoadjuvant and postoperative chemotherapy with paclitaxel plus cisplatin for the treatment of FIGO stage IB cervical cancer in pregnancy. Obstet Gynecol Sci 57: 539-543.
- Karam A, Feldman N, Holschneider CH (2007) Neoadjuvant cisplatin and radical cesarean hysterectomy for cervical cancer in pregnancy. Nat Clin Pract Oncol 4: 375-380.
- Boyd A, Cowie V, Gourley C (2009) The use of cisplatin to treat advanced-stage cervical cancer during pregnancy allows fetal development and prevents cancer progression: report of a case and review of the literature. Int J Gynecol Cancer 19: 273-276.
- Seamon LG, Downey GO, Harrison CR, et al. (2009) Neoadjuvant chemotherapy followed by post-partum chemoradiotherapy and chemoconsolidation for stage IIIB glassy cell cervical carcinoma during pregnancy. Gynecol Oncol 114: 540-541.
- Smyth EC, Korpanty G, McCaffrey JA, et al. (2010) Small-cell carcinoma of the cervix at 23 weeks gestation. J Clin Oncol 28: e295-e297.
- Rabaiotti E, Sigismondi C, Montoli S, et al. (2010) Management of locally advanced cervical cancer in pregnancy: a case report. Tumori 96: 623-626.
- Favero G, Chiantera V, Oleszczuk A, et al. (2010) Invasive cervical cancer during pregnancy: laparoscopic nodal evaluation before oncologic treatment delay. Gynecol Oncol 118: 123-127.
- Amant F, Halaska MJ, Fumagalli M, et al. (2014) Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer 24: 394-403.
- van Hasselt JG, van Calsteren K, Heyns L,et al. (2014) Optimizing anticancer drug treatment in pregnant cancer patients: pharmacokinetic analysis of gestation-induced changes for doxorubicin, epirubicin, docetaxel and paclitaxel. Ann Oncol 25: 2059-2065.
- Morice P, Uzan C, Gouy S, et al. (2012) Gynaecological cancers in pregnancy. Lancet 379: 558-569.
- Kim CH, Abu-Rustum NR, Chi DS, et al. (2012) Reproductive outcomes of patients undergoing radical trachelectomy for early-stage cervical cancer. Gynecol Oncol 125: 585-588.
- Berveiller P, Carbonne B, Mir O (2014) Cancer and pregnancy: an overview for obstetricians and gynecologists. Am J Obstet Gynecol 211: 82.
- Salani R, Billingsley CC, Crafton SM (2014) Cancer and pregnancy: an overview for obstetricians and gynecologists. Am J Obstet Gynecol 211: 7-14.
- Stan C, Megevand E, Irion O, et al. (2005) Cervical cancer in pregnant female: laparoscopic evaluation before delaying treatment. Eur J Gynaecol Oncol 26: 649-650.
- Alouini S, Rida K, Mathevet P (2008) Cervical cancer complicating pregnancy: implications of laparoscopic lymphadenectomy. Gynecol Oncol 108: 472-477.
- Sioutas A, Schedvins K, Larson B, et al. (2011) Three cases of vaginal radical trachelectomy during pregnancy. Gynecol Oncol 121: 420-421.
- Ferraioli D, Buenerd A, Marchiole P, et al. (2012) Early invasive cervical cancer during pregnancy: different therapeutic options to preserve fertility. Int J Gynecol Cancer 22: 842-849.
Corresponding Author
Dabao Wu and Ying Zhou, Department of Obstetrics and Gynecology, Anhui Provincial Hospital, Anhui Medical University, Hefei, 230001, China.
Copyright
© 2016 Xu F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.