Olaparib for Simultaneous Occurrence of Breast Cancer and Ovarian Cancer with BRCA1
Abstract
Olaparib, a poly ADP-ribose polymerase (PARP) inhibitor, is associated with a high tumor response rate among patients with breast cancer susceptibility (BRCA) genes. The efficacy and safety of olaparib in the treatment of patients with simultaneous occurrence of breast and ovarian cancers have not been reported on. We report the case of a 57-year-old woman who was treated for multiple lung metastases after resection of breast cancer. She was newly diagnosed with ovarian cancer, and a BRCA1 mutation was detected by genomic profiling for BRCA 1 and BRCA 2. Surgery for ovarian cancer and adjuvant chemotherapy were initially performed. Moreover, the patient received olaparib and bevacizumab as maintenance therapy for ovarian cancer, and palliative chemotherapy for breast cancer. The tumor was responsive to olaparib, and computed tomography revealed a partial response with multiple lung metastases and no recurrence of ovarian cancer. The patient is currently being treated with olaparib for ≥ 6 months. This is the first report on the efficacy of the PARP inhibitor olaparib in a patient with simultaneous occurrence of breast and ovarian cancer.
Key words
Breast cancer, Ovarian cancer, BRCA1/2 pathogenic variants, Olaparib
Abbreviations
PARP: Poly ADP-ribose Polymerase; BRCA: Breast Cancer Susceptibility; HBOC: Hereditary Breast and Ovarian Cancer; CT: Computed Tomography; PR: Partial Response; PFS: Progression-Free Survival
Introduction
Breast cancer is the most common cancer and the fifth leading cause of cancer-related mortality in women worldwide [1]. Approximately 10-15% of breast cancer cases diagnosed worldwide are hereditary and confer genetic mutations [2,3]. Tumor suppressor genes Breast cancer susceptibility (BRCA) 1 and BRCA2 are the main breast cancer susceptibility genes, and germline mutations in BRCA1/2 are associated with hereditary breast and ovarian cancer (HBOC) [4].
Poly ADP-ribose polymerase (PARP) inhibitors have been associated with a high tumor response rate among cancer patients with BRCA1/2 pathogenic variants [5,6]. Therefore, PARP inhibitors have been approved for breast and ovarian cancer patients harboring BRCA 1 or BRCA 2 mutations [7,8].
The efficacy and safety of olaparib in the treatment of patients with simultaneous occurrence of breast and ovarian cancers have not been reported. This report describes the case of a patient with simultaneous occurrence of breast and ovarian cancer that was successfully treated with olaparib.
Case Presentation
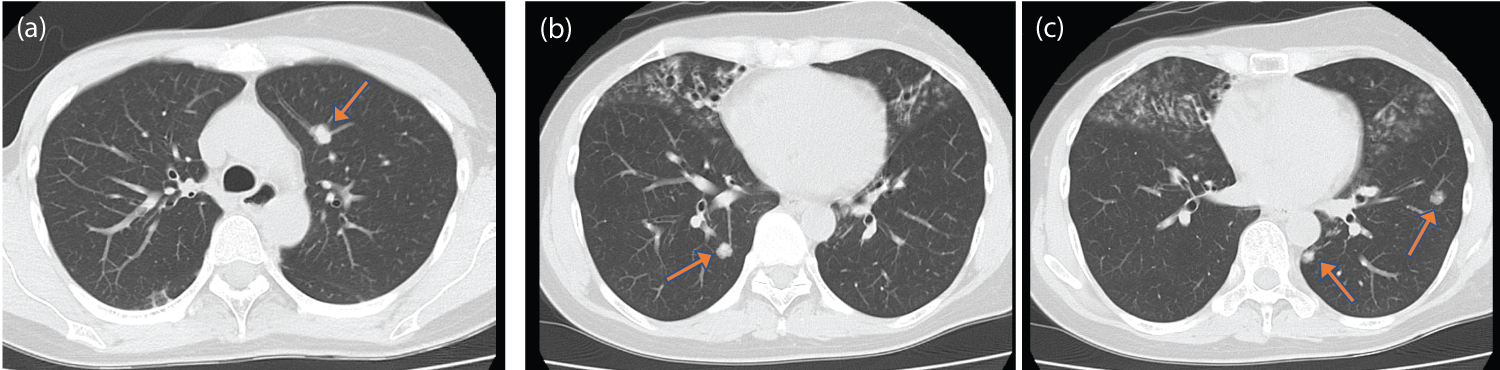
This patient was a 57-year-old Japanese woman who was initially diagnosed with locally advanced invasive ductal carcinoma of the left breast (hormone receptor-positive, HER2-negative) at 41 years of age. She underwent mastectomy of the left breast and axillary lymph node dissection (pathological T4N0M0StageIIIB according to the TMN classification, eighth edition) followed by adjuvant chemotherapy with doxorubicin, cyclophosphamide, and paclitaxel. However, she was diagnosed with breast cancer recurrence with multiple lung metastases at 54 years (Figure 1). Hormone receptor-positive, HER2-negative carcinoma was revealed from a biopsy specimen of the lung metastases. Therefore, she was administered anastrozole (1 mg once per day) and palbociclib (125 mg once per day). Fulvestrant (500 mg once per month) was replaced with anastrozole and palbociclib due to the progression of lung metastases on Computed tomography (CT) imaging 4 months after the first-line treatment.
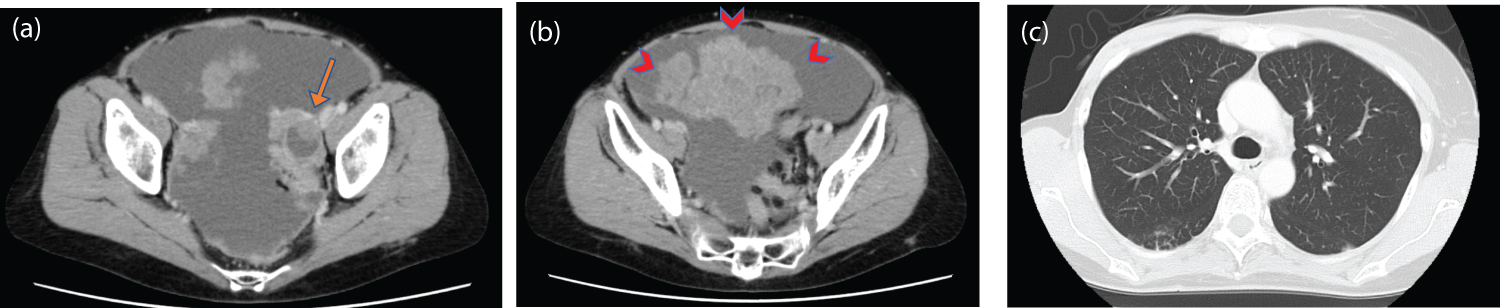
She received fulvestrant for 10 months without cancer progression; however, a follow-up CT scan revealed ovarian cancer and malignant ascites at 57 years (Figure 2). She then underwent ovarian cancer surgery (total abdominal hysterectomy, bilateral salpingo-oophorectomy, and omentectomy), and was diagnosed with pathological stage IIIB high-grade serous carcinoma. Subsequently, the patient received six cycles of adjuvant chemotherapy with carboplatin (AUC 6), paclitaxel (175 mg/m2), and bevacizumab (15 mg/kg).
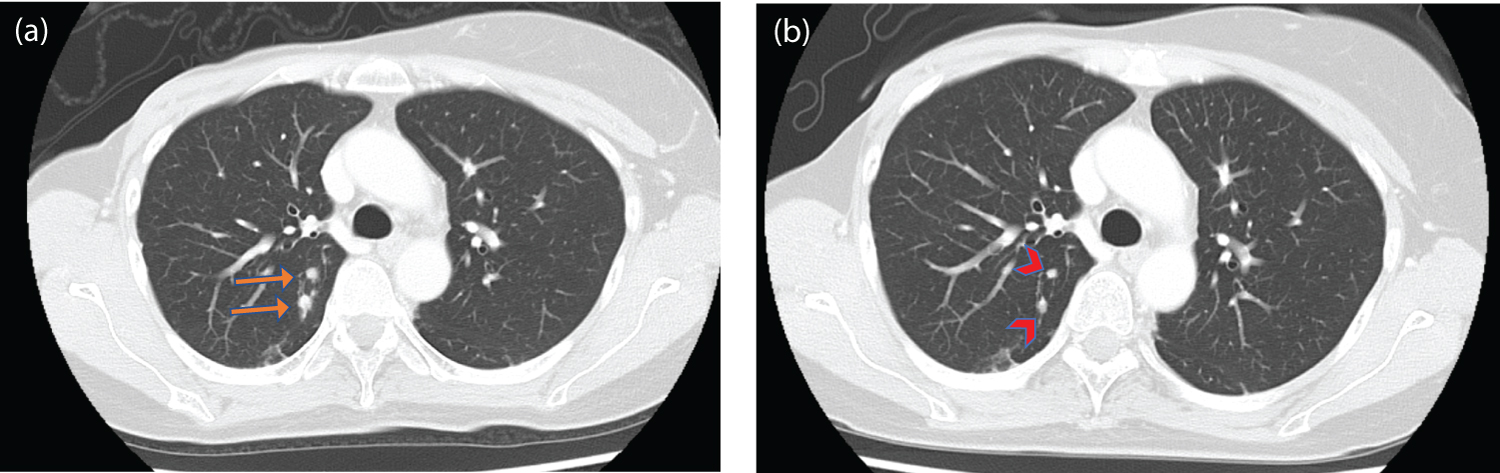
Follow-up CT images after adjuvant chemotherapy showed no residual or recurrence of ovarian cancer but showed tumor progression in the form of lung metastases (Figure 3a). Homologous recombination deficiency (HRD) testing was performed on tumor tissues from ovarian cancer. BRCA 1 mutation (c.188T > A; p. Leu63*) was detected as a pathogenic variant in this test. Additionally, the diagnosis of HBOC was established by the identification of a heterozygous germline pathogenic variant in BRCA1 using molecular genetic testing.
The patient was started on olaparib (300 mg twice daily) and bevacizumab (15 mg/kg) as maintenance therapy after adjuvant chemotherapy for ovarian cancer. The patient’s lung metastases, spread by breast cancer, were considered to be associated with the BRCA1 mutations. Therefore, the patient also received olaparib as palliative chemotherapy for recurrent breast cancer. She had grade 1 nausea but tolerated the dose. CT assessment on day 56 of treatment indicated a partial response (PR) of lung metastases and no recurrence of ovarian cancer to olaparib (Figure 3b). As of September 2022, the patient has been treated with olaparib without tumor progression.
Discussion
We present the case of a patient with simultaneous occurrence of breast cancer and ovarian cancer with a BRCA1 mutation, treated with olaparib. Our patient responded effectively to olaparib treatment for both cancer types. To our knowledge, this is the first report on the efficacy of olaparib in a BRCA1 mutation-positive patient with simultaneous occurrence of breast and ovarian cancer.
HBOC is an autosomal dominant disease caused by germline pathogenic variants of BRCA1 or BRCA2 and is associated with a high risk of breast and ovarian cancer. The cumulative risk in patients harboring BRCA1 or BRCA2 mutation is reported as 50-60% in breast cancer and 20-40% in ovarian cancer by the age of 70 years [9]. The characteristics of BRCA1- or BRCA2- mutated breast cancer is diagnosis at or before the age of 50 years, multiple primary breast cancers in either one or both breasts, and triple-negative breast cancer [10]. On the other hand, BRCA1- or BRCA2- mutated ovarian cancer is characterized by high-grade serous carcinoma, and advanced cancer (stage III or IV) at diagnosis accounts for more than 80% of cases [11]. HBOC also tends to be at a greater risk for male breast cancer, prostate cancer, pancreatic cancer, and melanoma [12,13].
PARP inhibitors may represent a potentially important new class of chemotherapeutic agents directed at targeting cancers with defective DNA damage repair, such as BRCA mutation-associated cancer. Recent clinical trials have reported the efficacy of PARP inhibitors in several types of cancer, including breast cancer, ovarian cancer, prostate cancer, and pancreatic cancer [5,6,14,15]. Therefore, BRCA gene testing has been approved for patients with these cancers.
Phase III studies have demonstrated the efficacy of PARP inhibitors in patients with breast cancer with BRCA mutations. The OlympiAD and EMBRACA trials showed that olaparib and talazoparib had a progression-free survival benefit compared to placebo [5,16]. The efficacy of olaparib resulted in 7.0 months progression-free survival and 59.9% overall response rate in the OlympiAD trial. PARP inhibitors have been approved for patients with breast cancer harboring BRCA mutations based on the results of these clinical trials.
Moreover, clinical trials have reported that olaparib is a maintenance therapy for ovarian cancer patients with a BRCA1/2 mutation who have a complete or partial clinical response after platinum-based chemotherapy [6,17-19]. A randomized phase III trial, SOLO-1, demonstrated that olaparib treatment as maintenance therapy in patients with newly diagnosed advanced ovarian cancer with a mutation in BRCA1/2 had a progression-free survival benefit compared to placebo [6]. Additionally, the PAOLA-1 study assessed the efficacy of combining maintenance olaparib and bevacizumab in ovarian cancer patients regardless of BRCA mutation status [17]. In patients with a tumor BRCA mutation, the olaparib plus bevacizumab treatment had a PFS benefit compared to that associated with placebo plus bevacizumab (median PFS: 37.2 months vs. 21.7 months, HR 0.31). According to these results, olaparib, or a combination of olaparib and bevacizumab, has been used as a maintenance treatment for patients with advanced ovarian cancer with BRCA mutations.
Based on the above results, PARP inhibitors, including olaparib, are effective against BRCA-positive breast and ovarian cancers. Therefore, we decided to administer olaparib to our patients with both breast and ovarian cancer. However, the optimal chemotherapy choice after disease progression in breast or ovarian cancer remains unclear.
The patient should receive cytotoxic chemotherapy as the next-line treatment should she experience recurrence of the ovarian cancer; a treatment for which there is no clinical data on the safety of its combination with olaparib. There is a concern that adverse effects, especially hematologic toxicity, were intensified by the combination of olaparib and cytotoxic chemotherapy. A recent phase II study evaluated the efficacy and tolerability of olaparib in combination with chemotherapy for recurrent platinum-sensitive ovarian cancer [20]. This study demonstrated the tolerability of combination therapy for adverse events, including hematological toxicity. Based on this result, our patient might have been able to receive a combination of olaparib and cytotoxic chemotherapy after disease progression of breast cancer or ovarian cancer. The next line of treatment should be carefully selected if the patient is refractory to olaparib treatment.
To our knowledge, this is the first report of the efficacy and safety of olaparib treatment in a BRCA1 mutation-positive patient with simultaneous occurrence of breast cancer and ovarian cancer. Further studies are warranted to evaluate the efficacy and safety of olaparib in these patients.
Compliance with Ethical Standards
Data availability
There is no available data.
Informed consent
Consent for publication of the case was provided by the patient.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest regarding the publication of this article.
Acknowledgments
None declared.
References
- Sung H, Ferlay J, Siegel RL, et al. (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71: 209-249.
- Hu C, Hart SN, Gnanaolivu R, et al. (2021) A population-based study of genes previously implicated in breast cancer. N Engl J Med 384: 440-451.
- Arai M, Yokoyama S, Watanabe C, et al. (2018) Correction: Genetic and clinical characteristics in Japanese hereditary breast and ovarian cancer: First report after establishment of HBOC registration system in Japan. J Hum Genet 63: 541-542.
- Yoshida R (2021) Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 28: 1167-1180.
- Robson M, Im SA, Senkus E, et al. (2017) Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377: 523-533.
- Moore K, Colombo N, Scambia G, et al. (2018) Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379: 2495-2505.
- Network NCC Breast cancer (2022) NCCN clinical practice guidelines in oncology, ver. 4.
- (2022) Network NCC Ovarian cancer including Fallopian tube cancer and primary peritoneal cancer. NCCN clinical practice guidelines in oncology, ver. 3.
- Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25: 1329-1333.
- Noguchi S, Kasugai T, Miki Y, et al. (1999) Clinicopathologic analysis of BRCA1- or BRCA2-associated hereditary breast carcinoma in Japanese women. Cancer 85: 2200-2205.
- Sekine M, Nagata H, Tsuji S, et al. (2001) Localization of a novel susceptibility gene for familial ovarian cancer to chromosome 3p22-p25. Hum Mol Genet 10: 1421-1429.
- Castro E, Goh C, Olmos D, et al. (2013) Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 31: 1748-1757.
- (2022) Network NCC Pancreatic adenocarcinoma. NCCN clinical practice guidelines in oncology, ver. 1.
- de Bono J, Mateo J, Fizazi K, et al. (2020) Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382: 2091-2102.
- Golan T, Hammel P, Reni M, et al. (2019) Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med 381: 317-327.
- Litton JK, Rugo HS, Ettl J, et al. (2018) Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379: 753-763.
- Ray-Coquard I, Pautier P, Pignata S, et al. (2019) Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381: 2416-2428.
- González-Martín A, Pothuri B, Vergote I, et al. (2019) Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381: 2391-2402.
- Coleman RL, Fleming GF, Brady MF, et al. (2019) Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med 381: 2403-2415.
- Oza AM, Cibula D, Benzaquen AO, et al. (2015) Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol 16: 87-97.
Corresponding Author
Akinori Sasaki, Department of Gastroenterology and Oncology, Tokyo Bay Urayasu Ichikawa Medical Center, 3-4-32 Toudaijima, Urayasu, 279-0001 Chiba, Japan, Tel: +81-47-351-3101, Fax: +81-47-352-6237
Copyright
© 2023 Tanaka R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.