Distribution of Toxic Plants in Zimbabwe: The Albizia Species
Abstract
Toxic plants are a major drawback to livestock production since they can bring about total loss or reduced productivity of affected animals. Intoxications from plants are generally listed last on clinician's differential diagnoses. There are no published articles specifically on the distribution of toxic plants in Zimbabwe despite the presence of these plants in the country. The effects and impacts of these toxic plants may therefore have been overlooked over the years. Thus, it was imperative that an assessment of the distribution of Albizia species of toxic importance in Zimbabwe be carried out. This was also necessary as it would unveil much information to the veterinary profession and farmers leading to the prevention of deaths of ruminants due to albiziosis. The purpose of the survey was to establish the distribution of the toxic Albizia species in Zimbabwe, the associated species, their habitats and the possible factors associated with their spread. Five toxicologists and a botanist participated in the survey. Plant species were identified to genus and species level. Confirmation of the plants was done by an experienced botanist. All the provinces and districts of Zimbabwe were visited by road. The species of the plants, the coordinates of the location of the toxic plants were recorded as well as the nature of the environment in which the plants were located or grew. Quantum GIS was used to plot the maps showing the distribution of the plants in Zimbabwe. Two major Albizia plant species of toxicological importance were encountered: Albizia tanganyincensis and Albizia versciolor. A.tanganyicensis was found mainly in the North Eastern Zimbabwe and the Southern parts of the country. Albizia versicolor was found mainly in the periphery of the central Highveld of Zimbabwe. The main associated species for A.tanganyicensis and A.versicolor is Sterculia africana, which served as an indicator plant as well. Terminalia sericea and Julbernardia globiflora were also associated with these albizia species. The spread of A. tanyanyicensis was found to be attributed to seed and adoption for domestic use as fencing poles and as ornamental plants. A. tanganyicensis and A. versicolor could be causing ruminant poisoning in Zimbabwe, however, cases may go undiagnosed due to the low index of suspicion among both the veterinary profession and poor knowledge among the general public. Albizia tanganyicensis and A. versicolor are well established in Zimbabwe. Veterinarians should have a high index of suspicion when investigating cases of sudden deaths in ruminants with signs of struggle. This study should provide veterinarians and farmers with a raised awareness on the probability of albiziosis in Zimbabwe. Training of farmers on prevention of deaths due Albizia species is of paramount importance.
Keywords
Albizia tanganyicensis, Albizia versicolor, Zimbabwe, Albiziosis, Toxic, Distribution
Introduction
Poisoning of livestock by plants result in considerable economic losses [1,2]. Plants that are poisonous to livestock can grow in anyplace and identification of the toxic plants that are common in each area can help reduce their economic impact on livestock farms [3]. Toxic plants are those plants which have active principles or chemical compounds that when animals or humans ingest, inhale or get into contact with, cause death, disease or injury [4]. Toxic plants have therefore a salient important impact on the health and productivity of grazing animals with cattle taking the most of the brunt due their non-selective grazing behavior. Such toxic plants are common in developing countries like Zimbabwe, however under reporting of poisoning cases could be due to lack of infrastructures and lack of knowledge on toxic plant taxonomy [5]. Underlying causes of exposures of animals to poisons are difficult to establish due to several reasons, which include as exposures going unnoticed by the owners or happening at night [6].
In Germany plant intoxications are responsible for the highest number of fatal cases among animals [7]. In the USA, between the years 2000-2010, exposures of animals caused 1371095 cases which represented approximately five percent of all cases of poisoning exposures reported on animals and humans [8]. Plants Poisonous as well as the secondary metabolites of these poisonous plants account for several cases of intoxication horses and cattle in Europe [9]. Albizia species are among the plants of toxic importance in Southern Africa including Zimbabwe [10].
The genus Albizia belongs to the family Fabaciae and is characterized by a root system with nitrogen fixing nodules comprising populations of Rhizobium bacteria, a characteristic that benefits both the species and other plants growing near its vicinity. Albizia species can increase the understorey grasses nutrient content due to the rapid turnover of their leaves and can result in great increases in the fertility of soil [11]. The Albizia (Fabaceae) genus includes almost 150 species, mainly shrubs and trees that are native to subtropical and tropical regions of Africa as well as Asia. The species of Albizia plants can contribute to ruminant poisoning if animals gain access to large amounts of unripe pods. Two species in the family Fabaciae, A. tanganyicensis and A. versicolor have been identified as the toxic species in Southern Africa whilst A. gumifera was also described as toxic in East Africa [12]. Aspects that are important in animal poisoning include: Ripening state of its fruits, time of year temperature, humidity, the physical conditions for its development, that is, type of soil, the age, the chemical substances present and its concentration, and the part of the plant ingested [13].
In Southern Africa consumption of the pods of A. tanganyicensis and A. versicolor cause cattle deaths, hypersensitivity, intermittent tetanic convulsions [10]. A. tanganyicensis is also called white paper bark Albizia [14]. The two neurotoxins from A. tanganyicensis were elaborated in 1987 by Steyn, et al., [15,16]. The principal toxin is 4-methoxy-pyridoxine and is similar in structure to that of the B6 vitamins, pyridoxine. It is a neurotoxin that is antagonist to vitamin B6 and interferes with coenzyme activity in the central nervous system [16,17]. Characteristics of animal poisoning in South Africa are similar the features of Albizia poisoning to those in other countries [2] including Zimbabwe. Native plants, like some Albizia species, are the primary causes of animal poisoning around the world [18]. The reported cases of animals exposed to poisonous plants, such as Albizia species, may represent only a fraction of the actual exposures [2] due to under reporting and misdiagnosis of poisoning cases.
Albizia versicolor is a tree that is medium to large that is usually with a height of about 10m and its crown is spreading or rounded [19]. A. tanganyicensis is a more upright tree usually only 3-8m tall that is medium sized, sparingly branched and has a thin, papery bark, brownish that is very characteristic [19]. The main characteristic which identifies this genus is the compound leaf which bears a glandular thickening or nodule on the petiole and small flowers borne in finger-shaped or globular clusters with stamens that are characteristically showy. The typical distribution of A. tanganyicensis is rocky granite hills and kopjies and watersheds for A. versicolor [10]. Despite A. versicolor having several medicinal uses in humans in various countries for the treatment of venereal diseases, as anti-helminthics, purgative and analgesic [20], It's unripe pods are very toxic to ruminants and cause albiziosis in cattle, which may lead to death in 48 hours of consumption [21].
There is little to no data specifically on the distribution of toxic plants in Zimbabwe. Thus, this study provides a comprehensive investigation on the distribution of Abizia species of toxicological importance in Zimbabwe. Knowledge on plants that are toxic is still inadequate and this makes it necessary to characterize plant species [13] and establish their distribution. As plants are important causative agents of animal poisoning, correct identification and classification of the plants based on taxonomic characteristics and active substances they contain is important for the diagnosis of species-specific toxicity in plant poisoning [18]. The goal of this research study was to establish the distribution of the Albizia species that are of toxic importance to animals, the associated species and habitats of plants and as well as the factors associated with their spread. This information is important as it raises the index of suspicion among veterinarians and cattle owners for the purpose of diagnosing and preventing albiziosis. Furthermore, this study will serve as a future reference for future research in the area of plant toxicology and albiziosis. In this study the distribution of the two species of Albizia that have toxicological importance are described, together with their associated habitats and plant species.
This research was imperative because there were no comprehensive distribution maps on toxic plants in Zimbabwe to guide veterinarians on the likelihood of Albizia poisoning cases occurring in their zones of operation. Additionally, this publication serves as a basis for future research and referencing as well as policy making in the field of animal and veterinary toxicology. The first step to managing animal plant toxicosis is to know where the species are and where they are not. This is the first compilation in Zimbabwe to offer a comprehensive coverage of the distribution of Albizia plants of toxicological importance based on a country-wide survey. The distribution maps will serve as the basis for teaching and learning on plant toxicology, especially the veterinary profession as the plant mainly affects animals.
Materials and Methods
Selection of study sites
A ground based exploratory field survey of the toxic plants was carried out in Zimbabwe. The main toxic plants of animals, some of which were reported by Kellerman, et al., [10] as occurring in Zimbabwe, were targeted, which included the Albizia species. All the 10 provinces in Zimbabwe were included as study sites. The entirety of all the districts of Zimbabwe was included in the survey. The places were randomly chosen and visited along navigable roads. The areas surveyed included farms, communal areas, mountains, valleys and riversides. Also road sides and riparian areas were scouted and sighting of toxic plants were noted. Accessible areas with dip tanks and homesteads were also visited. Furthermore, areas that were suspected to have toxic plants were intentionally visited to check and confirm the presence of such plants. Furthermore, a random spotting of toxic plants along the way resulted in further exploration of the area for similar and other toxic plants.
Data collection
All the provinces and the entirety of districts in Zimbabwe were visited and data collection done. Road transport was used and villages and farms and mountains visited for the presence of toxic plants. People who knew about the possible locations of the toxic plants were used as leads to the plant's locations. The locations and discoveries of most of the plants were de novo from various walk-through surveys throughout Zimbabwe. Each plant was identified to genus and/or species level. Six surveyors were involved in carrying out the study. The surveyors included five veterinary toxicologists from the University of Zimbabwe Faculty of Veterinary Science (UZFVS) and an experienced botanist from the National Hebarium of Zimbabwe. Surveyors operated in pairs while surveying distinct areas in the field but keeping a loose contact so as to ensure that most of the toxic plants were identified. Areas were scouted and presence of the plants were noted. Plants were examined in-situ and characterized using botanical/taxonomic features to genus and species level. Confirmation of the species identities was done by the experienced botanist from the National Herbarium of Zimbabwe who was part of the exploration team with members of the toxicology section of the University of Zimbabwe.
Photographs of toxic plants were taken in-situ. Plant and anthropogenic factors responsible for the spread of the Albizia species such as possible use of the plants were noted. Any unusual location of toxic plants was also recorded. For the habitat, the information on the soil type was noted and the terrain of the land. Associated plant species were also recorded. The GPS locations of the plants were determined using a GPS machine. All data was recorded on a data sheet for each location.
Permission and consent
Consent was sought for access of all places. Trespassing was avoided at all times. Owners of homesteads and farms, or their agents were approached on the day of survey for their consent only when no public rights of way were available. Surveying was carried out from footpaths, roads and other area where public rights of way was ensured.
Data analysis and data presentation
Data was captured onto excel spread sheet. Data recorded included the genus and species of each plant, the name of the place that the plants were sighted, their GPS locations, and the nature relative densities of the plant in the area- whether sparse, dense, or single/lone plant. The topographic category of the location was also noted as being one of the following: valley, slope, summit or ridge. The habitat type was designated using categories such as grassland, woodland, cropland, disturbed land, disused cropland, roadside or slope. The habitat in terms of soil type was noted as loam soil, clay soils, or rocky area well as the associated/indicator plants. Data was from the data sheet was captured into excel spread sheet. Data in the excel spread sheet was cleaned, analysed and converted into a quantum GIS data base. The distribution maps for each toxic plant were then created using the same software. Data on factors promoting the spread of the plants and the associated species, as well as the habitat were qualitatively analysed using themes.
Results
Types of toxic Albizia species in Zimbabwe
Two main Albizia species of toxicological importance were identified in Zimbabwe: Albizia tanganyicensis (Figure 1) and Albizia versicolor (Figure 2). A. tanganyicensis, common name paper bark Albizia, is a deciduous tree with a very distinctive pale grey to almost white trunk bearing brown papery bark that flakes off. The leaves are imparipinnate with 3-7 pairs of oblong shaped leaflets and bear a glandular nodule on the petiole. The position this glandular nodule is located varies from leaf to leaf on the same tree. Flowers are a pale green corolla with numerous stamens whitish green near the apex. The fruit is an oblong hairless brown dehiscent pod. Albizia versicolor, common name poison pod Albizia or large leafed false thorn is a medium to large deciduous tree 10m tall with a spreading crown often throwing branches close to the ground (Figure 2). The leaves are twice compound bearing 2-5 pairs of pinnae. The pinnae are large elliptic to ovate hairy above and rusty velvety below. The glandular nodule borne on the petiole of A.versicolor occurs on variable positions on the petiole. The flowers are a large powder puff of creamy-white heads with short duration lifespan and wither off November/December. The fruit is a dehiscent thin flat glossy pod.
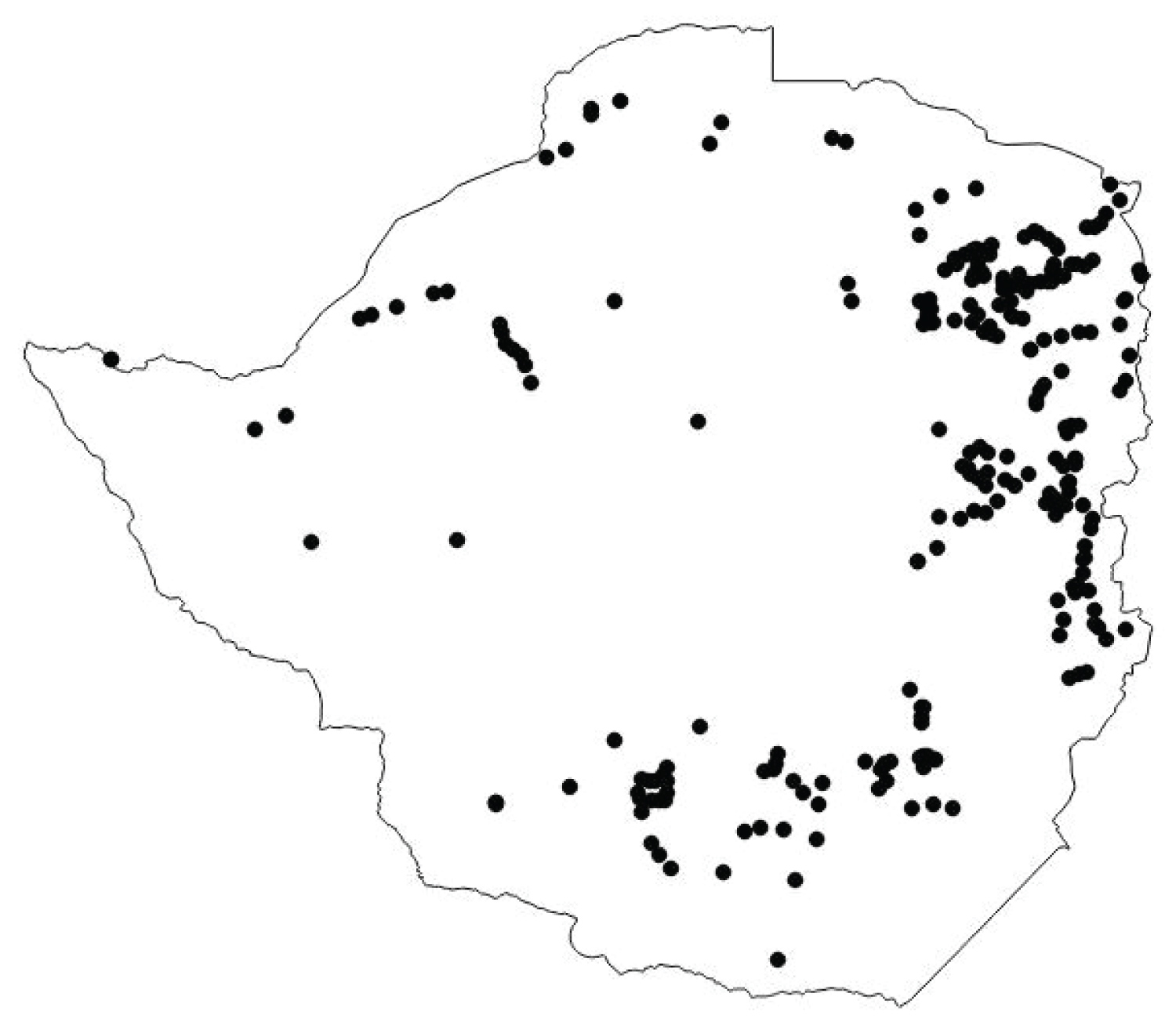
Distribution of A. tanganyicensis in Zimbabwe
Albizia tanganyicensis is more commonly found in the Eastern and Southern parts of Zimbabwe (Figure 3). In the Eastern parts of the country, the plant is located closer to the border and is more densely distributed than in any other region. In the central part of the country the tree is poorly distributed and absent in most parts of the country. In the north-western parts of the country A. tanganyicensis has a more scatter type of distribution.
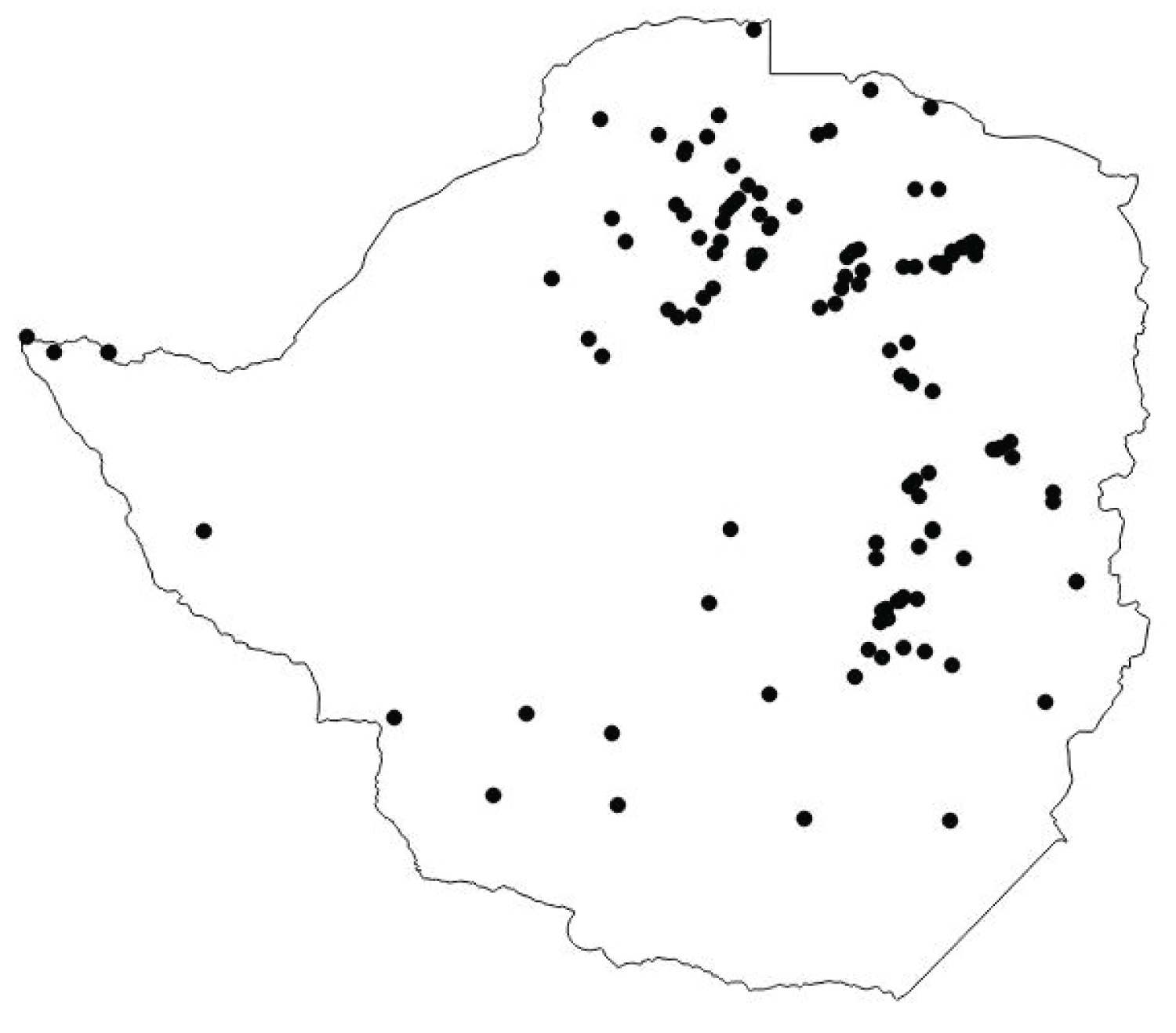
Distribution of Albizia versicolor in Zimbabwe
A. versicolor is found mainly on the eastern and northern parts of the central Highveld of the country (Figure 4). In the eastern parts of the country, A. versicolor is found more interior than A. tanganyicensis. A. versicolor is found in the Northern parts of the country of the country as well. In these areas, it was found mainly on waterways and near rivers and rivulets. It is more scattered in the southern parts of the country while in the western parts of the country the tree species is nearly absent. Along the eastern border areas there are very few A. versicolor plants and in most of the areas it is absent.
The nature and factors associated with the spread of the plants
A. tanganyicensis was found being adopted for domestic use. In Mutoko and Manical and A. tanganyicensis was found being used as live fencing poles. Propagation from cuttings was further explored and led to a flourishing tree on a rockery in a Harare suburb. The typical distribution sites for A. tanganyicensis, granite kopjes and hills, tended to have dense occurrence of the plant whereas the atypical distribution had fewer plants. A.tanganyicensis was also found being used for ornamental purposes as well. A. tanganyicensis and A. versicolor were found in flower August to October, young fruits in February to May, and from June to August the pods are ripening and drying. Nowhere in this survey was A. versicolor found being propagated by human beings.
Associated and indicator plants
The plants associated with A. tanganyicensis and A. versicolor included Steculia africana. Steculia africana species served as the main indicator species determinable from a distance due to its broad leaves. Other associated species included Terminalia sericea and Julbernardia globiflora (Table 1).
Associated Habitats
Albizia tanganyicensis was found typically on rocky granite kopjes and hills and atypically on sandy and loamy soils due to propagation. In Tsholotsho, this species was sighted on sandy soil with no visible rocky formation in the vicinity. A. versicolor was found mainly in the watershed and riverine areas with loamy soils but was also found on hills. Most sightings were on waterways, near rivers and rivulets. At Amanda's hill in Concession, Mazowe, both species were found occurring on top of the hill and one location had young trees of both species growing together. More A. versicolor trees were located all over this hill together with A. tanganyicensis and Steculia africana.
Discussion
Two major toxic Albizia plants were found in Zimbabwe: A. tanganyicensis and A. versicolor. These have been reported in South Africa as causing toxicities [2,10]. In this study, Both A. tanganyicensis and A. versicolor were found to have a wide distribution wider than traditionally believed. According to Mbanze, et al., [16], A. tanganyicensis and A. versicolor and are represented fairly well in the part of the African continent north of the Limpopo, and they occur together in Zimbabwe as well as in the far north parts of the Transvaal [16]. The distribution of the plants tends to be less dense from eastern parts of the Zimbabwe to the western parts of the country. This could be due to change in the rainfall pattern across the country. The rainfall pattern tends to decrease from the east to the west in Zimbabwe. The total number of species of plants, including the Albizia species, increase with increasing mean annual rainfall across the rainfall gradient [22]. For A. versicolor, the distribution could be decreasing due to decrease in the density of water sources from the eastern parts to the western parts of the country. Normally A. versicolor is found along rivers and rivulets [10]. Thus, the decrease in river density and water sources may result in decrease in the sightings of A. versicolor in Zimbabwe from East to west.
Albizia plant species can be used for nitrogen fixation as these show the symbiotic relationship with Rhizobium bacteria and fix atmospheric nitrogen used for its growth and also for the enrichment of the rhizosphere [23]. Nitrogen fixation and its roots that bind the soil render Albizia species, including the toxic species, useful for soil conservation and erosion management. It is recommended that Albiziosis be prevented by cutting down and destroying. However, nitrogen fixation may outweigh the usual measure of cutting down and destroying the Albizia plants. It was also established in the present survey that the plants were being used for domestic purposes such as ornamental and shade. Some of the toxic plants are ornamental plants [24]. It was also established elsewhere that Albizia plant species are also grown as an ornamental tree due to their pleasant appearance or shade [25]. When used as ornamental plants the attractiveness of these plants hides their poisonousness and the lack of knowledge among farmers potentiates these conditions [13].
A. tanganyicensis is found mainly on rocky kopjes and granite hills [10]. However, the finding of the plant in the loam soils means that the distribution of this tree is much wider than the granite rocky hills and kopjes. Propagation from cuttings was further explored and led to a flourishing tree on a rockery in a Harare suburb. This means that the distribution of this tree is much wider than reported previously by Kellerman, et al., [10]. A. tanganyicensis is found in the eastern and northern parts of Zimbabwe. In the North-western parts of the Transvaal in South Africa, A. tanganyicensis is predominant [10]. The finding of A. tanganyicensis in the Southern parts of Zimbabwe agree with Kellerman, et al., [10] who noted that A. tanganyicensis is also found in the Southern parts of Zimbabwe. The finding that the plant also occurs in various parts of the country means that albiziosis is could be more widespread than previously thought, that is, not only in the Southern parts of the country but in other parts of the country as well.
In South Africa, A. versicolor is distributed more easterly and stretches along the eastern parts of the Transvaal and then into Swaziland and eventually passes into the northern Natal as well as Zululand [10]. In Zimbabwe A. versicolor was found in the eastern parts of the country as well but A. tanganyicensis as a more easterly distribution than A. versicolor. More A. versicolor trees have since been sighted all over this hill together with A. tanganyicensis and Steculia africana tree. This finding indicates that both A. versicolor and A. tanganyicensis could actually be distributed much wider that is traditionally believed but is only indicative.
In this study, Steculia africana was found to be the indicator plant for both A. tanganyicensis and A. versicolor. In South Africa, the Commiphora species were established as the indicator plants [10]. Steculia africana serves as a good indicator plant because of its association with Albizia species and its broad leaves that can easily be identified from a distance.
The crown of A. tanganyicensis is normally held up such that leaves and pods are not easily accessed from the tree by cattle nor small ruminants. In southern Africa, consumption of the pods of A. tanganyicensis and A. versicolor by cattle is associated with irregular tetanic convulsions, hypersensitivity and mortality [10]. Plant toxicity differs from one plant to another and depends on various aspects such as the different chemicals substances that they have and their concentration, part of the plant that has been ingested, age of the plant, the physical conditions (time of year, soil type, temperature, humidity) for its development as well as the state of the ripening state of its fruits [4]. For A. versicolor and A. tanganyicensis it is the pods that are toxic to cattle. Strong winds that occur in winter and spring are associated with poisoning due to large amount of Albizia pods that fall as a result and become accessible to animals on the ground [10]. Poisoning occurs mainly on cattle and rarely on goats and sheep as a result of the consumption of the pods of A. versicolor and A. tanganyicensis [26].
In Zimbabwe, few cases of Albizia poisoning have been reported. Albizia toxicity could therefore be under diagnosed and under-reported. This could be due to low index of suspicion. Various challenges are associated with confirming underlying causes of animal poisoning exposures such as poisoning going unnoticed by the owners and the poisoning exposures occurring at night [6]. Such deaths can be ascribed to witchcraft and the veterinarians not called to attend to the post mortem or farmers salvage the meat instead of calling the veterinarian to attend since veterinarians would normally dispose dead animals by burning or burying. In both instances veterinarians are not called to make a diagnosis and advise accordingly. This might explain why there have not been confirmed cases of albiziosis in Zimbabwe since independence. The absence strong winds occurring in the country could also result in cases of albiziosis being absent. Furthermore, there must be a coincidence of strong winds and the availability of the pods when they a very toxic. In Zimbabwe, the pods are most toxic in February to May, when they are unripe and a time in which there are no strong winds. This means that the pods are less likely to fall and be exposed to cattle at their most toxic phase. Furthermore, it could be due to low suspicion index and poor diagnosis. Diagnosing the poisonings of livestock by plants is not always easy and simple, and relies on pathological lesions, evaluation of clinical signs, good case history as well as confirmatory laboratory tests [26]. Thus, the cases of albiziosis are probably being missed due to poor diagnosis. Mashobabne, et al., [2] also noted that the cases of poisoning appear to be under-reported in South Africa [2].
With Albizia poisoning, animals are usually found dead with evidence of struggle on the ground around the carcass as a result of violent convulsions before death. Animals observed in time can recover if treated with Vitamin B6 [19]. The toxic principle is 4-methoxypyridoxine, a vitamin B6 antagonist. Diagnosis is reached following post mortem examination and finding macerated pods in the rumen. Confirmation of source of the pods is reached from survey of the pastures where the causal trees would be found. Such deaths usually follow heavy winds that would have blown down unripe pods. History, clinical signs, post mortem findings, signs of plants being grazed and presence in the gastrointestinal tract of the remains of toxic plants, are important in diagnosis of plant poisoning including albiziosis [26].
In Zimbabwe, the occurrence of the windy season in August mainly may contribute to subclinical cases as the pods would be dry and with reduced toxicity. The most toxic are the young pods in which the toxin is concentrated in the cases of the pod and to a less common in the seeds [16]. Critical period to be wary of possible albiziosis in Zimbabwe is February to May when pods would still be unripe and most toxic, but during this period in Zimbabwe, there are no strong winds. In other countries, cattle albiziosis outbreaks usually occur in early spring or late winter when strong winds blow the pods from the trees [27].
Consumption of A. versicolor pods as little as 0.57 to 1.14 kg have shown to be fatal to cattle with 230 kg body mass while [27]. On post mortem examination there will be signs of disturbance of environment around the carcass which can be in good body condition. On opening up the carcass, macerated pods are found in the rumen. Confirmation is done by identification of source of the pods nearby. Care must be taken not to confuse these macerated pods with those of Acacia sieberiana which can be fed to ruminants when dry and being non-toxic then. When pods are found in the rumen survey of the pasture used the previous day would allow identification of source of the pods. In case of plant poisoning, including albiziosis, it is important to consider individual variability where aspects such as degree of exposure to the plant, age, physical condition and weight are important and at times diagnostic [13]. This variability, together with heavy winds, is responsible for clinical signs of albiziosis varying from sub clinical to an acutely fatal syndrome. Toxicities usually occur when cattle consume contaminated silage or hay [2]. Other causes are drought, recently burned pastures [28] and livestock/pastures management [29]. Poisoning cases are likely to occur when young pods are blown down by heavy winds and ruminants accessing these pods can succumb to death characterized by struggle. In Zimbabwe, August winds blow down dry pods and therefore the dose intake of the toxic principle, 4-methoxypyridoxine, per animal would be less than when young pods are blown down giving rise to sub-clinical cases. Clinical cases can be treated with pyridoxine injection.
Conclusion and Recommendations
Both A. tanganyicensis and A. versicolor were found to have a wide distribution wider than traditionally believed. A. versicolor should be understood as occurring not only along the riverbanks and flat lands but also to occur on hills. The veterinary profession should appreciate not only the botanical identification of the Albizia species but also that watersheds also occur on hills allowing A. versicolor a much wider distribution. The period during which pods would still be unripe and most toxic extends from February to May. Heavy winds during this period can blow down highly toxic pods. The seasonal winds encountered in August would only be blowing down ripe dry pods which are less toxic. Thus, during the period March to May winds would pose danger to animals and this is the time veterinarians should look out for albiziosis cases. Albizia tanganyicensis was found to be growing typically in the mountain areas and atypically as propagated on sandy and loam soils. Albizia versicolor was found growing on watershed and riverine soils as well as loamy soils on the hills. Local management of plants with the support of and participation of local communities is essential [30] for the prevention albiziosis optimal pasture management as well as well-informed farmers having a good knowledge of the local plants likely to cause poisoning and the circumstances under which this is likely to occur [26]. This work is expected to allow veterinarians in Zimbabwe to appraise themselves on the Albizia species identity and distribution and pave way to diagnosis of cases of poisoning in the future. Client education for the prevention of poisoning would assist in preventing any deaths should heavy winds occur during the critical season since these cases hardly allow intervention with pyridoxine injection. It is crucial that farmers are educated on the proper harvesting and disposal of pods from their live Albizia fencing poles to prevent cattle poisoning should heavy winds prevail. Thus, it is important that farmers be trained in the proper harvesting and disposal of the pods.
Author Contribution
Cecilia Masvingwe: Conception of the research project, data collection, data compilation, writing of the manuscript; Frank Kapungu: Data compilation and collation, data cleaning, plotting of the maps, writing and reviewing of the manuscript.
Conflict of Interest
The authors have no conflict of interests.
Acknowledgements
The authors would like to appreciate the following from University of Zimbabwe: Erick Kandiwa, Solomon Gove, Richard Tewe, Rangarirai Marumani and Antony Mapura from the National Herbarium of Zimbabwe for the assistance in data collection. The authors also greatly appreciate the funding provided by the European Union for the research.
Funding
Funding for data collection was provided by the European Union. Details of the funding are not available. The Funder played no part in the design of the study; data collection and analysis, results interpretation nor in the writing of the manuscript or in the decision to submit for publication.
References
- Kellerman TS, Naudé TW, Fourie N (1996) The distribution, diagnoses and estimated economic impact of plant poisonings and mycotoxicoses in South Africa. Onderstepoort J Vet Res 63: 65-90.
- Moshobane MC, Bertero A, Marks C, et al. (2020) Plants and mushrooms associated with animal poisoning incidents in South Africa. Vet Rec Open 7: 1-7.
- Torres P, Diaz GJ, Cárdenas E, et al. (2012) Ethnobotanical study of plants poisonous to cattle in Eastern Colombia. IJPPR 2: 14-19.
- Serrano R (2018) Toxic Plants: Knowledge, medicinal uses and potential human health risks. Env Ecol Res 6: 487-492.
- Pocock S, Collier T, Dandreo K, et al. (2004) Issues in the reporting of epidemiological studies: A survey of recent practice. BMJ 329: 883.
- McLean M, Hansen S (2012) An overview of trends in animal poisoning cases in the United States: 2002-2010. Vet Clin North Am Small Anim Pract 42: 219-228.
- Caloni F, Cortinovis C, Rivolta M, et al. (2012) Animal poisoning in Italy: 10 years of epidemiological data from the poison control centre of Milan. Vet Rec 170: 415.
- Buttke D, Schier J, Bronstein A, et al. (2012) Characterization of animal exposure calls captured by the National poison data system, 2000-2010. J Clin Toxicol 2: 117.
- Raimon G, Sachana M, Caloni F, et al. (2010) Animal poisoning in Europe. Part 3: wildlife. Vet J 183: 260-265.
- Kellerman TS, Coertzer JAW, Naude TW (1988) Plant poisonings and mycotoxicoses of livestock in Southern Africa. Oxford University Press, Cape Town, South Africa.
- Sundaravaili M, Paliwal K (2002) Effect of Albizzia lebbeck plantation on the nutrient cycling in a semi-arid grazingland. Trop Ecol 43: 305-314.
- Mwihia EW (2012) Toxicity of Albizia gumifera; A plant commonly used in ethnoveterinary medicine in Kenya.
- Mendieta MC, de Souza ADZ, Ceolin S, et al. (2014) Toxic plants: Importance of knowledge for realization of health education. J Nurs UFPE Online 8: 680-686.
- Nicolai V (1989) Thermal properties and fauna on the bark of trees in two different african ecosystems. Oecologia 80: 421-430.
- Steyn PS, Vleggaar R, Anderson LAP (1987) Structure elucidation of two neurotoxins from albizia tanganyicensis. S Afr J Chem 40: 191-192.
- Mbanze AA, Mário AM, Rivaes R, et al. (2019) Field data on vegetation structure and effects of human use of the dambos ecosystem in Northern Mozambique. Data Brief 26.
- Bateman NE (1985) Vitamin B6 comparative absorption studies. Int Clin Nutr Rev 5: 130-134.
- Caloni F, Cortinovis C, Rivolta M, et al. (2013) Plant poisoning in domestic animals: Epidemiological Data from an Italian Survey (2000-2011). Vet Rec 172: 580.
- Gummow B, Bastianello SS, Labuschagne L, et al. (1992) Experimental Albizia versicolor poisoning in sheep and its successful treatment with pyridoxine hydrochloride. Onderstepoort J Vet Res 59: 111-118.
- Kokwaro J (1976) Medicinal plants of East Africa. East African Literature Bureau 127-128.
- John B, Sandra B, Lotter M, et al. (2018) Trees and shrubs of mozambique. Print Matters (Pty) Ltd, Noordhoek, Cape Town.
- Shackleton CM (2000) Comparison of plant diversity in protected and communal lands in the Bushbuckridge lowveld savanna, South Africa. Biol Conserv 94: 273-285.
- Verma SC, Vashishth E, Singh R, et al. (2013) A review on parts of Albizia lebbeck (L.) Benth. Used as ayurvedic drugs. Research J Pharm Tech 6: 1307-1313.
- Morton J (1958) Ornamental plants with poisonous properties. Proc Florida State Hortic Soc 71: 372-380.
- Elzaki T, Shomeina SK (2012) Environment friendly alkaline pulping of Albizia lebbeck from Sudan. Nat Sci 10.
- Botha CJ, Penrith M (2008) Poisonous plants of veterinary and human importance in Southern Africa Poisonous plants of veterinary and human importance in southern Africa. J Ethnopharmacol 119: 549-558.
- Needham AE, Lawrence JA (1966) The toxicity of albizia versicolor. Rhod Agric J 63: 137-140.
- Cortinovis C, Caloni F (2015) Alkaloid-Containing plants poisonous to cattle and horses in Europe. Toxins 7: 5301-5307.
- Penrith M, Botha C, Tustin R (2015) Plant poisonings in livestock in Brazil and South Africa. J S Afr Vet Assoc 86: 2-4.
- Tidsell CA (1995) Issues in biodiversity conservation including the role of local communities. Environmental Conservation 22: 216-222, 228.
Corresponding Authors
Frank Kapungu, Faculty of Veterinary Science, University of Zimbabwe, Harare, Zimbabwe, Tel: +263715848067
Copyright
© 2022 Masvingwe C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.