DNA Biosensors Techniques and Their Applications in Food Safety, Environmental Protection and Biomedical Research: A mini-review
Abstract
Biosensors are analytical devices that convert a biological response into an electrical signal. Recently, Deoxyribonucleic acid (DNA) based biosensors (genosensors) have been developed significantly to detect the environmental pollutions, food analysis, clinical diagnosis, drug discovery and biomedical research. Different types of DNA biosensors have been optimized and introduced. In this study, we explain the DNA hybridization, aptamers and electrochemical DNA as DNA-based biosensors. In addition, we describe the application of DNA biosensors in food analysis (e.g. detection of toxins and pathogens), environmental monitoring and biomedical research such as detection of SARS-COV-2 virus responsible for the COVID-19 pandemic disease and cancer associated-genes. This mini-review provides evidence about importance and application of DNA biosensors in different fields.
Keywords
DNA biosensors, Hybridization, Aptamers
Introduction
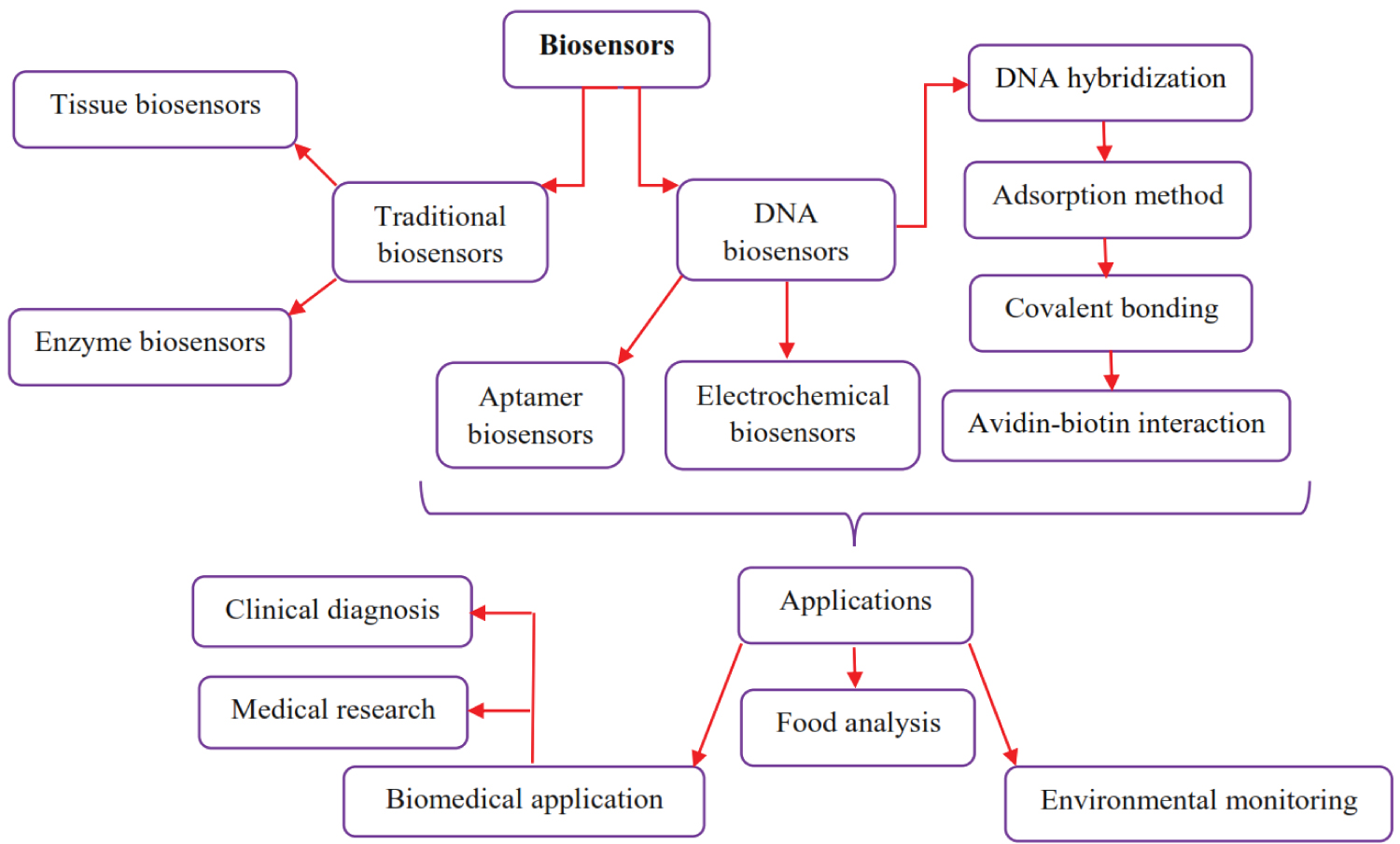
A biosensor is defined as a device that is able to measures biological or chemical reactions by generating signals proportional to the concentration of an analyte in the reaction [1]. Biosensors contribute in wide applications such as disease monitoring, environmental monitoring and food analysis. In general, biosensors include five different parts. 1. Analyte: A substance of interest that needs recognition. 2. Bioreceptor is known as a molecule that specifically recognizes the analyte. 3. Transducer is an element that converts one form of energy into another. In fact, transducer has main role in convert the bio-recognition event into a measurable signal. 4. Electronics contribute to processes the transduced signal and prepares it for display. 5. Display usually as a computer provides understandable curve or numerical value [2]. There are various types of biosensors such as enzyme-based, tissue-based, immunosensors, DNA biosensors, thermal and piezoelectric biosensors which are available to apply in different investigations. However, an interesting biosensor must have several features such as, sensitively, fast and provide reliable results [3]. Biosensors can be classified in traditional and novel biosensors. Although, in the past decade traditional biosensors (e.g enzyme and thermal biosensors, etc.) have been used in different fields but they had many problems. It should be noted that usually traditional biosensors were too time consuming and must performed by high skill personnel. The first biosensors were reported in the 1960s by the pioneers Clark and Lyons. In general, the most important traditional biosensors are clustered in enzymes and tissues biosensors. The first enzyme-based sensor was reported by Updike and Hicks in 1967. An enzyme biosensor is an analytical device that combines an enzyme with a transducer to produce a signal proportional to target analyte concentration. This signal can result from a change in proton concentration, release or uptake of gases, such as ammonia or oxygen, light emission, absorption or reflectance, heat emission, and so forth, brought about by the reaction catalyzed by the enzyme [4]. The tissue-based sensors arise from plant and animal sources. Biosensors based on tissue structures in living animals can be used to detect and measure hormones, drugs, and toxins. The potential use of tissue-based biosensors extends to such diverse fields of biomedical science as physiology, pharmacology, and biodefense. Biophotonics provides the most versatile basis for tissue-based biosensors. Light output from biosensor cells can be in the form of fluorescence or bioluminescence [5]. Fast screening and sensitivity are two main factors for an ideal biosensor. Traditional biosensors usually depend on experimental protocols and it can be too time consuming and expensive. Most importantly, the results accuracy of these biosensors are not completely reliable and must be repeated for several times. In contrast, novel biosensors especially DNA biosensors can provide reliable results in short time with low cost. Therefore, they are becoming a powerful monitoring tool in different fields [6]. There are many investigations, which show the application of DNA biosensors in various fields such as, drug discovery, food analysis, environmental monitoring and biomedical research. Table 1 shows the application of DNA biosensors in different fields.
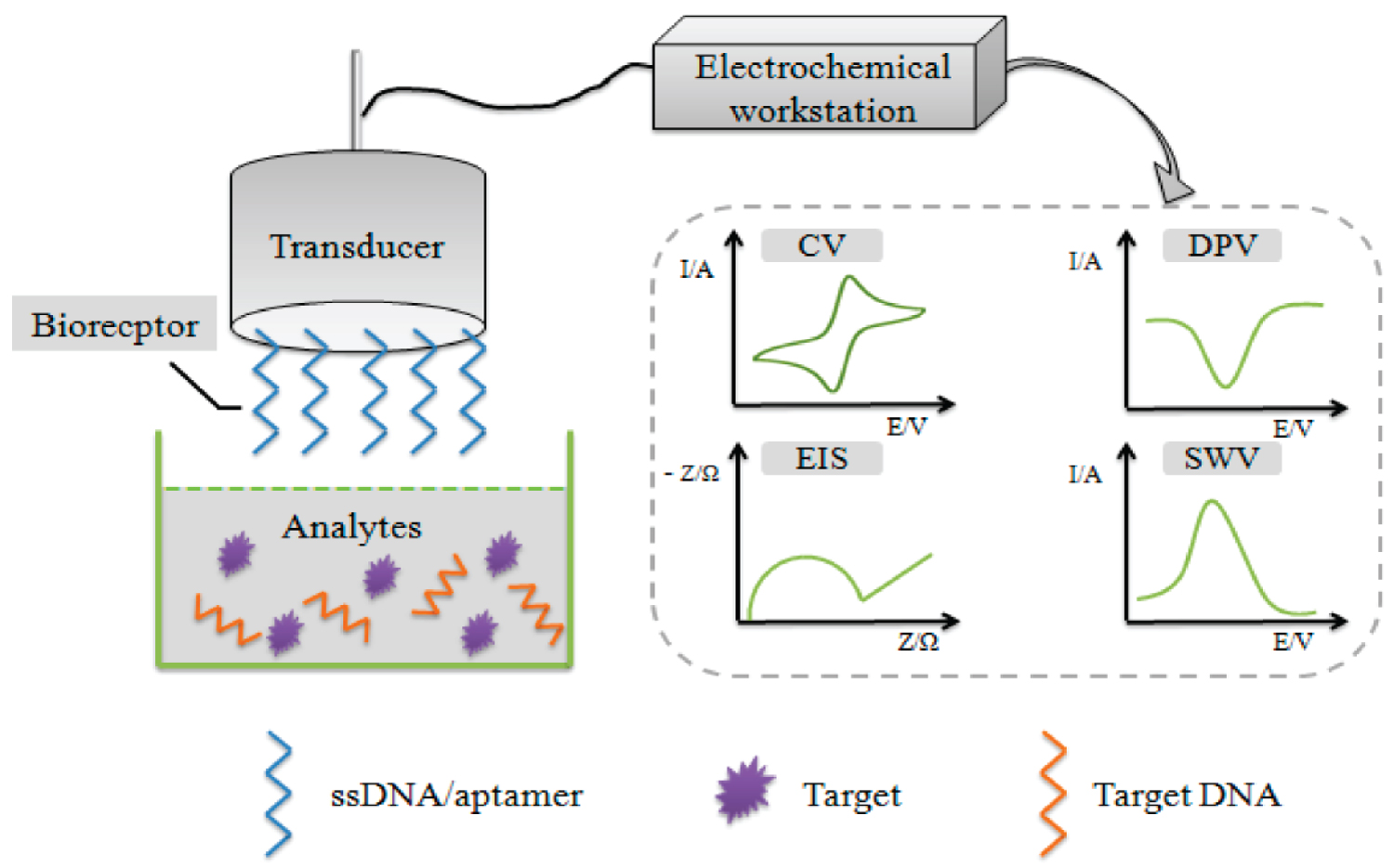
In this mini-review we describe different types of DNA biosensors and explain their applications in food analysis, environmental monitoring and biomedical research. Figure 1 shows the graphical abstract and summarize the contents of this investigation.
DNA-Based Biosensors
DNA biosensors are known as applicable biosensor, play critical role in different subjects such as environmental monitoring, food control, drug discovery, forensics and biomedical research. Here, we describe the different types of DNA biosensors and review its application in various contexts.
DNA hybridization biosensors
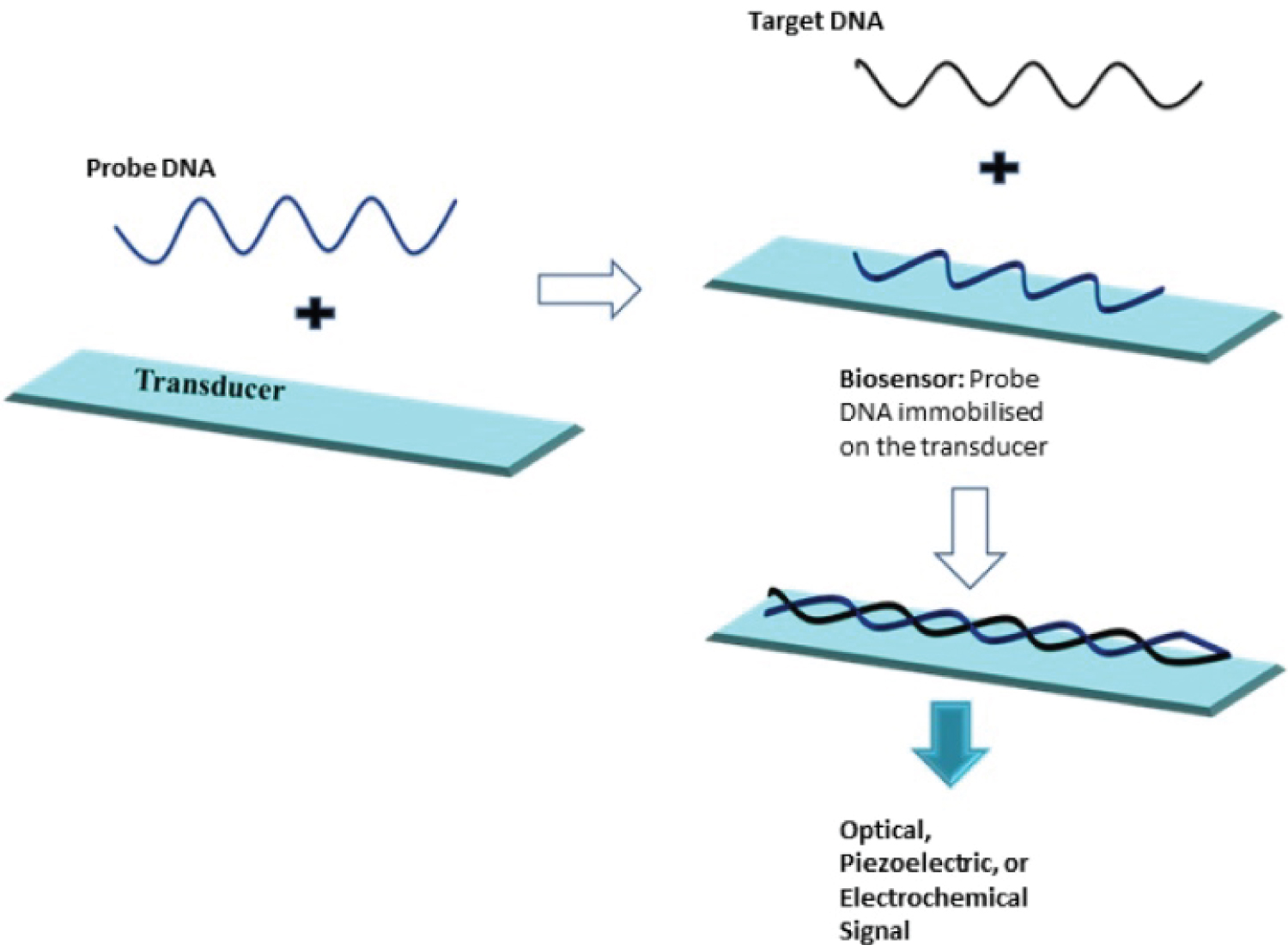
This approach works based on DNA complementary. In this method, the detection layer of the biosensor consists of short single-stranded DNA (ssDNA probe) able to form duplex with complementary target nucleic acid fragment with high efficiency and specificity. The probe is associated with a transducer translating hybridization event into a physically measurable value [7,8]. Figure 1 shows the mechanism the DNA hybridization biosensors method [9].
In this approach, Short single-stranded DNA segments are immobilized on the electrode surface. The DNA fragments have to be immobilized in a way that retains their stability, reactivity, accessibility to target analytic and optimal orientation. An electrical signal is produced when target DNA binds to the complementary sequence of the capture or probe DNA in a process called hybridization.
Several points must be considered for designing the ideal probs. First, probe nucleic acids hybridizes specifically and selectively to the target sequence nucleic acids; second probe must not self-complementary nor should the probe hybridize to non-target sequence nucleic acids in a sample mixture of nucleic acids and finally the non-target cells should not have the targeted sequence of nucleic acids. The most important advantages of hybridization biosensors include, low cost, sensitive and selective detection of target DNA fragments, and can be used for routine tests [10].
DNA hybridization biosensors strategies
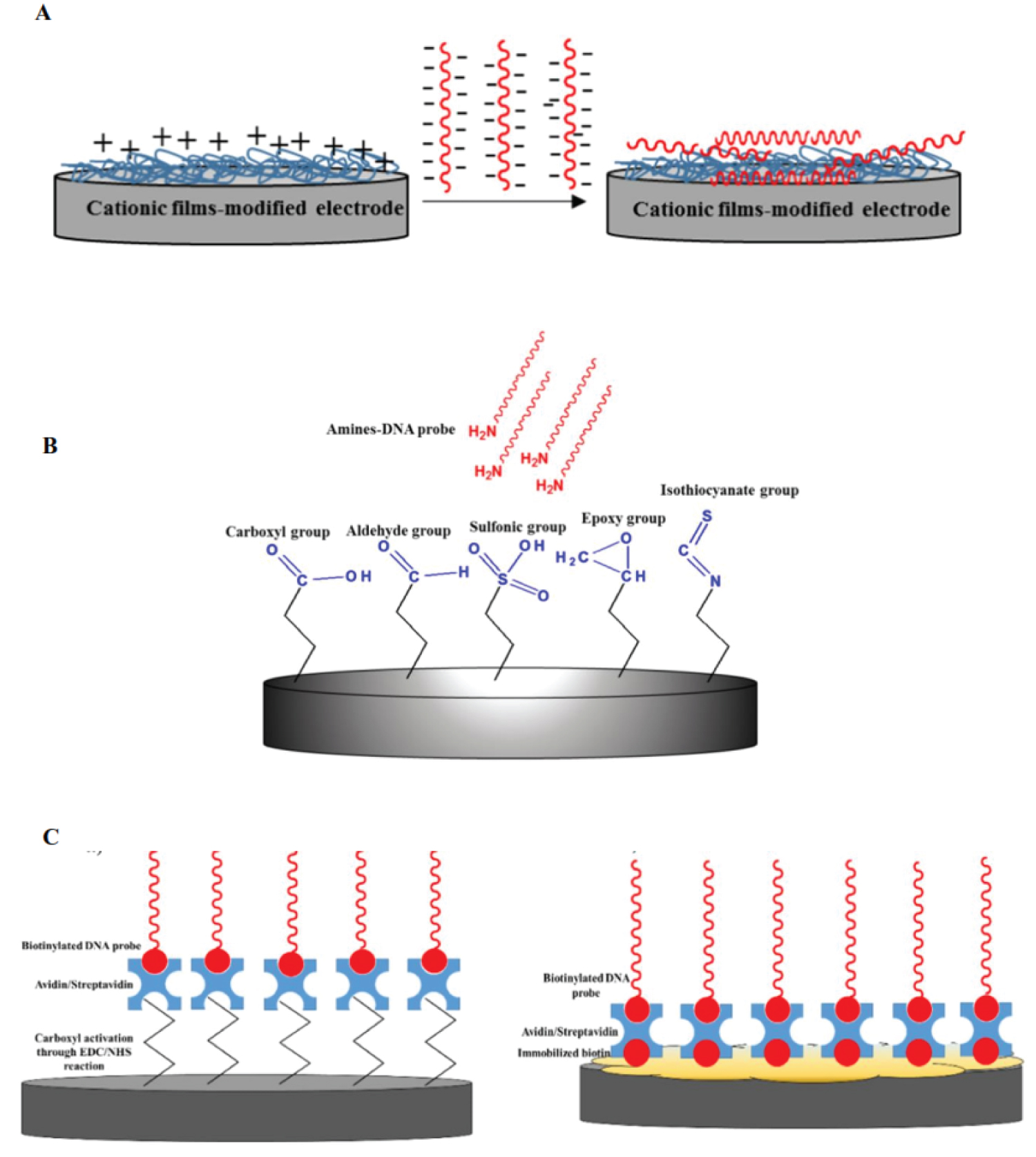
Several DNA probe immobilization techniques have been developed in electrochemical DNA sensing including adsorption methods, covalent bonding and avidin-biotin interaction. Adsorption is the simplest technique for DNA probes immobilization on working electrode surface, which does not require any chemical reagents and DNA probes modification [11]. DNA probes are immobilized on the working electrode surface via electrostatic adsorption between a negatively charged phosphate group of DNAs on the positive charged films-modified electrodes.
Unlike adsorption technique, DNA probes immobilization technique via covalent bonding demonstrated a good stability, flexible, highly binding strength and prevent desorption of DNA probe monolayer from the electrode surface. In covalent bonding technique, the synthesized DNA probe is typically linked with the group of thiols (S H) or amines (NH2) at the end of 3' or 5' to bind covalently to the metal surface or specific functional group introduced to the electrode surface. This procedure led to high specific attachment of DNA probe onto the electrode surface and can prevent non-specific binding [12].
Another strategy for non-covalent immobilization of DNA probes on the electrode surface is based on the formation complex of avidin (either streptavidin)-biotin. The interaction between SA (streptavidin) and biotin is one of the strongest non-covalent interactions in nature. It should be noted that, avidin/streptavidin, a large tetrameric protein (70 kDa) could provide four binding sites of biotin molecule and these tetrameric interactions could be exploited to immobilize DNA probes onto the solid electrode surface. This could be done by modifying the end of 3' or 5' of DNA probes sequence with biotin molecule and later introducing it to the avidin/streptavidin-modified electrode [13,14]. Figure 2 shows the schematic process of different types of DNA hybridization biosensors [15].
Aptamer Biosensors
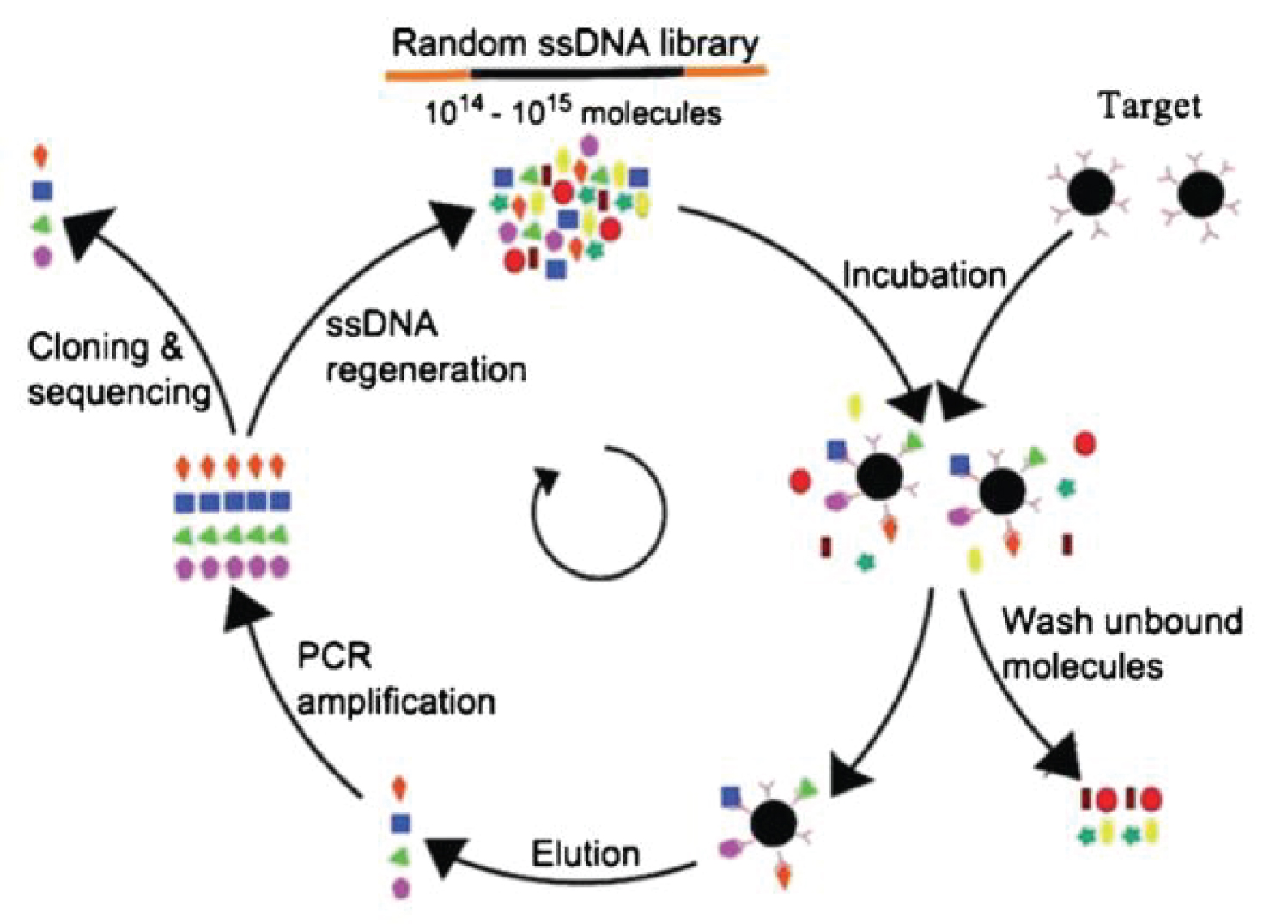
Aptamers are short fragment of single-strand DNAs (ssDNAs)/ RNAs selected in vitro and can bind with a broad range of target molecules like amino acids, drugs, proteins and other molecules. They are small in size, chemically stable and cost effective [16]. Additionally, aptamers offer remarkable flexibility and convenience in the design of their structures, which has led to novel biosensors that have exhibited high sensitivity and selectivity. Recent advances have shown that, the combination of aptamers with novel nanomaterials has significantly improved the performance of aptamer-based sensors [17]. Systematic Evolution of Ligands by Exponential enrichment (SELEX) is the most popular aptamer biosensor system. SELEX is firstly introduced in 1990, as an in vitro selection tool to discover a new kind of nucleic acid ligands, named aptamers. Briefly, SELEX is performed in several steps. First, incubation of target molecules with the random sequence pools second, the subsequent separation of unbound oligonucleotides and the elution of bound oligonucleotides and third, PCR amplification of bound aptamers [18,19]. The summary of SELEX is shown in Figure 3.
Electrochemical DNA Biosensors
The principle of electrochemical DNA biosensor is that the biological reaction between bioreceptor and target can produce or consume ions or electrons, which changes the electric current, potential, or other electrical properties of the solution. The biological signal can be converted into a detectable electrical signal proportional to target concentration by transducer and displayed on a computer [20]. The basic view of an electrochemical DNA biosensor is presented in Figure 4 [21].
Application of DNA Biosensors
The advantageous of DNA biosensors were explained in the introduction. The most important benefits of DNA biosensors are summarized in reliable results, fast tests and low cost. However, DNA biosensors are not without problem. In general, in DNA-based biosensors materials must interact with DNA at atomic level. In this way, identification of novel nanomaterials can interact with DNA is the main stage of DNA biosensors developments. In fact, nanomaterials must bind to DNA strongly, under uncontrolled conditions. Therefore, find these nanomaterials is the undeniable challenge in the application of DNA biosensors [22].
Food analysis
Agriculture products are widely transferred among countries. In this way, a reliable, applicable and accurate system is needed to screen the safety, quality and authenticity. Food safety monitoring is a very important aspect for dealing with threats to human health and well-being of the population. Usually, food monitoring is performed for pathogen and toxin pollutions. Detection of contaminated food by pathogenic microorganism is an important concern for ensuring food safety, security and public health. Food contamination by pathogenic bacteria like, Escherichia coli, Salmonella typhimurium, Staphylococcus aureus, Bacillus cereus and etc., can causes several foods borne illnesses and are responsible for approximately 90% of all food borne diseases [23]. Conventional methods for bacterial identification usually involves various culturing techniques and different biochemical tests which are very time consuming and usually require 2-4 days [24]. However, electrochemical biosensors have been provided reliable system for pathogen screening in a short time. For example, detection of Staphylococcus aureus was reported exploring single-walled carbon nanotubes in pig skin [25]. In addition, [26] reported an electrochemical DNA-based biosensor for Bacillus cereus in milk and infant formula. It should be noted that the detection of Salmonella typhimurium was performed by gold nanoparticles and carbon nanotubes [27].
Biosensors are widely used to determination of small molecule biological toxins especially mycotoxin and neurotoxin. Mycotoxins are a diverse group of organic compounds produced by fungal species and neurotoxin is one of the most toxic non-protein substances known, that is produced by a several of marine algal species and contaminated shellfish [28]. Previously, typical methods such as high-performance liquid chromatography (HPLC) with ultra-violate (UV) and fluorescence detection (FLD) have been utilized [29]. However, these techniques are not without problem. For example, they are very laborious, and not really suitable for screening large numbers of samples for fieldwork. In addition, they are too time consuming and need highly skilled user. Unlike, the mentioned techniques, biosensors as a rapid, sensitive and routine monitoring of food play a significant role for food screening [30]. As an example, Ye, et al. [31] showed the application of marine toxins detection by biosensors based on aptamers. Similarity, Fetter, et al. [32] established the role of electrochemical aptamer scaffold biosensors for detection of botulism and ricin toxins.
Environmental monitoring
Development of various industries, farming and growth rate of population in the world, have caused the distribution of different types of pollutions into environment. As an example, the worldwide production in 1985 of just one chemical that is released into the environment pentachlorophenol was more than 50,000 tons [33]. In this way, a large scale screening method is necessary to detect and remove the pollution compounds. There are several investigations, illustrated the application of biosensors for environmental monitoring. Chiti, et al. [34] described the role of electrochemical DNA biosensor for environmental monitoring. They explained the determination of toxic aromatic amines by electrochemical DNA biosensor. In addition, it was observed DNA hybridization sensors for sequences related to microbial or viral pollutions, and DNA-modified carbon electrodes for monitoring low molecular weight priority pollutants interacting with DNA [35]. Interestingly, in a major study, a biomonitoring tool was developed in Fish Bile as DNA biosensor. It was shown the that electrochemical DNA biosensor is proposed as a screening device for the rapid detection of polycyclic aromatic hydrocarbons (PAH) metabolites in fish bile samples. PAH has negative effects on aquatic biota due to their persistence and potential for accumulation [36].
Biomedical research
The biomedical application of DNA biosensors can be classified as the clinical diagnosis for disease (e.g. COVID-19 detection) and medical research.
The COVID-19 pandemic is an ongoing pandemic of coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Currently, quantitative real-time polymerase chain reaction (qRT-PCR) is the most popular technique detect COVID-19 from various types of biological specimens, however, qRT-PCR is time-consuming, labor-intensive and may not be rapidly deployable in remote or resource-limited settings. Interestingly, Samson, et al. [37] suggested the rapid detection of COVID-19 based on nucleic acid biosensors. They reported several existing of current diagnostic detection methods based on biosensors such as, nucleic-acid based, aptamer-based, Antigen-Au/Ag nanoparticles-based electrochemical biosensor, optical biosensor. These biosensors could provide effective tools for rapid, authentic, portable, and more promising diagnosis in the current pandemic that has affected the human health. In another studies, [38] developed a fiber-optic biosensor to detect the nucleocapsid (N) protein, a specific SARS-CoV antigen as a faster and more sensitive alternative to the qRT-PCR and serological tests. Roh and Jo [39] using quantum dots-conjugated RNA aptamer immobilized over a designed chip to recognize the same N protein from SARS-CoV, and it is, to date, the lowest limit of detection found for a coronavirus through biosensor devices. Moreover, Ribeiro, et al. [40] reported another biosensor such as enzymatic biosensors, whole-cells biosensors, immunosensors could contribute to rapid detection of in respiratory viruses.
Fast and early diagnosis is one the main key in the cancer treatments. DNA biosensors are the most sensitive tools and provide information to assist clinicians in making successful treatment decisions and increase the patient survival rate [41]. In a major study, Senel, et al. [42] showed the application of electrochemical DNA detection strategy for the detection of a gene relevant to breast cancer. They result showed that the studied biosensor is highly sensitive and selective in the detection of the target DNA. The results also have shown highly motivating for exploring DNA biosensing technology in the diagnosis of breast cancer caused by mutation of the BRAC1 gene.
Concluding Remarks
The rapid development in the field of biosensors over the past decades provides new perspective to investigate the application of biosensors. Future work about DNA biosensors is due mainly to clarifying the mechanism of interaction between nanomaterials and biomolecules, identification of novel nanomaterials can interact with DNA and enhanced manipulation and processing precision at the atomic, by specific molecular tools such as ligases, nucleases and other DNA-processing enzymes is another remarkable feature. It should be noted that nanotechnology would have a major impact on future advances of biosensing technology.
Conflict of Interest
The author has none to declare.
References
- Teles FRR, LP Fonseca (2008) Trends in DNA biosensors. Talanta 77: 606-623.
- Bhalla N, Jolly P, Formisano N, et al. (2016) Introduction to biosensors. Essays Biochem 60: 1-8.
- Mehrotra P (2016) Biosensors and their applications - A review. J Oral Biol Craniofac Res 6: 153-159.
- Mulchandani A (1998) Principles of enzyme biosensors. In: Enzyme and Microbial Biosensors. Humana Press, 3-14.
- Acha V, Andrews T, Huang Q, et al. (2010) Tissue-based biosensors. In Recognition Receptors in Biosensors Springer, New York, NY, 365-381
- Sin ML, Mach KE, Wong PK, et al. (2014) Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev Mol Diagn 14: 225-244.
- Palecek E, Fojta M (2005) In: Willner, I, Katz, E,Bioelectronics.
- de-los-Santos-Álvarez P, Lobo-Castanon MJ, Miranda-Ordieres AJ, et al. (2004) Current strategies for electrochemical detection of DNA with solid electrodes. Anal Bioanal Chem 378: 104-118.
- Hlongwane GN, Dodoo-Arhin D, Wamwangi D, et al. (2019) DNA hybridisation sensors for product authentication and tracing: State of the art and challenges. South African journal of chemical engineering 27: 16-34.
- Tichoniuk M, Ligaj M, Filipiak M (2008) Application of DNA hybridization biosensor as a screening method for the detection of genetically modified food components. Sensors (Basel) 8: 2118-2135.
- Pividori MI, Merkoci A, Alegret S (2000) Electrochemical genosensor design: immobilisation of oligonucleotides onto transducer surfaces and detection methods. Biosens Bioelectron 15: 291-303.
- Wang L, Chen X, Wang X, et al. (2011) Electrochemical synthesis of gold nanostructure modified electrode and its development in electrochemical DNA biosensor. Biosens Bioelectron 30: 151-157.
- Lee HE, Kang YO, Choi SH (2014) Electrochemical-DNA biosensor development based on a modified carbon electrode with gold nanoparticles for influenza A (H1N1) Detection: Effect of spacer. Int J Electrochem Sci 9: 6793-6808.
- Singh R, Verma R, Sumana G, et al. (2012) Nanobiocomposite platform based on polyaniline-iron oxide-carbon nanotubes for bacterial detection. Bioelectrochemistry 86: 30-37.
- Rashid JIA, Yusof NA (2017) The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sensing and Bio-Sensing Research 16: 19-31.
- Kavita V (2017) DNA Biosensors-A Review. J Bioeng Biomed Sci 7: 222.
- Song S, Wang L, Li J, et al. (2008) Aptamer-based biosensors. TrAC Trends in Analytical Chemistry 27: 108-117.
- Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX-a (r) evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24: 381-403.
- Wu J, Zhu Y, Xue F, et al. (2014) Recent trends in SELEX technique and its application to food safety monitoring. Mikrochim Acta 181: 479-491.
- Gaudin V (2017) Advances in biosensor development for the screening of antibiotic residues in food products of animal origin-A comprehensive review. Biosens Bioelectron. 90: 363-377.
- Wu Q, Zhang Y, Yang Q, et al. (2019) Review of electrochemical DNA biosensors for detecting food borne pathogens. Sensors (Basel) 19: 4916.
- Tuantranont A (2013) Applications of nanomaterials in sensors and diagnostics. Springer series on chemical sensors and biosensors (Springer, Berlin Heidelberg, 2014).
- Mishra GK, Barfidokht A, Tehrani F, et al. (2018) Food safety analysis using electrochemical biosensors. Foods 7: 141.
- Cabral JP (2010) Water microbiology. Bacterial pathogens and water. Int J Environ Res Public Health 7: 3657-3703.
- Zelada-Guillén GA, Sebastián-Avila JL, Blondeau P, et al. (2012) Label-free detection of Staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosensors and bioelectronics, 31: 226-232.
- Izadi Z, Sheikh-Zeinoddin M, Ensafi AA, et al. (2016) Fabrication of an electrochemical DNA-based biosensor for Bacillus cereus detection in milk and infant formula. Biosensors and Bioelectronics 80: 582-589.
- Ma X, Jiang Y, Jia F, et al. (2014) An aptamer-based electrochemical biosensor for the detection of Salmonella. J Microbiol Methods 98: 94-98.
- D'Mello JF (2003) Food Safety: contaminants and Toxins. CABI.
- Kim EK, Maragos CM , Kendra DF (2004) Liquid chromatographic determination of fumonisins B1, B2, and B3 in corn silage. J Agric Food Chem 52: 196-200.
- Wang XH, Wang S (2008) Sensors and biosensors for the determination of small molecule biological toxins. Sensors 8: 6045-6054.
- Ye W, Liu T, Zhang W, et al. (2019) Marine toxins detection by biosensors based on aptamers. Toxins (Basel) 12: 1.
- Fetter L, Richards J, Daniel J, et al. (2015) Electrochemical aptamer scaffold biosensors for detection of botulism and ricin toxins. Chemical Communications 51: 15137-15140.
- Glick BR, Patten CL (2017) Molecular biotechnology: Principles and applications of recombinant DNA 34.
- Chiti G, Marrazza G, Mascini M (2001) Electrochemical DNA biosensor for environmental monitoring. Analytica Chimica Acta 427: 155-164.
- Wang J, Rivas G, Cai X, et al. (1997) DNA electrochemical biosensors for environmental monitoring. A review Anal Chim Acta 347: 1-8.
- Lucarelli F, Authier L, Bagni G, et al. (2003) DNA biosensor investigations in fish bile for use as a biomonitoring tool. Analytical Letters 36: 1887-1901.
- Samson R, Navale GR, Dharne MS. (2020) Biosensors: Frontiers in rapid detection of COVID-19. 3 Biotech 10: 385.
- Huang JC, Chang YF, Chen KH, et al. (2009) Detection of severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in human serum using a localized surface plasmon coupled fluorescence fiber-optic biosensor. Biosensors and Bioelectronics 25: 320-325.
- Roh C, Jo SK (2011) Quantitative and sensitive detection of SARS coronavirus nucleocapsid protein using quantum dots-conjugated RNA aptamer on chip. J Chem Technol Biotechnol 86: 1475-1479.
- Ribeiro BV, Cordeiro TAR, e Freitas GRO, et al. (2020) Biosensors for the detection of respiratory viruses: A review. Talanta Open 2: 100007.
- Sohrabi N, Valizadeh A, Farkhani SM, et al. (2016) Basics of DNA biosensors and cancer diagnosis. Artif Cells Nanomed Biotechnol 44: 654-663.
- Senel M, Dervisevic M, Kokkokoglu F (2019) Electrochemical DNA biosensors for label-free breast cancer gene marker detection. Anal Bioanal Chem 411: 2925-2935.
- De Abreu FC, De Paula FS, Ferreira DC, et al. (2008) The application of DNA-biosensors and differential scanning calorimetry to the study of the DNA-binding agent berenil. Sensors (Basel) 8: 1519-1538.
- Monošík R, Stred'anský M, Šturdík E (2012) Application of electrochemical biosensors in clinical diagnosis. J Clin Lab Anal 26: 22-34.
- Diculescu VC, Chiorcea-Paquim AM, Oliveira-Brett AM (2016) Applications of a DNA-electrochemical biosensor. TrAC Trends in Analytical Chemistry 79: 23-36.
- Tripathy S, Singh SG (2020) Label-free electrochemical detection of DNA hybridization: A method for COVID-19 diagnosis. Trans Indian Natl Acad Eng 25: 1-5
- Bunney J, Williamson S, Atkin D, et al. (2017) The use of electrochemical biosensors in food analysis. Current Research in Nutrition and Food Science 5: 183-195.
- Rodriguez-Mozaz S, Marco MP, De Alda ML, et al. (2004) Biosensors for environmental applications: Future development trends. Pure Appl.Chem 76: 723-752.
- Mehta J, Rouah-Martin E, Van Dorst B, et al. (2012) Selection and characterization of PCB-binding DNA aptamers. Anal Chem 84: 1669-1676.
Corresponding Author
Ali Esmailizadeh, Department of Animal Science, Faculty of Agriculture, Shahid Bahonar University of Kerman, Kerman, PB 76169-133, Iran.
Copyright
© 2020 Koopaee HK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.