Effects of Biochar Soil Amendment on the Rooting and Early Growth of African Mahogany Species: Khaya Ivorensis And Khaya Grandifoliola
Abstract
Biochar application as growing medium is being recognized globally in forest and crop restoration. Biochar soil amendment was proposed to improve rooting media characteristics for effective rooting of mahogany stem cuttings in vegetative propagation system. A study was undertaken at Forestry Research Institute of Ghana to assess effects of different biochar (20v/v and 50v/v) amendment to riversand, loamy soil (80v and 50v), and mixtures of riversand + loam (40v/v and 25v/v) on rooting performance of K. ivorensis and K. grandifoliola cuttings in non-mist propagators. Thirty single node cuttings of both mahogany species were distributed per treatment in completely randomized block design with 3 replicates. Mean number roots per rooted cutting, root length and survival rate were assessed ten weeks after propagation. Species specific responses to biochar soil amendment was observed as K. ivorensis recorded maximum mean survival rate (80.08%) and root length (6.9 cm) whiles K. grandifoliola had maximum roots per cuttings (7.6). The 50v biochar + 25 v/v riversand + loam significantly yielded maximum number of roots formed per cutting (6.8), root length (7.03) and survival (75.08%) for both K. ivorensis and K. grandifoliola. Riversand recorded lowest roots per cutting for both species (3.48) and survival (45%) while loamy soil yielded lowest root length (4.02cm). Increasing biochar (50v/v) and lower mixtures of riversand and loam (25v/v) had profound impact on rooting K. ivorensis and K. grandifoliola cuttings. 50v biochar + 25 v/v riversand + loam is recommended as suitable medium for producing mahogany stock-plants for effective restoration and conservation.
Keywords
African mahogany, Biochar-soil amendment, Rooting, Stock-plants, Restoration
Introduction
African mahogany is highly desirable and important export commodity in the global timber business and Sub-Saharan Africa respectively [1-5]. The prime mahoganies include species in the genera Khaya, Entandrophragma, Toona, Guarea and others in the family Meliaceae which are widely distributed in Cameroun, Niger, Cote D’Ivoire, Uganda, Gabon and found in Ghana within the semi deciduous, moist and wet evergreen, transitional forest zones [2,6]. Globally, African mahogany are well recognized for their versatile uses for construction of furniture, veneer, and good medicinal value of the bark extracts [6-10]. The rapid declining of many economically important indigenous species in the main land tropics have been emphasized in many studies [11-13]. The African mahogany species in Ghana have faced overexploitation pressures from their natural population in the quest to meet the ever-increasing demand for their hardwood which has triggered scarcity of the resource base, diminishing tree species diversity and loss of valuable germplasm which are needed for restoration [14-17]. Consequently, the rate of their extraction exceeds their natural regenerative capacity. Matured seeds harvested from the tree after fruiting may have good germination potential (90%), however the viable seeds that fall on the natural forest floor reduces its viability and are also preyed on by some insects and seed feeding mammals [16,18,19]. These problems in turn impedes successful natural regeneration of mahogany species. Several reports indicate that conservation of biodiversity components including genes, ecologically resilient species and ecosystems enhances sustainability of prime species which genetic resources are eroding from their natural ranges in the tropical forest [11,20]. Plantation culture is promoted as the viable way to restore the declining economic species; however, plantation establishment of African mahogany species are often thwarted by the devastating pest Hysipyla robusta [2,21]. The pest attacks actively growing dominant shoots, causing multiple lateral branching which deforms the tree and eventually reduces the quality of tree stem [8,22]. Strategies to propagate the mahoganies through selection and breeding of quality tolerant varieties which could facilitate integrated pest management in plantations are advocated for sustainability of the species [1,8,17,23,24]. Efforts of developing cultivars that are genetically and ecologically adapted to the adverse environments and Hypsipyla pest incidence are important to conserving valuable germplasm and sustainable mahogany plantation culture [1,15,24]. Previous studies assessed the influence of cutting length, IBA, age of stock plant, species, and position of cuttings on shoots on the rooting efficiency of Khaya and Entandrophagma species in the vegetative propagation system [4,5,10,16,19,25-27].
Among other factors, the rooting medium is particularly significant to any vegetative propagation techniques developed as it influences root initiation, growth, and survival of stock-plants for effective regeneration [16,27,28]. Production of quality seedlings of the African mahogany species correlates to increasing stock potential of the established plantations. The choice of a specific technique in regeneration efforts may depend on availability of materials and personnel, cost and the easiness to adopt especially by local people [19,27]. Simple low-cost propagation strategies that maximize growth in the tropical forest tree improvement is preferable to the highly costly techniques [24,29]. Different growing substrate such as sand, topsoil, sawdust, peat moss, vermiculite, and gravel are well documented in the propagation of mahogany with variable responses [16,19,24]. Usually, the suitable medium mixtures are added to soil medium to improve the soil nutrient content for increased vigor of rooting and quality seedlings production.
Currently, biochar is primarily being promoted as soil amendment for forest restoration due to frequent nutrient depletion of many tropical soils [30-32]. The use of biochar as potting and growing substrate are linked to improvement of soil conditions which includes nutrient, soil cation exchange capacity and organic carbon in poorer soils with considerable positive impacts on crop growth [32-35]. Biochar used as soil amendment has potential for carbon sequestration, soil water retention, enhanced mycorrhizal fungi activities and organic matter in soils [36-39]. Compared to other soil amendments, combining different levels of biochar in mixtures of soil needs to be experimented as few evidences on their performance on tropical trees seedling growth are known. Biochar soil mixtures as substrate is likely to improve the rooting efficiency and survival of mahogany leafy cuttings at their early growth stages in the vegetative propagation however ideal rates of biochar application are unclear. Some studies found increased plant uptake of available nutrients with biochar incorporation ranging from lower rates in volume of 4%, 30% and a maximum of 70% [40-42]. The insignificant impact of biochar application rates on growth and yield of crops has also been reported in other studies [43]. Biochar additions to soil media for propagation may differ across species and their environmental ranges, presenting interesting advances for research. Our study was commissioned to assess the effects of different biochar soil amendment on the rooting ability of African mahogany species K. ivorensis and K. grandifoliola.
Materials and Methods
Propagation set up and stem cuttings production
Simple non mist propagators modified by Leakey, et al. 1990 (31), was utilized for the trial. It was made up of a constructed wooden frame of about 200cm length, width;100cm and height; 5 m. A transparent polythene was used to tighten the base of the propagator and filled with sequential layers of large stones diameter (6-10cm) to a depth (10-15cm), small stones (3cm level), and gravels of diameter (0.1-1cm) to a depth (about 5cm). The set up was watered uniformly. The tightly covered base was to prevent penetration by other roots from the ground and the layers of successive stones and gravels enhanced water retention necessary for adventitious root formation by the leafy stem cuttings.
There was a slight variation in the rooting media application as two separate experiments were involved in the study. However, in all experiments, the rooting media were pasteurized by heating over an open cast metal plate [28]. The media were evenly distributed on the propagator based on the measured quantities and covered with a 0.5mm thick polythene sheet. Non-mist propagators used in the green house were constructed under Tectona grandis stand covered with shaded net which ensured light irradiance of 82% - 85% to prevent excessive temperatures. Stock plants were obtained from approximately 15-year-old trees severed to coppicing for about 6 weeks to develop epicormic shoots. Leafy stem cuttings of length (10-30cm) of K. ivorensis and K. grandifoliola were collected in the morning hours (between 7-8 am) from shoots of the coppiced trees of both species and then enclosed in polythene bags to maintain a humid condition. Single node cuttings of length (6cm) were made from each spp. stems maintaining at most two (2) leaves, and the leaf area was trimmed to 30cm2 using a graphed paper template. The apical succulent portion of shoots (approximately 5cm) were discarded as they were prone to rot within a short time in the propagator. The experiment was conducted at the nursery site of CSIR-Forestry Research Institute of Ghana (6°44'N, 1°30'W and relief 280 m above sea level). The site is within the moist semi-deciduous forest zone of Ghana with annual precipitation of 1200-1800mm.
Techniques used in the rooting of the stem cuttings
Leafy stem cuttings of K. ivorensis and K. grandifoliola were inserted to a depth of 1-2cm in each rooting medium. The rooting media consisted of 20v biochar and 50v biochar addition to riversand, mixtures of riversand and loam, and loamy soil to evaluate their rooting abilities for the mahogany species. The biochar was derived from pyrolysis of wood and agricultural residues including poultry litter feedstock and pieces of woody biomass at about 150-250°C for 60 minutes. The cuttings were watered daily using a fine mist knapsack sprayer to maintain adequate moisture needed for rooting. Filling point of the media was monitored to prevent excessive water retention and possible leaves dehydration. The temperature within the propagators was 28-30°C and the humidity was about 70-80% after watering.
Experimental design
Fifteen coppiced trees (stock plants) were selected for each of the two species. The slender tips of the leafy cuttings were discarded as they were prone to rotting within a short time. Single nodes about (6cm) were made from the remaining stems and the leaf area was trimmed to 50cm2 using a graphed paper template. Twenty cuttings of each of the two species were collected and distributed uniformly into the treatment with three replicates. Each treatment with replicate consisted of 60 cuttings (1plants x 1trtmts x 3replicates x 20cuttings) hence a total of 720 cuttings were utilized in the experiment. The cuttings were inserted in the 20v and 50v biochar treatments added to riversand, loamy soil, and the mixture of riversand and loam media, and their controls in a randomized completely block design.
Two experiments were conducted in a complete randomized block design with variation in the media mixtures. The treatments consisted of 20v biochar and 50v biochar additions to riversand, mixture of riversand and loam, and loamy soil. The first experiment consisted of 20v biochar + 80vriversand, 20v biochar + 40v/v riversand + Loam, 20v biochar + 80v loamy soil. The second experiment consisted of 50v biochar + riversand, 50v biochar + 25v/v riversand and loam, and 50v biochar + 50v loamy soil. In both experiments, control treatments consisting of riversand, 50v/v riversand and loam, and loamy soil was set up for comparison. Adequate water necessary for rooting was ensured. The cuttings were considered rooted when at least 1 primary root (≤ 2cm) was formed (Figure 1). However, observations on dead cuttings and callus formation were made daily after watering.
Data collection and Analysis
Data on number of roots formed root length and percentage survival of the cuttings were taken 8 weeks after insertion into the rooting media. However, observance of dead cuttings, callus formation was made daily after watering. Data collected were subjected to one-way analysis of variance at P < 0.05. Where differences between means were significant, they were further separated with the Tukey's range test of statistiXL version 15.
Results
Experiment 1
Effects of 20v/v biochar on rooting performance of the African mahogany cuttings
K. ivorensis recorded maximum mean number of roots formed (5.73) and root length (5.28cm) in the 20v biochar + 80v loamy soil (Table 1). There were no marked differences among the media with biochar additions for number of roots formed and root length growth of K. ivorensis cuttings (P > 0.05) but remarkable differences existed between the 20v biochar combined with either 80v riversand or 80v loamy soil. Riversand medium yielded the lowest number of roots formed (3.68) by K. ivorensis whereas the lowest root length was recorded for loamy soil (3.91cm). K. grandifoliola recorded maximum mean number of roots formed per rooted cuttings (5.40) and root length (5.51cm) in the 20v biochar + 40v/v riversand + loam which differed significantly from all the control media and the 20v bio + 80v riversand treatment. No significant interaction existed between 20v biochar + 40v/v riversand + loam and the 20v biochar + 80v loamy soil for number of roots formed per cutting and root length growth of K. grandifoliola (Table 1).
The 20v biochar + 40v/v riversand + loamy soil recorded the highest survival for both K. ivorensis and K grandifoliola at values of 58.5% and 67.5% respectively. Major differences were recorded in rooting performance among the media between the 20vbiochar + 40v/v riversand and loam, and the other companion biochar media as well as the media without biochar except the 20v biochar + 80v loamy soil. The lowest survival rate for both K. ivorensis (about 47%) and K. grandifoliola (45.8%) was recorded in loamy soil. Comparison of species rooting ability shows that with respect to 20v biochar treatments and their controls, loamy soil recorded highest number of dead cuttings (over 50%) for both K. ivorensis and K. grandifoliola.
Experiment 2
Effects of 50v/v biochar soil amendment on roots formed per cutting, root length growth and survival rate of the African mahogany cuttings
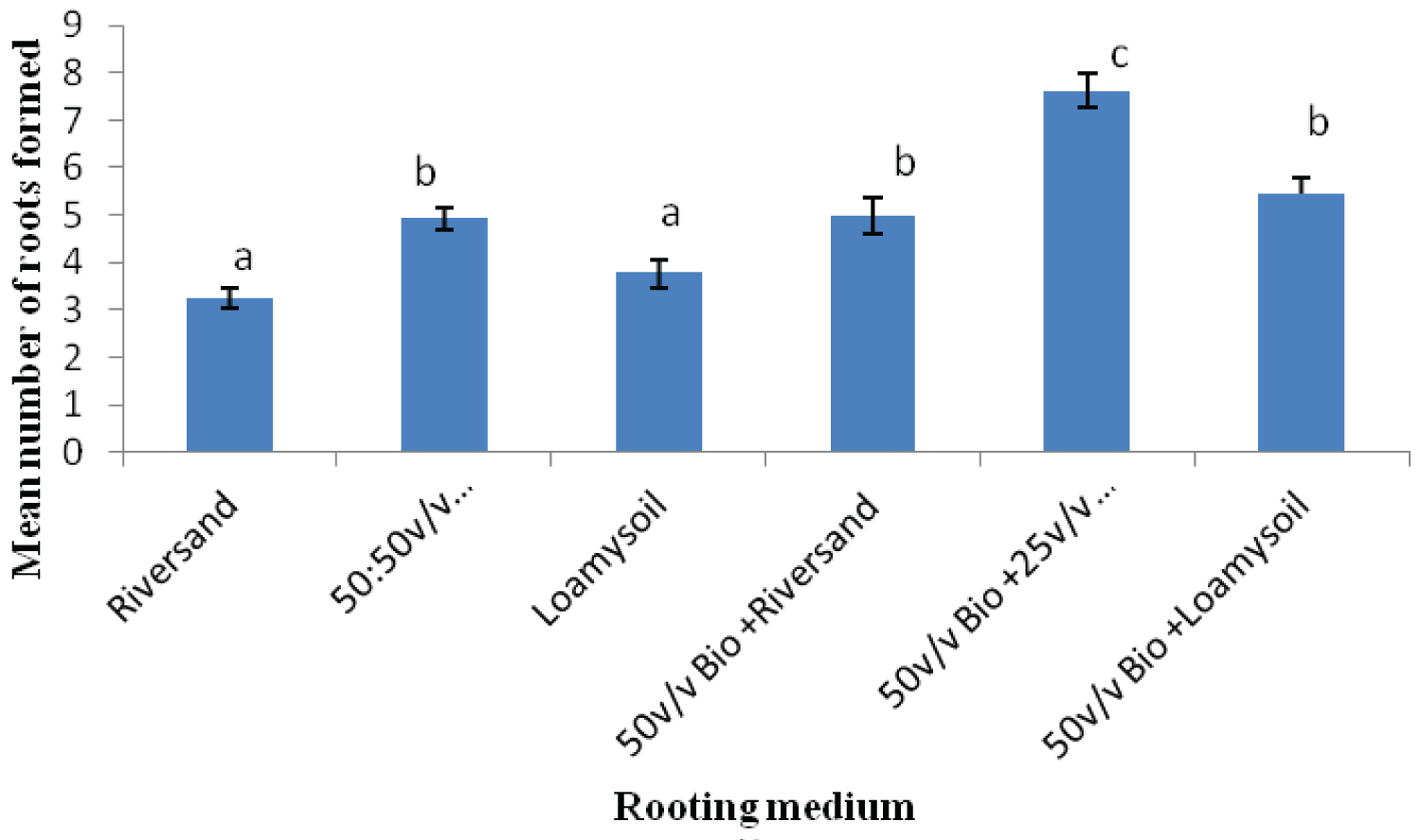
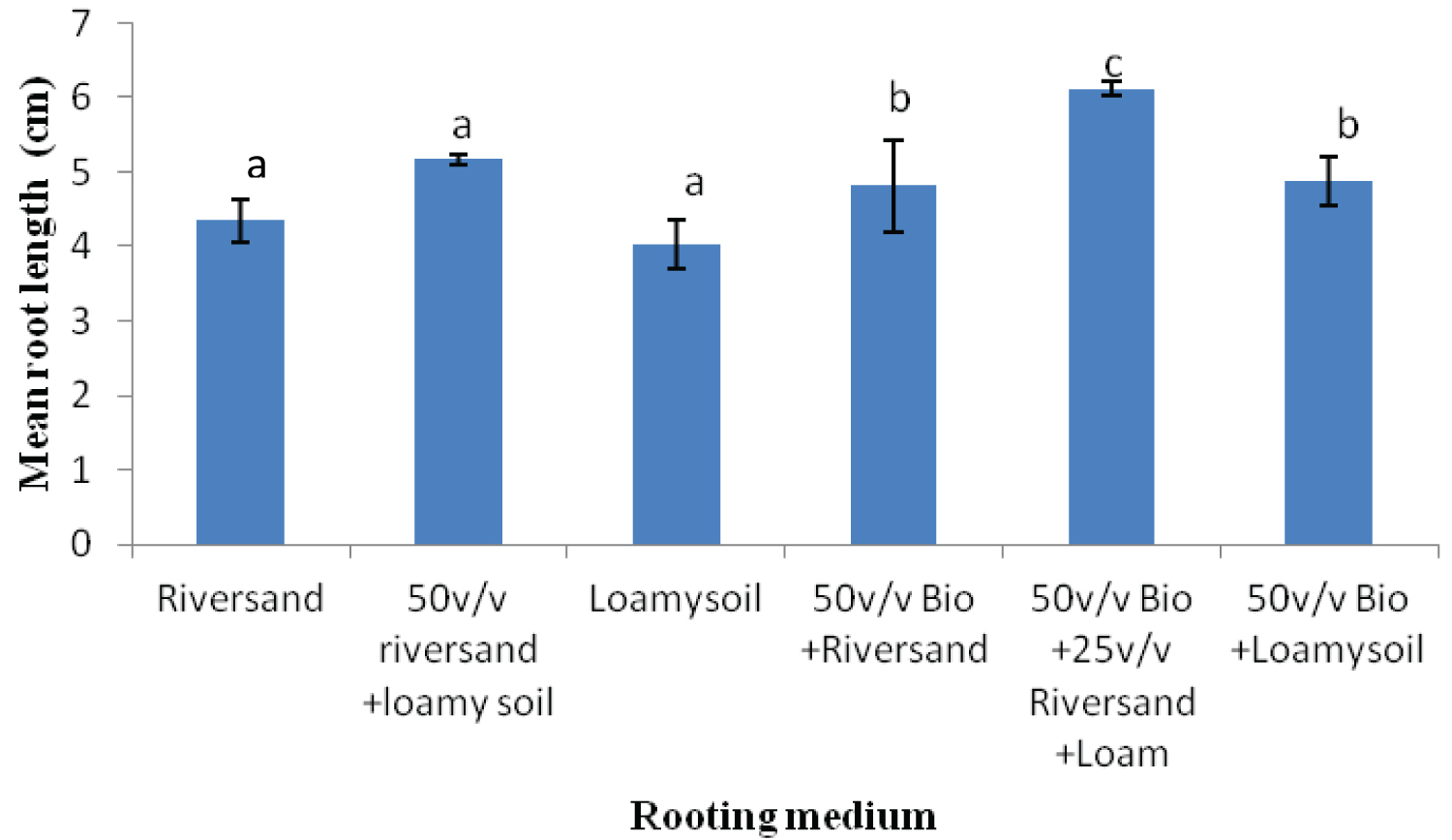
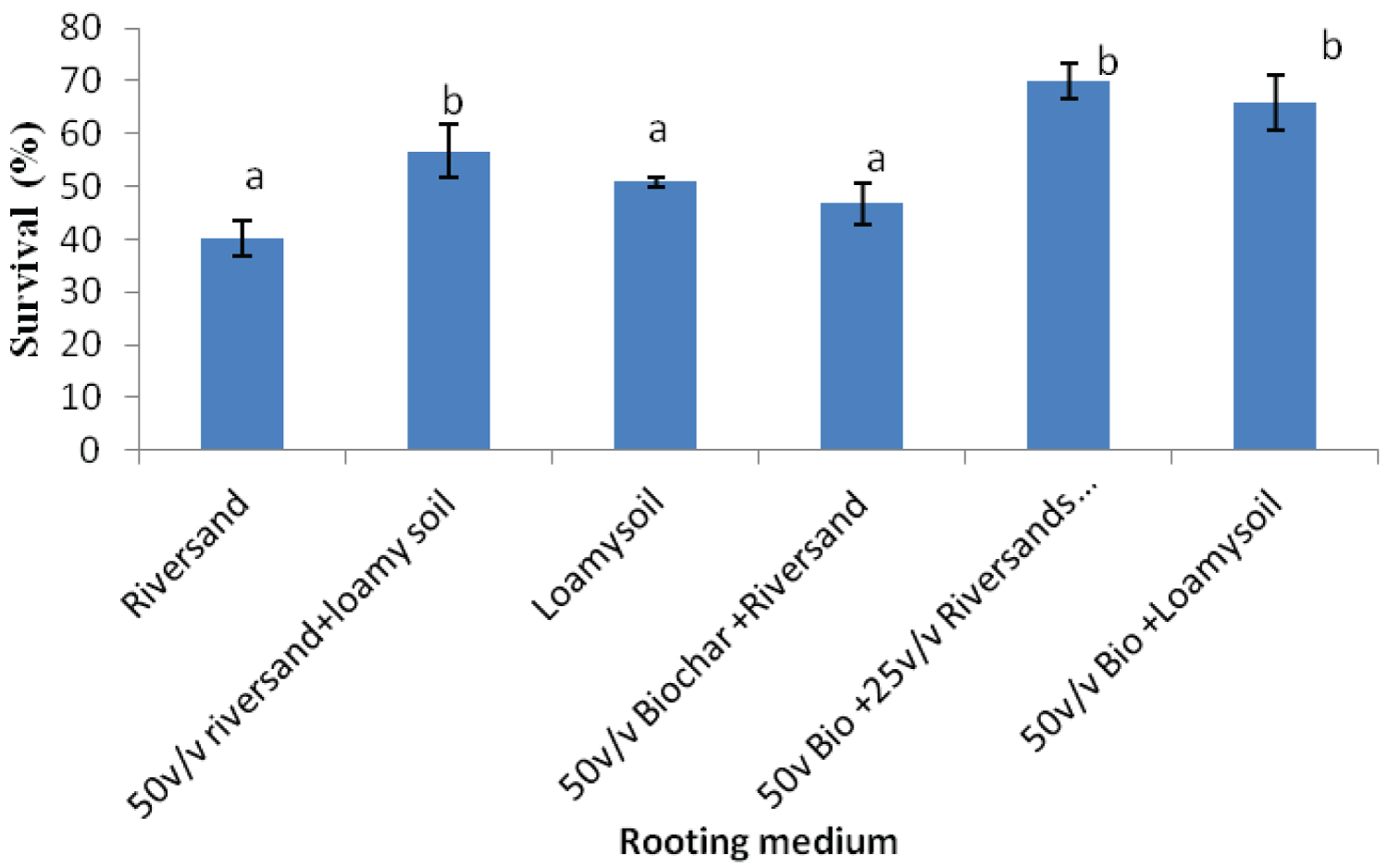
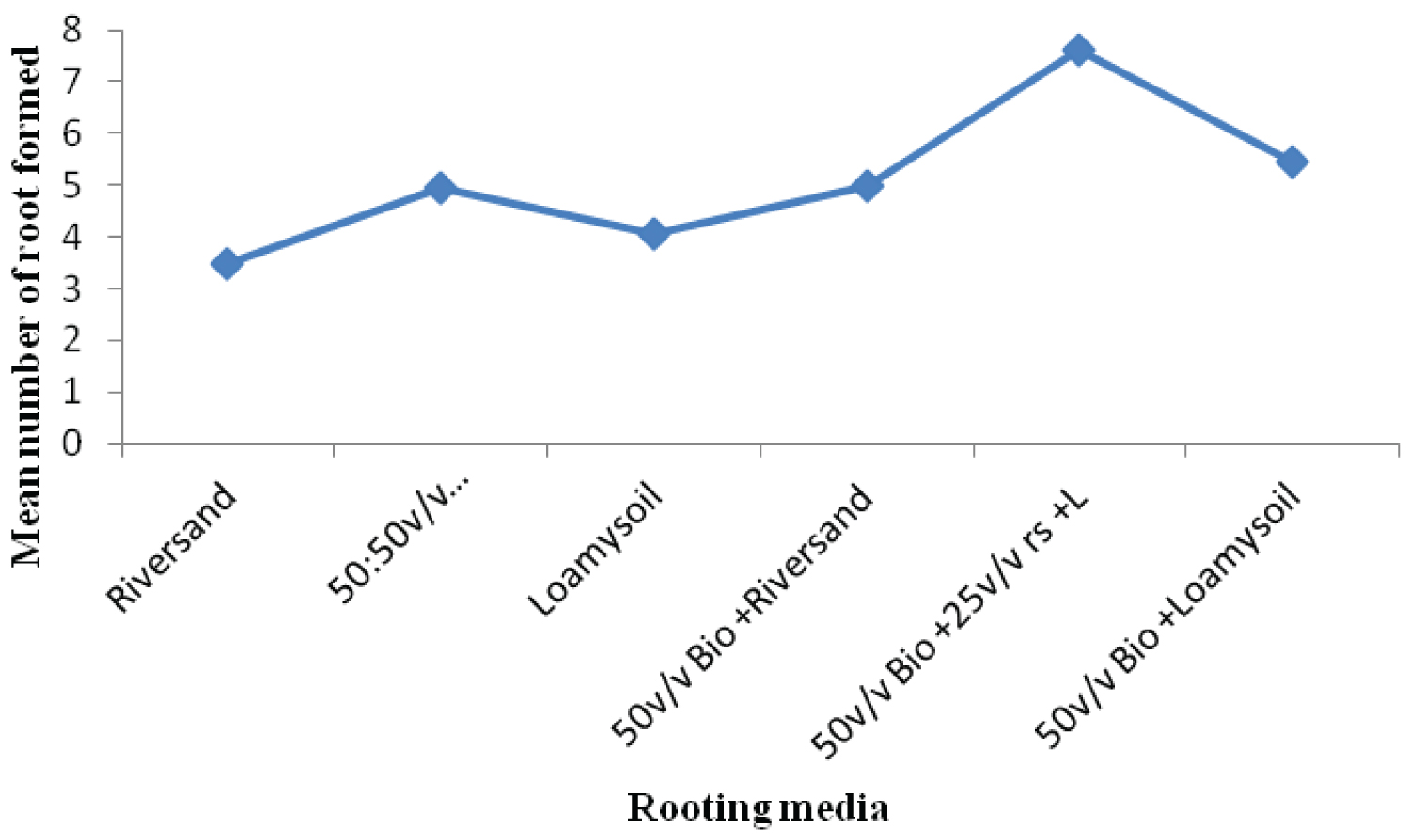
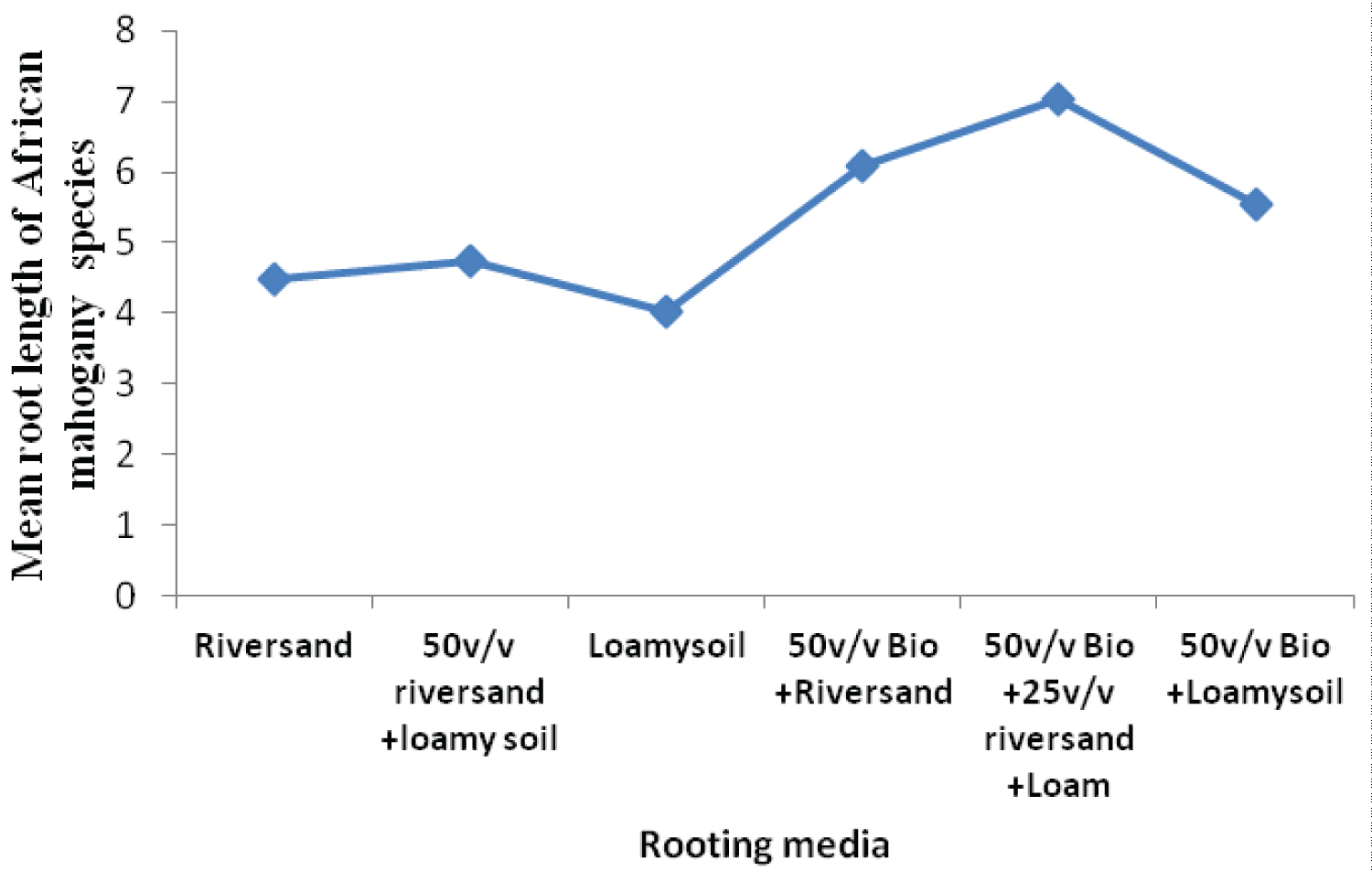
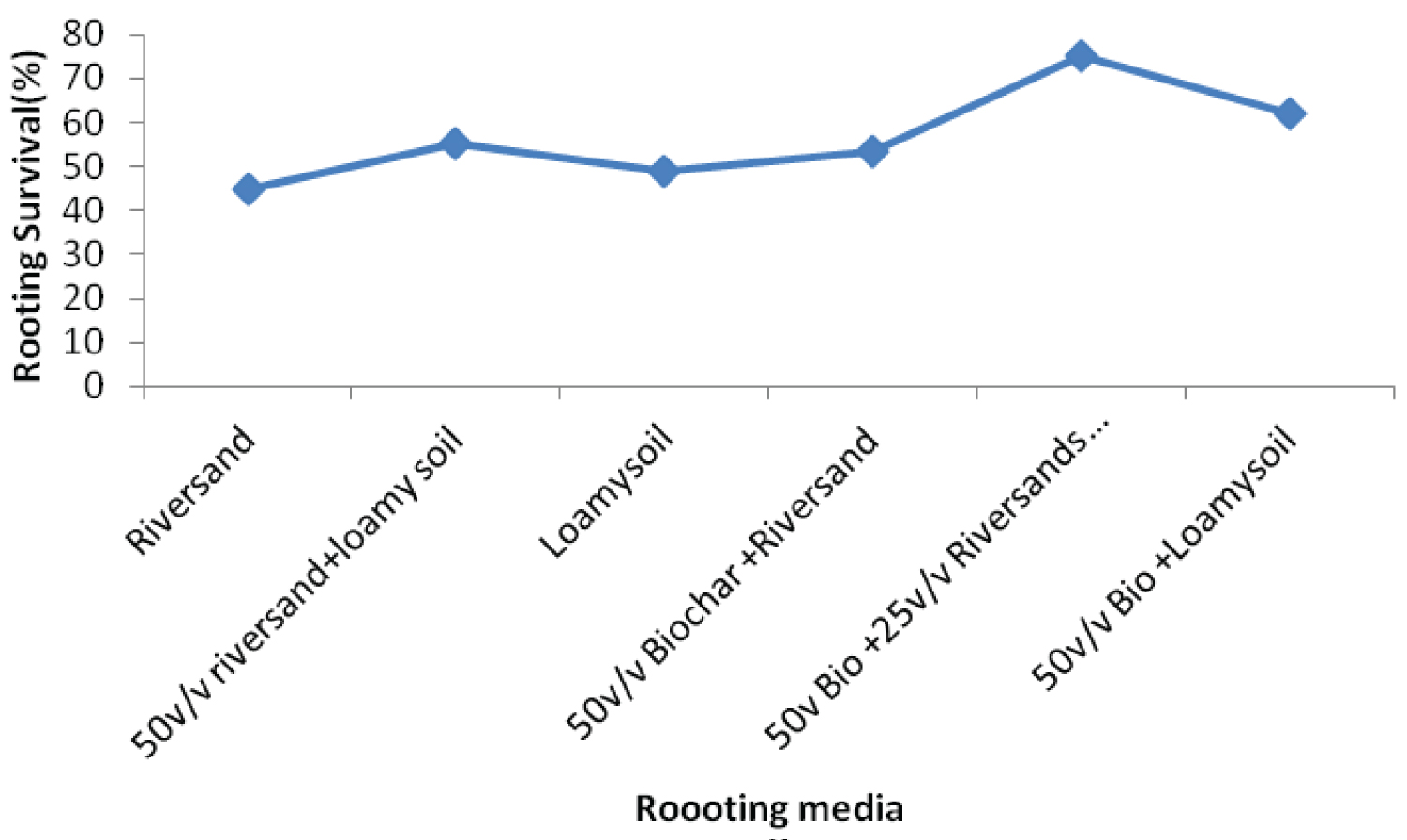
The rooting media influenced the rooting response of cuttings of both Khaya grandifoliola and Khaya ivorensis. Results from our study shows variation in species performances and rooting ability to the biochar soil mixtures and their control treatments assessed in our study. K. grandifoliola yielded significantly higher mean roots formed per cutting than K. ivorensis leafy stem cuttings within the same propagation environment. K. grandifoliola yielded maximum mean number of roots formed (7.6), root length (6.11cm) and percentage survival (70%) in the 50v biochar + 25v/v riversand and loamy soil (Figure 2, Figure 3, Figure 4). There was significant variation among the rooting performance of K. grandifoliola in the various media with the 50v biochar + 25v/v riversand and loamy soil differing markedly from the other media rooting ability (P < 0.05). Comparison between the media performance showed highly significant differences between the 50v/v biochar + 25v/v riversand and loam, and the relative biochar media 50v/v bichar + loam (P ≤ 0.001), and 50v/v biochar + riversand (P ≤ 0.001). Major differences did not occur for number of roots formed by K. grandifoliola cuttings in riversand and loam media (P > 0.05, Figure 2). A similar trend was recorded for root length growth of K. grandifoliola leafy cuttings (Figure 3). Riversand control medium yielded the lowest number of roots formed for K. grandifoliola (3.27), and survival (40%) whereas loamy soil recorded the least mean root length (4.03cm) per cutting (Figure 2, Figure 3, Figure 4). There was however no considerable variation (P > 0.05) among the media performances for average number of roots formed per cutting and survival rate of the spp. in the control treatment (Figure 2, Figure 4).
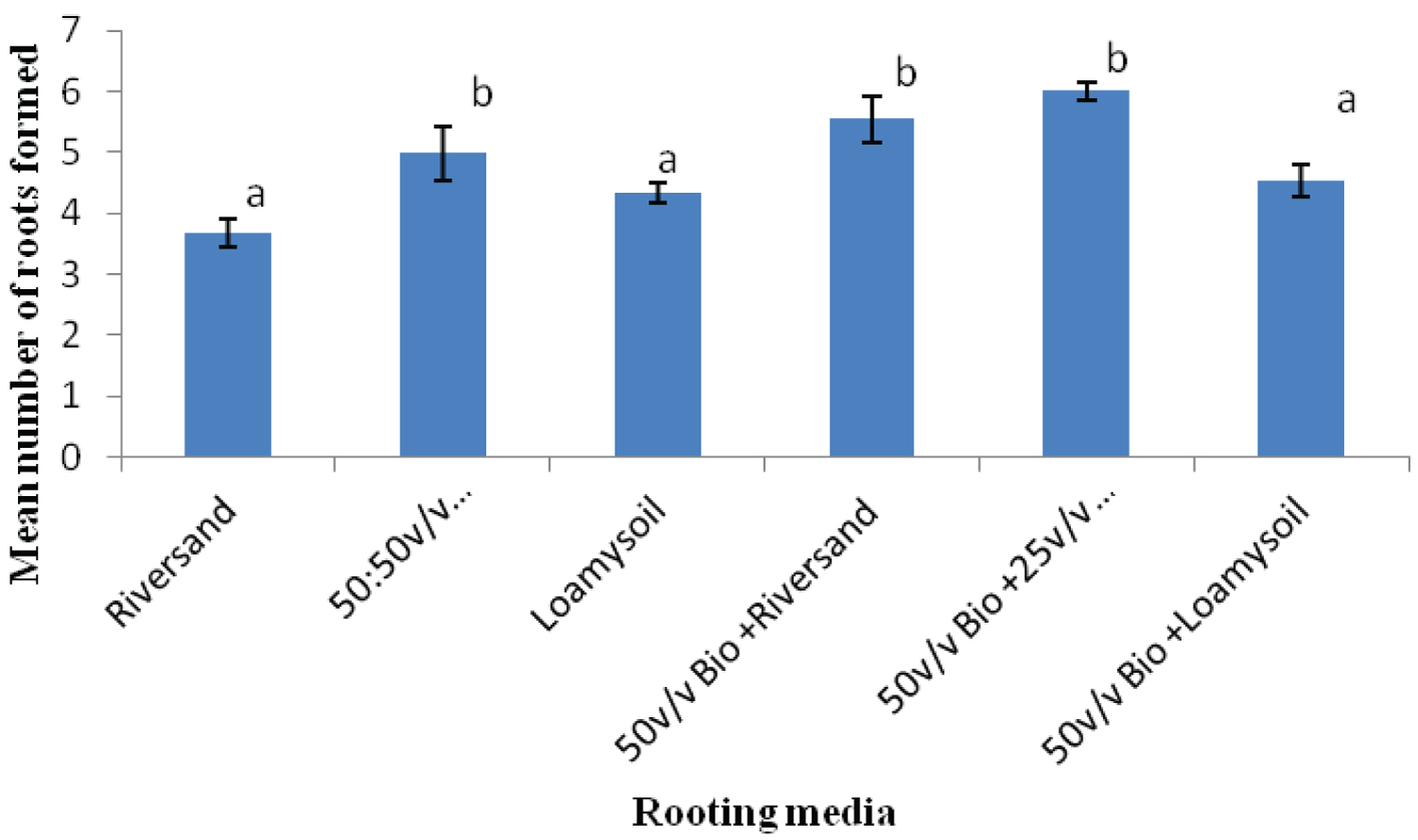
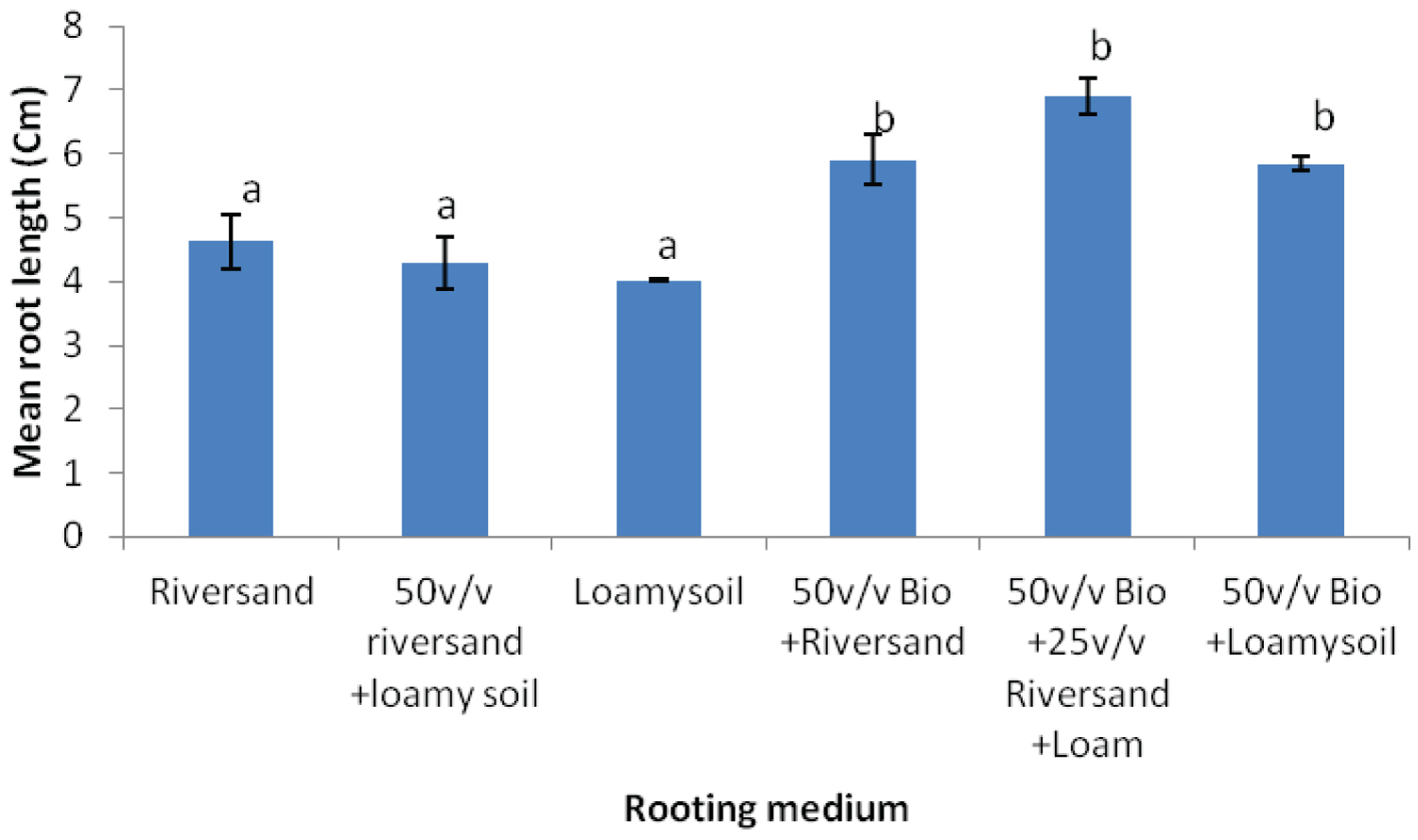
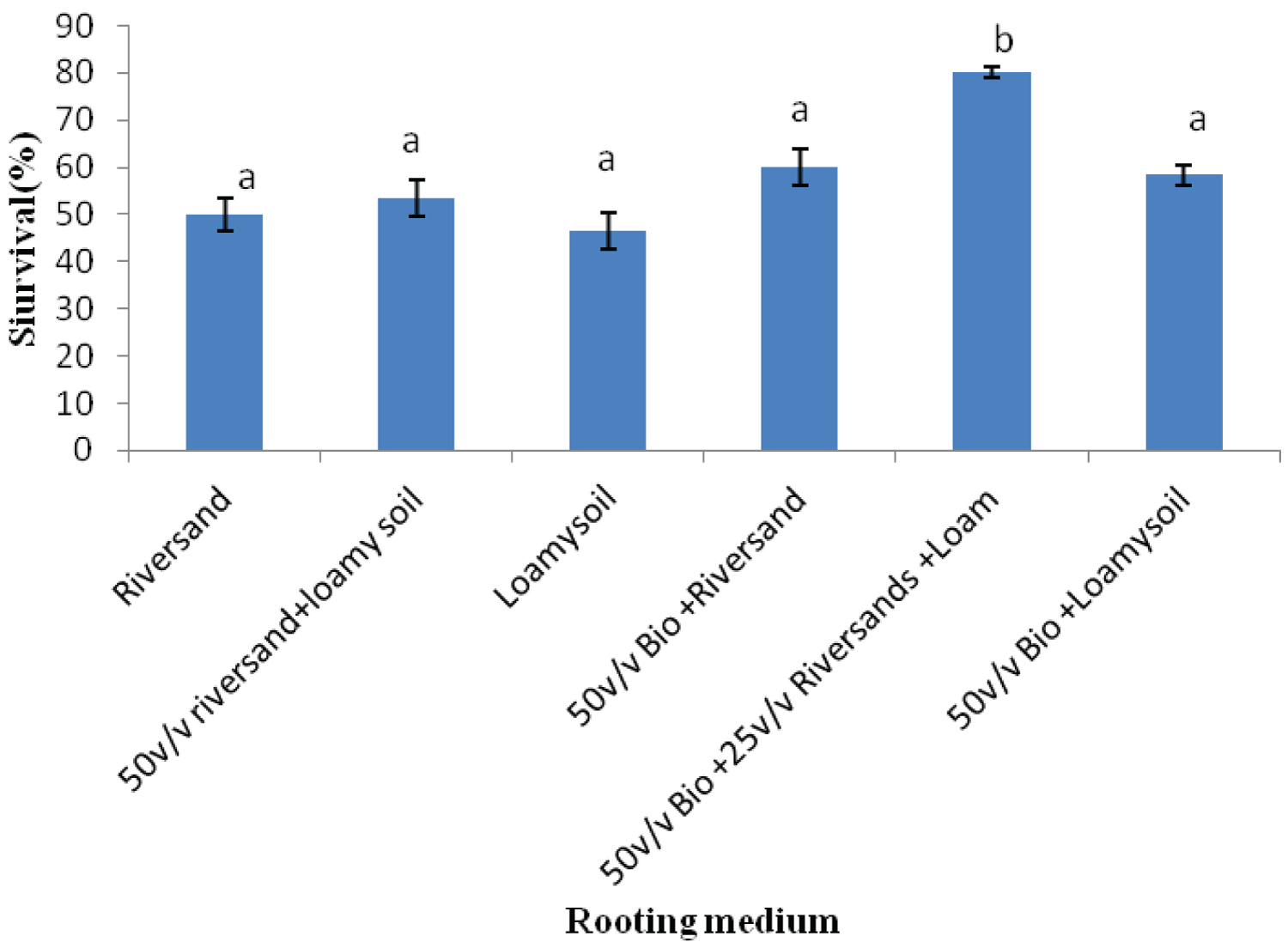
K. ivorensis leafy cuttings recorded highest mean values of roots formed per cutting (6.0), root length (6.9cm) and survival (80.20%) in the 50v biochar + 25v/v riversand and loamy soil (Figure 5, Figure 6, Figure 7). The highest rooting ability in the K. ivorensis cuttings for number of roots formed and survival in the 50v biochar + 25v/v riversand and loamy medium differed remarkably (P < 0.05) from the relative values of parameters recorded for the spp. in the companion biochar media and their control treatment. Comparison of species performances showed that K. grandifoliola yielded the overall highest number of roots formed per cutting (7.6; Figure 1), whiles the greatest mean values of root length (6.9cm) and survival of the cuttings (over 80%) were recorded for K. ivorensis leafy cuttings rooted in the 50v biochar + 25v/v riversand and loamy soil which varied remarkably from the rooting responses in the other media (Figure 3, Figure 7, P < 0.05). The study also found that the 50v biochar + 25v/v riversand and loam varied greatly (P < 0.05) from the other media rooting response for most parameters assessed for both K. ivorensis and K. grandifoliola with mean maximum number of roots formed per cutting (6.8), root length (7.03cm) and percentage survival of the cuttings (75.08%) (Figure 8, Figure 9, Figure 10). Generally, the control treatment riversand recorded the lowest mean number of roots (3.48) and survival (45%) for both K. grandifoliola and K. ivorensis while loamy soil yielded the least root length (4.02cm) (Figure 8, Figure 9, Figure 10). There were no remarkable differences for number of roots formed by K. ivorensis leafy stem cutting propagated in riversand and the other companion media without biochar additions; the 50v/v riversand + loam, and loamy soil (Figure 5, P > 0.05). However, root length growth of K. ivorensis in the biochar media varied slightly from the control media without biochar additions (Figure 6, P < 0.05). Apart from the 50v Biochar + 25v/v river-sand and loam, which differed significantly from the other media for survival rate of K. ivorensis cuttings, there were no major interaction between the control media and the other two biochar treatments: 50v/v biochar + riversand, and 50v/v biochar + loamy soil (Figure 7).
Discussion
Several factors have been investigated to influence rooting of cuttings of African mahogany species in the vegetative propagation system. These include age of stock plant, leaf area and auxin on K. ivorensis and K. anthotheca [25,19], cutting position of stock-plant in Khaya and Entandrophragma spp. [16], growth regulators on K. senegalensis [4], Indole-3-Butyric Acid on K .ivorensis [10, 44].
The rooting media influences the early development, roots formation and survival of stock plants in the vegetative propagation [16,28,29,45]. There is no universally recommended soil media for propagation of elite mahogany genotype, hence techniques of improving the growing media characteristics for adequate growth of the stock-plants is paramount to ensuring availability of planting materials for regeneration of the species. In our study, the best rooting ability of K. ivorensis and K. grandifoliola leafy stem cuttings was recorded in the 50v biochar + 25v/v riversand and loamy soil. The rooting performance of both mahogany species in the 50v biochar + 25v/v riversand and loamy soil was generally greater than the 20v biochar + 40v/v riversand and loamy soil, as well as the other media with and without biochar mixtures. The 50v biochar + 25v/v riversand and loamy soil significantly yielded highest mean number of roots per cutting, root length and survival rate of cuttings in both K. ivorensis and K. grandifoliola.
Leafy stem cuttings of many tropical tree species successfully develop root and shoots in a variety of rooting medium [16,14,29,45,46]. In related studies [16], recorded highest rooting percentage, and roots formed per cuttings of Khaya species in the 50v/v mixtures of river-sand and topsoil whereas the top-soil medium yielded best rooting response of Entandrophragma species under the same propagation environment.
Studies on propagation of other tree species using various soil subtrates yielded maximum rooting percentage of Milicia excelsa, Irvingia gabonensis and Cordia alliodora cuttings Simmondstia chinensis cuttings [14,45-47].
The relationship between species response to rooting and the substrate performances may possibly relate to genetic characteristics of the species, propagation environment and their ability to promote root formation, root elongation and survival rate of stem cuttings. The requirement for rooting, growth and survival of stem cuttings may differ among plant species [14,28,46]. Earlier reports indicate that the optimum characteristics of a suitable rooting medium such as rich nutrient content, good aeration, water holding capacity and drainage among others could facilitate maximum and effective rooting ability of stock-plants in the vegetative propagation system [27,28,43,48]. In addition, soil texture is found to mediate plant water absorption and availability for plants rooting, further growth, functioning and development [49,50]. Suitable medium therefore provides a balanced environment to increase the rooting efficiency and maximum growth of the softwood cuttings during propagation [27,28,51].
The significantly highest rooting response in our study observed in the 50v biochar and 25v/v riversand and loam could therefore be attributed to the relatively good and balanced characteristics of the media in mixtures for sufficient nutrient supply, water holding ability and aeration which favored adequate rooting capacity of both K. grandifoliola and K. ivorensis cuttings. Thus, the different levels of biochar application (20v and 50v biochar) to the soil media riversand, loamy soil and mixtures of riversand and loam exhibited greatest rooting ability than their control media without biochar. The use of biochar as soil amendment or growing substrate is linked to soil water retention, enhanced beneficial soil micro-organisms and organic matter in soils needed for growth and development of crops and plant species [32,34,36,37,39,52].
Different amounts of biochar amendment to soil for nursery plants and crops are noted to increase plant vigor and nutrient absorption potential for growth and production of good quality seedlings [32,41,42,53]. Some studies reported that biochar have few or no major effect on crop growth [54]. It is however emphasized that depending on the type of substrate, crop plant and the purpose of application, biochar addition ranging from 15v/v to 70v/v to soils could have considerable impact on plants growth [40,42,52]. While Conversa, et al., [40] found remarkable seedling growth of Pelargonium zonale plants with increasing biochar from zero to 70v/v biochar addition to soil, Vaugh, et al., [41] observed few differences in plant growth but marked height growth of tomato cultivars (Solanum lycopersicum) with 15v/v biochar growing medium.
Our findings agree with other studies that biochar have a considerable influence on rooting efficiency of mahogany species when suitable levels of biochar (50v/v) were added as a catalyst to enrich the soil for maximum rooting. Usually, suitable soil media provides favorable physical conditions and adequate nutrients to the cuttings for growth and survival. Riversand medium recorded lowest mean number of roots and survival rate for both K. grandifoliola and K. ivorensis whereas loamy soil yielded the least root length. As widely known, the large pore space of riversand medium enhances oxygen diffusion, and loamy soil is noted for its rich organic matter [28,51], probably a suite of these elements was required to initiate root formation, root length and survival. In previous studies, lowest percentage rooting in M. excelsa, K. grandifoliola and Endrophrama angolense occurred in riversand medium [14,16]. Thus, compared to the mixed medium, perhaps the low water holding capacity of riversand and low oxygen diffusion in loamy soil might have resulted in the low rooting performances of mahogany stem cutting utilized in the present study. The young cuttings could be exposed to moisture stress in a medium with low water retention capacity and dense media without proper aeration may also impede the ability to form roots and grow successfully.
The study further showed a clear variation in African mahogany species performance to rooting among the biochar-soils and control media. In the same propagation system, K. grandifoliola cuttings yielded highest number roots per cutting but maximum length of rooted cuttings and survival rate were recorded for K. ivorensis cuttings. stem cuttings of the same age of stock-plant in the same propagation environment.
The maximum survival of cuttings and root length growth of K. ivorensis in the 50v biochar + 25v/v riversand and loamy soil than K. grandifoliola could perhaps be attributed to their intrinsic genetic characteristics which reflected their response to rooting in the different media. There were no significant differences in the rooting performance of the 20v/v biochar treatments to soil and the control media, nevertheless K. grandifoliola yielded better rooting responses than its related species K. ivorensis.
K. ivorensis yielded significantly higher survival rate, roots formed per rooted cuttings where as K. grandifoliola recorded the highest length of rooted cutting in the 50v biochar + 25v/v riversand and loamy soil. Species response to propagation treatments are thus influenced by the distinct genetic compositions and the environmental conditions [16,17,19,28]. Naturally, K. ivorensis grows well in wet/moist soils where as K. grandifoliola is found in the moist semi-deciduous and sparsely in the transition and dry zones [2,6,7,15]. The stock plants used in the study were collected from the semi-deciduous forest zone in which both species co-occur. K. ivorensis and K. grandifoliola yielded best rooting ability in the 50v biochar + 25v/v riversand + loam suggesting that the microclimate within the propagator was suitable for growth of both mahogany species. Thus, The main differences in the values of rooting response of the mahogany species under the same propagation environment thus reflects their distinct genetic make-up which influences their adaptation to propagation techniques.
Conclusion
The study has demonstrated that biochar soil amendment can be used as a catalyst to increase the rooting efficiency of the African mahogany species, K. grandifoliola and K. ivorensis leafy stem cuttings in the vegetative propagation system. Biochar incorporation in restoration of mahogany in 20 and 50 by volume additions to soil media river-sand, loam, and mixtures of river-sand up to 100% performed relatively better in rooting efficiency than their companion control soils without biochar. The 50v biochar and 25v/v riversand and loam consistently yielded maximum number of roots, root length growth and mean survival rate of both K. grandifoliola and K. ivorensis. 50v biochar and 25v/v riversand and loam medium is remarkably suitable and therefore recommended for vegetative propagation of these mahogany species for efficient restoration of the species in plantations and for conservation of superior genotypes. K. ivorensis recorded highest survival rate of cuttings which was 5% higher than K. grandifoliola in the 50v biochar + 25v/v riversand and loamy soil. On the other hand, K. grandifoliola also yielded a greater number of roots per cutting and remarkable root length than its related species in the same 50v biochar + 25v/v riversand and loamy soil medium. This depicts a high species variation to biochar propagation treatments. The study strongly supports the addition of (50v/v) biochar to mixed soils of riversand and loam to boost rooting vigor, root length and survival of the vegetative cuttings for effective seeding production, conservation and sustainable management of the African mahogany species; K. ivorensis and K. grandifoliola.
Acknowledgments
We are grateful to the International Tropical Timber Organization project 'PD 528 Rev 1F' and Fellowship Ref 166/15S. We thank the vibrant field team at the Entomology Lab of CSIR-Forestry Research Institute of Ghana who helped in setting up the experiment.
References
- Nikles DG, DI Bevege, GR Dickinson, et al. (2008) Developing African mahogany (Khaya senegalensis) germplasm and its management for a sustainable forest plantation industry in northern Australia: progress and needs. Australian Forestry 71: 33-47.
- Opuni-Frimpong E, Khaya grandifoliola CDC (2008) In: Louppe D, Oteng-Amoako AA, Brink M, Plant resources of Tropical Africa 7(1). Timbers 1. PROTA Foundation, Wageningen, Netherlands/ Backhuys Publishers, Leiden, Netherlands/CTA, Wageningen, Netherlands, 329-333.
- Dzacka R, S Pentsil, J Korang (2016) A decade and half of Ghana’s trade in African Mahogany: A Review. JENRM 3: 1-7.
- Rodrigo Tenório de Vasconcelos, SV Valeri, A Martins, et al. (2016) Rooting of African mahogany (Khaya senegalensis A. Juss.) leafy stem cuttings under different concentrations of indole-3-butyric acid. African Journal of Agricultural Research 11: 2050-2057.
- de Oliveira TPDF, Siqueira DP, Lamônica, K, et al. (2018) Stock plants productivity and mini cuttings rooting of Khaya ivorensis A. Chev treated with IBA. CERNE 24: 114-120.
- Oteng-Amoako AA (2006) 100 tropical African timber trees from Ghana: Tree description and wood identification with notes on distribution, ecology, silviculture, ethnobotany and wood uses. Kumasi, Ghana: Forestry Research Institute of Ghana, KNUST.
- Opuni-Frimpong E, DF Karnosky, AJ Storer, et al. (2008) Silvicultural systems for plantation mahogany in Africa: Influences of canopy shade on tree growth and pest damage. Forest Ecology and Management 255: 328-333.
- Opuni-Frimpong E, DF Karnosky, AJ Storer, et al. (2008) Relative susceptibility of four species of African mahogany to the shoot borer Hypsipyla robusta (Lepidoptera: Pyralidae) in the moist semideciduous forest of Ghana. Forest ecology and management 255: 313-319.
- Ky-Dembele, Catherine, Mulualem Tigabu, Jules Bayala, et al. (2011) Clonal propagation of Khaya senegalensis: The effects of stem length, leaf area, auxins, smoke solution, and stockplant age. International Journal of Forestry Research.
- Barroso Deborah Guerra, Taiane Pires de Freitas de Oliveira, David Pessanha Siqueira, et al. (2018) Mini-stumps productivity and rooting of khaya ivorensis A. CHEV MINI-CUTTINGS TREATED WITH IBA. Cerne 24: 114-120.
- Assessment, FAO Global Forest Resource, and On Statistical Data. (2010) "Key Findings." FAO, Rome, Italy.
- Meyfroidt Patrick, Eric F Lambin (2011) Global forest transition: Prospects for an end to deforestation. Annual review of environment and resources 36: 343-371.
- Oduro KA, GMJ Mohren, M Pena-Claros, et al. (2015) Tracing forest resource development in Ghana through forest transition pathways. Land Use Policy 48: 63-72.
- Ofori DA, AC Newton, RRB Leakey, et al. (1996) Vegetative propagation of Milicia excelsa by leafy stem cuttings: Effects of auxin concentration, leaf area and rooting medium. Forest Ecology and Management 84: 39-48.
- Opuni-Frimpong E, SM Opoku, AJ Storer, et al. (2013) Productivity, pest tolerance and carbon sequestration of Khaya grandifoliola in the dry semi-deciduous forest of Ghana: A comparison in pure stands and mixed stands. New forests 44: 863-879.
- Owusu Sandra A, Emmanuel Opuni-Frimpong, Charles Antwi-Boasiako (2014) Improving regeneration of mahogany: Techniques for vegetative propagation of four African mahogany species using leafy stem cuttings. New forests 45: 687-697.
- Opoku Esther M, Emmanuel Opuni-Frimpong, Daniel Dompreh (2018) Developing sustainable regeneration techniques for four African mahogany species: Grafting methods for success and growth. New Forests.
- Grogan James, Jurandir Galvão (2006) Factors limiting post‐logging seedling regeneration by big‐leaf mahogany (Swietenia macrophylla) in Southeastern Amazonia, Brazil, and implications for sustainable management. Biotropica: The Journal of Biology and Conservation 38: 219-228.
- Opuni-Frimpong E, DF Karnosky, AJ Storer, et al. (2008) Key roles of leaves, stockplant age, and auxin concentration in vegetative propagation of two African mahoganies: Khaya anthotheca Welw. and Khaya ivorensis A. Chev. New Forests 36: 115-123.
- Rudel Thomas K (2002) Paths of destruction and regeneration: Globalization and forests in the tropics. Rural Sociology 67: 622-636.
- Griffiths MW (1998) The biology and ecology of Hypsipyla shoot borers. In. ACIAR PROCEEDINGS, ACIAR, 74-80.
- Floyd Robert B, Caroline Hauxwell (2001) Hypsipyla shoot borers in Meliaceae: Proceedings of an International Workshop held at Kandy, Sri Lanka 20-23 August 1996. In: Hypsipyla shoot borers in Meliaceae: Proceedings of an International Workshop held at Kandy, Sri Lanka 20-23 August 1996. Australian Centre for International Agricultural Research (ACIAR), Australia.
- Newton AC, Leakey RRB, Powell W, et al. (1994) Domestication of mahoganies. In: Leakey RRB, Newton AC, Tropical trees: the potential for domestication and the rebuilding of forest resources. London, HMSO, 256-266.
- Mayhew JE, Newton AC (1998) The Silviculture of Mahogany. CABI, Publishing, New York, NY, USA.
- Tchoundjeu Z, RRB Leakey (1996) Vegetative propagation of African mahogany: Effects of auxin, node position, leaf area and cutting length. New Forests 11: 125-136.
- Tchoundjeu Z, RRB Leakey (2000) Vegetative propagation of Khaya ivorensis (African mahogany): Effects of stockplant flushing cycle, auxin and leaf area on carbohydrate and nutrient dynamics of cuttings. Journal of Tropical Forest Science 77-91.
- MacDonald B (1993) Practical woody propagation for nursery growers. Timber Press Inc. Portland, Oregon, USA.
- Hartmann HT, Kester DE, Davies Junior FT, et al. (2002) Plant propagation: Principles and practices. (7th Edn) New Jersey: Prentice Hall.
- Leakey Robert RB, JF T Mesen, Z Tchoundjeu, et al. (1990) Low-technology techniques for the vegetative propagation of tropical trees. The Commonwealth Forestry Review 69: 247-257.
- Glaser Bruno, Johannes Lehmann, Wolfgang Zech (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal-A review. Biology and fertility of soils 35: 219-230.
- Warnock Daniel D, Johannes Lehmann, Thomas W Kuyper, et al. (2007) Mycorrhizal responses to biochar in soil-concepts and mechanisms. Plant and soil 300: 9-20.
- Thomas Sean C, Nigel Gale (2015) Biochar and forest restoration: A review and meta-analysis of tree growth responses. New Forests 46: 931-946.
- Chan KY, L Van Zwieten, I Meszaros, et al. (2007) Agronomic values of green waste biochar as a soil amendment. Soil Research 45: 629-634.
- Kimetu Joseph M, Johannes Lehmann, Solomon O Ngoze, et al. (2008) Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 11: 726-739.
- Novak Jeffrey M, Isabel Lima, Baoshan Xing, et al. (2009) Characterization of designer biochar produced at different temperatures and their effects on a loamy sand. Annals of Environmental Science 3.
- Lehmann Johannes, John Gaunt, Marco Rondon (2006) Bio-char sequestration in terrestrial ecosystems-a review. Mitigation and adaptation strategies for global change 11: 395-419.
- Yamato Masahide, Yasuyuki Okimori, Irhas Fredy Wibowo, et al. (2006) Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil science and plant nutrition 52: 489-495.
- Warnock DD, J Lehmann, TW Kuyper, et al. (2007) Mycorrhizal responses to biochar in soil- concepts and mechanisms. Plant and Soil 300: 9-20.
- Woolf Dominic, James E Amonette, F Alayne Street-Perrott, et al. (2010) Sustainable biochar to mitigate global climate change. Nature communications 1: 56.
- Conversa Giulia, Anna Bonasia, Corrado Lazzizera, et al. (2015) Influence of biochar, mycorrhizal inoculation, and fertilizer rate on growth and flowering of Pelargonium (Pelargonium zonale L.) plants. Front plant sci 6: 429.
- Vaughn Steven F, Fred J Eller, Roque L Evangelista, et al. (2015) Evaluation of biochar-anaerobic potato digestate mixtures as renewable components of horticultural potting media. Industrial Crops and Products 65: 467-471.
- Dumroese R, Jeremiah Pinto, Juha Heiskanen, et al. (2018) Biochar can be a suitable replacement for Sphagnum peat in nursery production of Pinus ponderosa seedlings. Forests 9: 232.
- Tchoundjeu Z, ML Avana, RRB Leakey, et al. (2002) Vegetative propagation of Prunus africana: effects of rooting medium, auxin concentrations and leaf area. Agroforestry systems 54: 183-192.
- Barbosa Filho Joamir, Maria Angélica Di Carvalho, Leandro Silva de Oliveira, Enéas Ricardo Konzen, and Gilvano Ebling Brondani. (2018) Mini-cutting technique for Khaya anthotheca: selection of suitable IBA concentration and nutrient solution for its vegetative propagation. Journal of forestry research 29: 73-84.
- Mesén F, Adrian C Newton, Robert RB Leakey (1997) Vegetative propagation of Cordia alliodora (Ruiz & Pavon) Oken: the effects of IBA concentration, propagation medium and cutting origin. Forest Ecology and management 92: 45-54.
- Shiembo PN, AC Newton, RRB Leakey (1996) Vegetative propagation of Irvingia gabonensis, a West African fruit tree. Forest Ecology and Management 87: 185-192.
- Eed Ahmed M (2016) Effect of various potting media on percent survival and growth of jojoba (simmondsia chinensis) rooted cuttings. Int J Curr Microbiol App Sci 5: 454-461.
- Wazir MG, NU Amin, M Ishtiaq (2003) Effects of different potting mixtures and nitrogen source on the performance of Brassaia seedlings-I. Sarhad J 19: 463-468.
- Sperry JS, UG Hacke (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Functional Ecology 16: 367-378.
- Hultine KR, DF Koepke, WT Pockman, et al. (2006) Influence of soil texture on hydraulic properties and water relations of a dominant warm-desert phreatophyte. Tree Physiology 26: 313-323.
- Hacke UG, JS Sperry, BE Ewers, et al. (2000) Influence of soil porosity on water use in Pinus taeda. Oecologia 124: 495-505.
- Graber Ellen R, Yael Meller Harel, Max Kolton, et al. (2010) Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant and soil 337: 481-496.
- Major Julie, Marco Rondon, Diego Molina, et al. (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant and soil 333: 117-128.
- Spokas Kurt A, Keri B Cantrell, Jeffrey M Novak, et al. (2012) Biochar: A synthesis of its agronomic impact beyond carbon sequestration. Journal of environmental quality 41: 973-989.
Corresponding Authors
Esther Mensah Opoku, Department of Forest Science, University of Energy and Natural Resources, P.O. Box 214, Sunyani, Ghana; Department of Silviculture and Forest management, Kwame Nkrumah University of Science and Technology, Ghana
Opuni-Frimpong E, Department of Forest Science, University of Energy and Natural Resources, P.O. Box 214, Sunyani, Ghana; CSIR-Forestry Research Institute of Ghana
Copyright
© 2022 Opoku ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.