GC-MS Composition of Morinda Lucida Benth. and Phytotoxicity Activity of Ethyl Acetate Extract of the Bark
Abstract
Morinda lucida Benth is a well-known medicinal plant used for the treatment of various ailments across Africa and other parts of the world. Herein, we examined the phytochemical components of the ethyl acetate extract by GC-MS and also established the phytotoxic activity of the extract by using lettuce seed ( Lactuca sativa ). In the phytotoxicity experiment using Petri-dish lined with Whatman™ No 41 filter papers, three treatments(TR) were prepared in triplicates; TR1, TR2 and TR3, with concentrations 19.61µg/µL, 39.22µg/µL and 78.44µg/µL respectively. Data visualization was done using a bar chart by plotting the treatments against the Shoot and root lengths. It was observed after 7days that TR3 exhibited the highest phytotoxic activity against the seed growth with a reduction of 80% and 85% of the shoot length and root length respectively. The GC-MS data revealed 24 compounds with the major components being Ergosta-4,22-dien-3-one and Cholest-4-en-3-one constituting 14.2% and 12.9% respectively. Other compounds reported to possess phytotoxic activity identified in the extract are 4(1H)-Quinazolinone, 5-(p-hydroxyphenyl)-5-phenyl- and 9-octadecenoic acid.
Keywords
Phytotoxicity, Medicinal plant, Extract, GC-MS
Introduction
Morinda lucida Benth. (Rubiaceae) is a medium size tree with short curved branches which is used as a medicinal plant in Ghana for the treatment of ailments such as diabetes, hypertension, cerebral congestion, dysentery, stomach-ache, leprosy and gonorrhoea [1,2] It is common and can be found throughout the year. There is an increased use of its parts for treatment of ailments. Ethno botany claims on the healing properties of this plant is most often taut to cultural reasons, long-term use, affordability, safety and efficacy. Several researchers have reported the biological activity of this plant and its associated identified compounds. The many benefits derived from Morinda lucida is owed to the high contents of active secondary metabolites such as vitamins A, K and E, alkaloids, tannins, anthraquinones, sterols, saponins, polyphenols, terpenoids and cardiac glycosides. These group of compounds are reported to have antioxidant, which are effective as free radical scavengers, have anti-allergic, anti-inflammatory, anti-viral, anti-proliferative and anti-carcinogenic properties [3-5]. Figure 1 shows the Morinda lucida plant.
The use of Morinda lucida in crop protection has not been reported in literature. The plant is part of nature which encompasses several other organisms which act as the largest chemical laboratory providing several essential compounds which are beneficial to human health with some serving as hit to development of drugs [6]. Literature from the Chinese, Egyptians and the Mesopotamians indicated that plants used as medicine date back to 3000 to 4000 years ago. The Greek, Latin and Arabs who inherited ancient civilization emboldened the knowledge of medicinal plants [7]. The use of plants for crop protection was not left out, several years ago, agricultural practices depended heavily on crop rotation or mixed crop planting with the aim of controlling pests and also boost production. The idea of pest control in agricultural has its roots since man adopted farming as means of survival. The Greek and Roman scholars in the (371BC-79AD) wrote about various ways of pest management in agriculture with different approaches. The Chines in the (300 AD) came in with elaborations on biological pest management. They conducted a survey which revealed potential plants with pesticidal activities. The work of these scholars really gave birth to the exploitation of nature for solutions to pests’ management [8].
Plants that are used as pest management tool, produce secondary metabolites that exhibit the effects on the pests. Those used for weed control does that through a process known as Allelopathy [9]. Allelopathy is a phenomenon by which the growth or germination of plants are interfered or inhibited due to the release of secondary metabolites from plants or living organisms. The secondary metabolites involved in these interactions are called allelochemicals [10,11]. Allelochemicals comprise of compounds such as phenolic, terpenoids, flavonoids, benzoxazinoids, tannins alkaloids etc. can be found in various parts of plants [12]. On the field, these allelochemicals might enter the environment through different routes like leaching, volatilisation, root exudation, seed-coat exudation after imbibition and decomposition of different parts of the plant [13].
Knowledge of allelopathy/allelochemical is important since it act as a guiding principle in the identification and development of herbicides. Herbicides are phytotoxic chemicals used for controlling various weeds [14]. Currently, over 1,000 acttive herbicidal ingredients are in use worldwide, many of which are synthetic and contain non-degradable chemicals. These herbicides are considered to have a significant environmental impact, as their non-biodegradability and tendency to disperse through air and water make them environmentally unfriendly. Globally, over 4.10 million tons of pesticides are used annually, with herbicides making up 47.5%, followed by insecticides at 29.5%, fungicides at 17.5%, and other types accounting for 5.5% [15]. There is a pressing need to develop alternative weed control methods utilizing more environmentally sustainable and biodegradable chemicals.
Significant attention has shifted toward plants in the search for new active ingredients for herbicide production. Nature is believed to hold solutions to many unresolved challenges, particularly the non-biodegradability of organochlorine-based herbicides and the resistance that weeds have developed against these chemicals. In recent years, secondary metabolites have emerged as a significant source of leads for developing active pharmaceutical ingredients (APIs) and are recognized as a reliable reservoir of potential compounds for the herbicide industry. Building on this premise, we aim to investigate the medicinal plant Morinda lucida for active allelochemicals that inhibit the germination of lettuce seeds. These compounds could serve as hits for identifying potent phytotoxic principles with potential applications in herbicides development.
Results and Discussion
Phytotoxic experiment
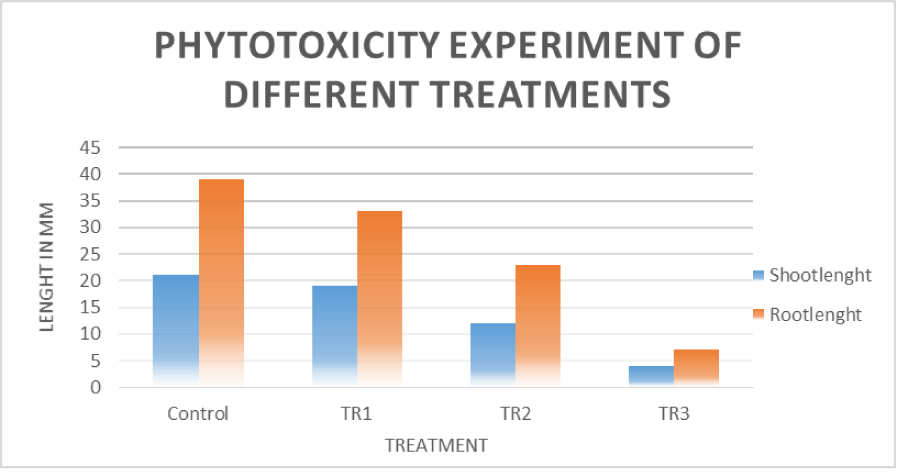
The results indicate that ethyl acetate extract of Morinda lucida caused inhibition of germination and growth of both the root and shoot compared to the control. Treatment one (TR1) exhibited relatively mild growth inhibition, likely due to a lower concentration of phytotoxic compounds in this treatment. A 10% reduction in shoot length and 18% reduction in root length were observed.
Treatment 2 (TR2) showed a further decline in growth, in this treatment, two-fold concentration of TR1 was used. This further reduction, could be due to a two-fold increase in phytotoxic compound found in the extract. A closer analysis revealed a shoot length reduction of 45% and a root length reduction of 43% with respect to the control. In Treatment 3 (TR3), shoot and root lengths were further reduced by 80% and 85%, respectively, compared to the control. This treatment involved a two-fold increase in concentration compared to TR2. The observed inhibition is likely due to the higher levels of phytotoxic compounds in TR3 resulting from the increased concentration. Figure 2 shows a bar chart of the control and the three treatments.
The determination of the percentage reduction of the root and shoot lengths is given by the relation below:
Where, N c = Length of the shoot/root in control experiment
N e = Length of the shoot/root in experimental set-up
The roots of the seeds were greatly affected, this is shown through the three treatments with respect to the control. This could be due to the fact that the first point of contact of the phytotoxic compound is the root [16]. Translocation of the phytotoxic compound may occur through the stem and would require a number of days to carry out this biological process. Water and nutrient uptake may be disrupted due to the phytotoxic principle in the extract. The difference in the rate of reduction in these two parameters (Root length and Shoot length) could also be a result of structural differences in the two.
Identification and Analysis of Potential Allelopathic Chemicals in Root Extracts of Morinda lucida
The extract was subjected to GC-MS analysis to ascertain the types of compounds present in the extract which could be responsible for the phytotoxic activities. Table 1 below shows the list and characteristics of compounds identified in the extract.
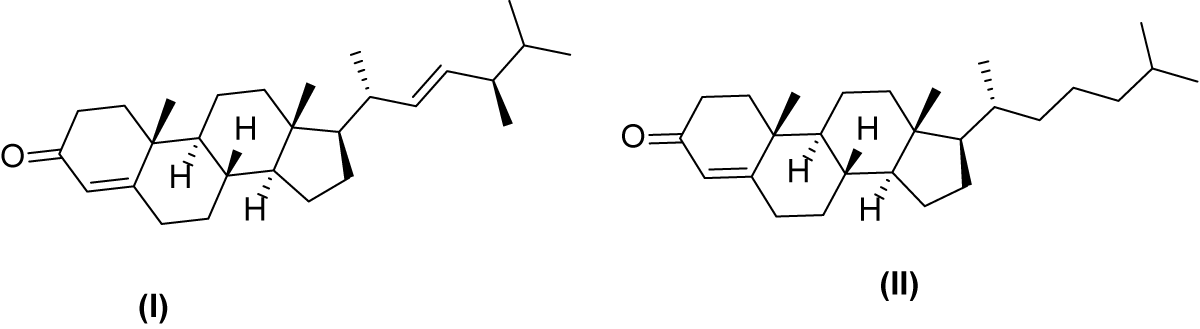
A total of 24 compounds were identified with their structures shown in [Table 1] of which Ergosta-4,22-dien-3-one (I) forms the major component of the extract representing 14.2% of the total component identified. Literature revealed that this compound has exhibited anti-microbial [17,18], antiviral and antitumor activity [19]. The second major component in the metabolite identified is Cholest-4-en-3-one (II) it constitutes 12.9% of the total components identified. It is an important synthetic intermediate in steroids transformation and has been found to be effective against obesity, liver disease, and keratinization [20]. Figure 3 show the structures of Ergosta-4,22-dien-3-one and Cholest-4-en-3-one.
Recent research revealed the presence of cholest-4-en-3-one in human cells migration. In other studies, the compound has shown that dietary cholest - 4 - en - 3 - one exerts anti-obesity and lipid-lowering effects in mice [21]. Studies from Ramesha, et al., revealed the allelopathic effect of cholest-4-en-3-one isolated from Endophytic Fungi [22].
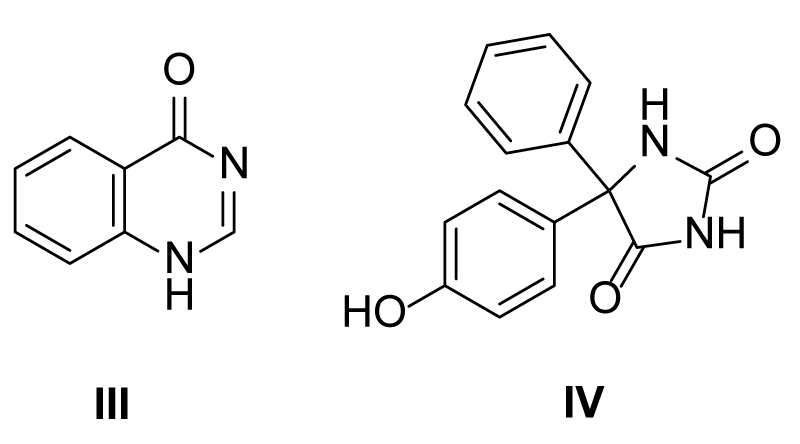
The quinazolinone 4(1H)-Quinazolinone ( III )) moiety attached to the synthesis of herbicidal compounds have shown great prospect. Na Li, et al., revealed that aryloxyphenoxypropionate herbicides were developed with much improved activity using quinazolinone moiety [23]. In other studies, 5-(p-hydroxyphenyl)-5-phenyl- (IV) have been reported to exhibit some allelopathic properties by disrupting plant growth by way of interaction with the plant hormones. Figure 4 shows the structures of the compounds mentioned in text.
Jin and colleagues identified an active fraction from Eichhornia crassipes roots containing a saturated fatty acid and pelargonic acid, which reduced chlorophyll levels in target species by up to 95.3% [24]. Similarly, rice husks demonstrated allelopathic effects on barnyard grass, attributed to a fraction containing 9-Octadecenoic acid, 7-Octadecenoic acid, 5,8,11-Heptadecatriecenoic acid, and Androstan-17-one. This fraction fully inhibited seed germination at 200 ppm with an MIC of 50 ppm [25]. The current study detected 9-Octadecenoic acid, constituting ~1% of the ethyl acetate extract, potentially contributing to allelopathic activity, as literature highlights similar compounds' phytotoxic effects.
Previous research has shown that Morinda lucida contains several biologically active compounds in its extract with some isolated compounds exhibiting antimicrobial properties [26-28]. Investigating the allelopathic properties of its ethyl acetate extract revealed that the plant produces secondary metabolites with allelopathic effects, in addition to its notable medicinal usages.
Material and Methods
General Experimental Procedure
Plant Material: The bark of the plant ( Morinda lucida ) was collected in June 2023 at Akim Oda in the Eastern region of Ghana. Specimen identification was done by the curator of the UCC Botanical Garden. The plant samples were air-dried and grinded into powder using an electric blender.
Reagents and chemicals used in this study were purchased from Merck, Ghana and all organic solvents were redistilled and dried according to standard procedures before being used. Petri-dish™ and Whatman No 41 filter papers were purchased from the market. Seeds were purchased from seeds store in Ghana
GC-MS data were recorded on an Agilent GC-MSD apparatus equipped with a DB-5SIL MS (30 m x 0.25 mm i.d., 0.25 µm film thickness) fused silica capillary column. He (2 mL min-1) was used as a carrier gas and ethyl acetate was used to dissolve the sample. The injector was kept at 250ºC and the transfer line at 280 ºC. The column temperature was held at 50ºC for 2 min, and then ramped to 280ºC at 20ºC min -1 where it was held for 15 min. The MS was operated in the EI mode at 70 eV.
Extraction procedure
The air-dried samples were crushed and blended into powder using an electric blender. The dry mass obtained was 200g.
Maceration method was employed for the extraction process. A total of 200 g of the powdered sample was placed in a flat-bottom flask, followed by the addition of 500 mL of pure ethyl acetate. The flask's mouth was sealed to prevent solvent evaporation, and the mixture was left to stand for 24 hours [29]. Afterward, the contents were filtered using Whatman™ No. 41 filter paper. This procedure was repeated twice, and the combined filtrates were concentrated using a rotary evaporator, Figure 5 below. The crude extract obtained weighed 56.33g, yielding 27% of the product.
Preparation of Extract for GC-MS Analysis
In the preparation of the extract for GC-MS analysis, 1mg of the extract was taken and dissolved in 1mL of ethyl acetate (GC grade) making a concentration of 0.001mg/mL (1µg/µL)
Phytotoxicity Experimental Set-up
In this set-up, 1g of the extract was taken and dissolved in 1mL of neat ethyl acetate solution making a stock concentration of 1000mg/mL. To prepare treatments TR1, TR2 and TR3, the following was done;
TR1 ; 100µL of the stock solution was taken and diluted with 5mL ethyl acetate making a final concentration of 19.61µg/µL.
TR2 ; 200µL of the stock solution was taken and diluted with 5mL ethyl acetate making a final concentration of 39.22µL.
TR3 ; 400µL of the stock solution was taken and diluted with 5mL ethyl acetate making a final concentration of 78.44µL.
Three Petri dishes were prepared, each lined with double layer of Whatman™ No. 41 filter paper. The filter papers were impregnated with plant extracts and left in a fume hood for 24 hours to allow the ethyl acetate to evaporate. Afterward, 5mL of distilled water was added to each Petri dish, and 20-30 lettuce seeds ( Lactuca sativa ) were randomly placed on the filter paper. A control group was set up alongside the experimental treatments, where the filter papers were treated with only ethyl acetate, allowed to evaporate to dryness, and then 5mL of distilled water was added, followed by 20-30 seeds. The set-up was kept at room temperature for 7days. Germination was considered successful when the radicle emerged more than 3 mm. The experiment was repeated three times. The root and shoot lengths were measured and their averages taken as indicators of phytotoxicity.
Conclusion
Crude ethyl acetate extract of Morinda lucida possesses strong allelochemicals capable of inhibiting the germination and growth of seeds. The study revealed the steady reduction in both shoot and root length with steady increment in extract concentration. At TR3 which has an extract concentration of 78.4µg/µL showed 80% and 85% reduction in the length of both the shoot and root respectively. The GC-MS analysis revealed 24 compounds with Ergosta-4,22-dien-3-one constituting the largest component of 14.2%. A number of phytotoxic compounds such as Cholest-4-en-3-one, 9-Octadecenoic acid, 4(1H)-Quinazolinone and 5-(p-hydroxyphenyl)-5-phenyl- acid have all been identified which could be attributed to the phytotoxic property of the extract. Bio-guided isolation approach is needed to establish a single principle responsible for the activity, this contributes to literature by establish that the ethyl acetate extract of the medicinal Morida lucida contains allelochemicals and serve as hit to the development new herbicides.
Competing interests
The authors report no financial or personal conflicts of interest.
References
- Ayertey F, Ofori-Attah E, Antwi S, et al. (2021) Anti-inflammatory activity and mechanism of action of ethanolic leaf extract of Morinda lucida Benth. J Tradit Complement Med 11: 249-258.
- Apenteng JA, Mintah DN, Klu MW, et al. (2017) In vitro anti-infective and antioxidant activities of Garcinia cola Heckel and Morinda lucida Benth. Journal of Medicinal Plants Research 11: 507-512.
- Adewole KE, Attah AF, Adebayo JO (2021) Morinda lucida Benth (Rubiaceae): A review of its ethnomedicine, phytochemistry and pharmacology. J Ethnopharmacol 276: 114055.
- Dah-Nouvlessounon D, Chokki M, Noumavo ADP, et al. (2023) Ethnopharmacological value and biological activities via antioxidant and anti-protein denaturation activity of morinda lucida benth and momordica charantia l. Leaves Extracts from Benin. Plants (Basel) 12: 1228.
- Adebayo JO, Adewole KE, Krettli AU (2017) Cysteine-stabilised peptide extract of Morinda lucida (Benth) leaf exhibits antimalarial activity and augments antioxidant defense system in P. berghei-infected mice. J Ethnopharmacol 207: 118-128.
- Nicolaou KC, Chen JS, Dalby SM (2009) From nature to the laboratory and into the clinic. Bioorg Med Chem 17: 2290-2303.
- Naboulsi I, Aboulmouhajir A, Kouisni L, et al. (2018) Plants extracts and secondary metabolites, their extraction methods and use in agriculture for controlling crop stresses and improving productivity: A review. Acad J Med Plants 6: 223-240.
- Dayan FE, Cantrell CL, Duke SO (2009) Natural products in crop protection. Bioorganic & Medicinal Chemistry 17: 4022-4034.
- Han M, Yang H, Huang H, et al. (2024) Allelopathy and allelobiosis: Efficient and economical alternatives in agroecosystems. Plant Biol 26: 11-27.
- Latif S, Chiapusio G, Weston LA (2017) Chapter Two - Allelopathy and the role of allelochemicals in plant defence. Advances in Botanical Research Becard, G. Ed, Academic Press 82: 19-54.
- Hierro JL, Callaway RM (2021) The ecological importance of allelopathy. Annual review of Ecology, Evolution, and Systematics 52: 25-45.
- Palanisamy CP, Gunasekaran VP, Dominic S, et al. (2020) Phenolic allelochemicals from crops and weed management. Plant Phenolics in Sustainable Agriculture 1: 183-199.
- Iqbal A, Shah F, Hamayun M, et al. (2019) Plants are the Possible Source of Allelochemicals that can be Useful in Promoting Sustainable Agriculture. Fresenius Environmental Bulletin 28: 1040-1049.
- Gupta PK (2014) Herbicides and fungicides. In. Biomarkers in Toxicology. Gupta RC, Academic Press 409-431.
- Sharma K, Tripathy V, Gopal M, et al. (2018) Good agricultural practices and monitoring of herbicide residues in India. Herbicide Residue Research in India 443-465.
- Mota Filho TMM, da Silva Camargo R, de Menezes CWG, et al. (2024) Fumigant toxicity of Cymbopogon flexuosus lemon grass (Poaceae) essential oil to Sitophilus zeamais maize weevil (Coleoptera: Curculionidae) and phytotoxicity to Zea mays (Poaceae). Cereal Research Communications 52: 215-220.
- Ramos-Ligonio A, López-Monteon A, de la Soledad Lagunes-Castro M, et al. (2017) In vitro expression of toll-like receptors and proinflammatory molecules induced by ergosta-7, 22-dien-3-one isolated from a wild mexican strain of Ganoderma oerstedii (Agaricomycetes). Int J Med Mushrooms 19: 203-211.
- Hai P, Gao Y, Yang L, et al. (2023) Two new compounds from the endophytic fungi of dryopteris crassirhizoma and their antimicrobial activities. Molecules 28: 8043.
- Agboke AA, Nwosu C, Ubak FI, et al. (2021) In vitro evaluation of Anti–MRSA properties and GC-MS bioactive compunds of methanol extract fractions of Moringa oleifera Lam. Root Bark. European Journal of Biotechnology and Bioscience 2321-9122.
- Wu K, Li W, Song J, et al. (2015) Production, Purification, and Identification of Cholest-4-en-3-one Produced by Cholesterol Oxidase from Rhodococcus sp. in Aqueous/Organic Biphasic System. Biochem Insights 8: 1-15.
- Higuchi M, Okumura M, Mitsuta S, et al. (2024) Dietary cholest-4-en-3-one, a cholesterol metabolite of gut microbiota, alleviates hyperlipidemia, hepatic cholesterol accumulation, and hyperinsulinemia in obese, diabetic db/db mice. Metabolites 14: 321.
- Alurappa R, Chowdappa S, Narayanaswamy R, et al. (2018) Endophytic Fungi and Bioactive Metabolites Production: An Update. In. Microbial Biotechnology: Volume 2. Application in Food and Pharmacology. Patra JK, Das G, Shin H.S, Springer Singapore 455-482.
- Li N, Chen K, Han S, et al. (2024) Synthesis, Herbicidal Activity, and Molecular Mode of Action Evaluation of Novel Aryloxyphenoxypropionate/Amide Derivatives Containing a Quinazolinone Moiety. J Agri Food Chem 72: 9445-9456.
- Jin Z, Zhuang Y, Dai S, et al. (2003) Isolation and identification of extracts of Eichhornia crassipes and their allelopathic effects on algae. Bull Environ Contam Toxicol 71: 1048-1052.
- Ko J, Eom SH, Kim Myong Jo, et al. (2005) Allelopathy of rice husk on barnyardgrass. Journal of Agronomy 4: 288-292.
- Del Vecchio G, Zhang L, Sinan KI, et al. (2025) Different extraction methods shape the phenolic signature and biological activity of Morinda lucida extracts: A novel source of bioactive compounds preparing functional applications. Food Chemistry 462: 140956.
- Achukwu N, Enweani-Nwokelo I, Urama E (2024) In vitro evaluation of Morinda lucida root extracts against multi-drug resistant bacterial pathogens isolated from diabetic foot ulcers: Évaluation in vitro d'extraits de racine de Morinda lucida contre des agents pathogènes bactériens multirésistants isolés d'ulcères du pied diabétique. African Journal of Clinical and Experimental Microbiology 25: 438-445.
- Aiyonoguan I, Iyekowa O, Oghomwen RO, et al. (2024) GC-MS Analysis and Antifungal Activity of Morinda Lucida Extract. NIPES-Journal of Science and Technology Research 6.
- Gori A, Boucherle B, Rey A, et al. (2021) Development of an innovative maceration technique to optimize extraction and phase partition of natural products. Fitoterapia 148: 104798.
Corresponding Author
Mahama Alhassan, Department of Chemistry Education, University of Education, Winneba.
Copyright
© 2025 Amenano J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.