Dormancy and Light Requirement for Germination of Sprawling Bauhinia (Tylosemafassoglense) Seeds
Abstract
Tylosemafassoglense (Fabaceae) is a tuber producing vine, endemic to sub-Saharan Africa, where it's used as food, fodder and medicine. However, its domestication may be hampered by limited information on its seed dormancy and germination ecology. This study evaluated seed-coat dormancy on three seed lots, collected from Busia, Migori and Siaya counties in Kenya, and the role of light on germination. Seed-coat dormancy was studied by water imbibition and germination percentage at 25℃ on scarified and non-scarified seeds from each seed lot. Additionally, the germination percentage of scarified seeds from each seed lot was evaluated under 12/12 and 0/24 hours photoperiod at 25℃. Dormancy level of non-scarified seeds from Migori differed significantly (p < 0.05) compared to both Busia and Siaya seed lots. Mean amount of water imbibed by non-scarified seeds was highest in seeds from Busia (1.07 g), followed by Siaya (0.89 g) and lowest in Migori (0.15 g). Mean germination percentage of non-scarified seeds from both Busia and Siaya was 70% and 30% in seeds from Migori. Scarification caused a significant (p < 0.05) increase for the three seed lots in both water imbibition and germination to above 3.40 g and 90%, respectively. Seed germination was insensitive to light and the relative light germination index was 0.46-0.59. This study suggests that T. fassoglense possess different levels of physical dormancy that can be attributed to seed source temperature while germination/regeneration can occur on or under the soil surface. The findings of this study are useful in propagation of T. fassoglense to benefit small-holder farmers of Kenya through food security and sustainable agriculture.
Keywords
Domestication, Imbibition, Light sensitivity, Scarification, Seed lot, Tylosemafassoglense
Introduction
Tylosemafassoglense Kotschyex.Schweinf. [Family: Fabaceae-Caesalpinioideae] is a tuber producing vine, endemic to the arid and semi-arid regions of sub-Saharan Africa [1]. In Kenya, T. fassoglense is widespread from the Coast, through Eastern, Rift Valley to the Lake Victoria Basin. It grows in scattered bushland, open grassland and along roadside on well drained sandy-loam soils [2]. The leaves are large, bilobed while flowers are creamy-yellow and dimorphic/heterostyllus [3]. Flowering and seeding phenology differs from one region to another in Kenya. The pods bear 1 or 2 large black-brown seeds. Tylosemafassoglense has been exploited for food (seeds, flowers and tubers), fiber (vines), fodder (leaves) and medicine (tubers) over decades [2, 4]. The seeds which contain oil (20-40%), protein (30-40%) and micronutrients are consumed as a snack or beverage [5-7]. The tuber which acts as water reservoir is rich in starch and it's heavily exploited for medicinal purpose [2, 4]. The extracts of T. fassoglense tuber have been reported to have antibacterial and antifungal activity [8]. Thus, seeds and tuber may serve as a valuable resource in modern nutraceutical and pharmaceutical industries. However, the extraction of the tuber and seed collection from the wild is not only unsustainable but also threatens its population and natural regeneration.
Tylosemafassoglense is widely distributed in Kenya and found in different habitats from open grasslands to roadsides and scattered bushlands [2]. Previous studies have reported on ethnobotanical information, potential uses as well as nutritional composition of seeds. However, there is no documented information on seed biology and germination ecology of T. fassoglense to guide its propagation and domestication. The reproductive cycle of plant species in the wild depends on seed production, dispersal, germination and soil seed bank [9]. Therefore, knowledge of seed dormancy and germination ecology is not only important in understanding plant regeneration in the wild but also the conditions conducive for artificial germination or production under cropping system.
The seeds of several plant species have evolved dormancy, which is adaption to environmental heterogeneity that synchronizes germination to suitable conditions in time and space [9, 10]. Seed dormancy; simply defined as the failure of a viable seed to germinate under suitable conditions for the species [11]. The seed dormancy level has been reduced in cultivated legume crops, Fabaceae- Papilionoideae allowing rapid and uniform germination as well as crop management [12]. However, seed dormancy remains a major biological constraint in propagating wild species including Fabaceae sub-families Caesalpiniodeae and Mimosoideae [9, 13]. The five classes of seed dormancy are; physical dormancy PY (i.e. presence of impermeable seed/fruit coat that prevents embryo imbibition); physiological dormancy PD (seed requiring special environmental condition to germinate); morphological dormancy MD (seed with underdeveloped embryos) and combinational dormancy (i.e. morphophysiological MP+PD and physiophysical PD + PY) [9].
Physical dormancy which is caused by the presence of tightly packed Malphigian cells that are impregnated with chemicals (e.g callose, suberin, phenolic compounds), that prevents water imbibition and/or gaseous exchange has been reported in 18 angiosperm plant families including Fabaceae [9]. Travlos, et al. (2007) [14] reported moderate physical dormancy in Tylosema esculentum (Fabaceae-Caesalpinioideae), however the presense of physical dormancy in one member of a family orgenus does not mean all the species have this type of dormancy [9]. Therefore, the presence or absence of physical dormancy should be evaluated on the other species in this genus including T. fassoglense. Seeddormancy is genetically controlled [15] and intra-species variations have been reported due to seed source temperature, soil salinity and nutrient level as well as seed collection year [16-22]. Under natural conditions, high and alternating temperatures and microbial action are known to break physical dormancy allowing imbibition and germination [23]. However, a number of artificial techniques have been developed for breaking physical dormancy in seeds that include manual and chemical scarification as well as alternating or high temperatures [14, 24-29]. This information has not been generated for T. Fassoglense to help in its propagation, domestication or use in conservation programmes.
The seeds of T. fassoglense have been reported to germinate well at temperature ranges of 15-35℃ and zero Megapascal water potential [30], however light and oxygen availability can also impact seed germination [9]. Light requirement is an environmental factor influencing seed germination, soil seed bank and dispersal in a number of species [23]. The role of light on germination is influenced by phytochrome B (light condition) and phytochrome A (dark condition) in imbibed seeds [9]. According to Baskin and Baskin, et al. (2014) [9] three groups of plants exist based on light requirement: those that require light (positive photoblastic), those not sensitive to presence/absence of light (neutral photoblastic) and those that do not require light (negative photoblastic). Milberg, et al. (2000) [31] also proposed relative light germination (RLG) index ranging from zero (negative photoblastic) to one (positive photoblastic) based on proportion of seed germinating under light and dark. The role of light in seed germination is also strongly linked to seed mass and shape, which limits seed burial depth that will permit seed germination and seedling emergence [31,32]. The concept of the role of light on germination has been explored on some wild species and vegetables [33-37]. The knowledge of the role of light on germination is important in propagation and understanding germination ecology of wild germplasm [9]. Tylosemafassoglense has the potential as a future crop in Kenya; however, information on seed germination response to light to guide sowing under cultivation has not been generated.
Therefore, the objective of this study was to investigate the presence or absence of physical dormancy and the role of light on germination in three seed lots of T. Fassoglense collected from Busia, Migori and Siaya counties in Kenya.
Material and Methods
Seed source information
Seeds of T. Fassoglense were collected from three populations (later referred to as seed lots) along the Lake Victoria basin in Busia, Migori and Siaya counties in Kenya where large populations have been reported [7]. The pods were sampled randomly from seeding plants between December 2017 and January 2018 in Busia (0.259770°N, 34.062437°E) and Migori (0.949194°S, 34.474791°E) sites and between April and June 2018 in Siaya (0.128960°N, 34.319916°E) site (Table 1). Harvest maturity indicator of T. Fassoglense was determined by pod physiological maturity when the pods colour turned from green to brown or dark brown and rattled when shaken. On hot sunny days, the mature pods snapped during harvesting to yield brown to dark brown or black hard seeds. The pods collected from these sources were put in paper bags and transported to Kenya Forestry Research Institute (KEFRI-Seed Centre) Muguga for processing. Seeds were extracted from the pods by drying at 25-30℃ in the glasshouse to allow them to dehisce naturally while those that failed to dehisce were opened manually by hand. Seeds were allowed to dry further in the glasshouse for a couple of weeks, sorted manually by hand and then stored at 10℃ for between three and eight months. The seed lots were shipped to the Millennium Seed Bank of the Royal Botanic Gardens, Kew, in the United Kingdom where the experiments were conducted.
Seed lot characteristics
Immediately the seeds were received at Millennium Seed Bank-Laboratories in the UK, the seed lot assessment by x-ray radiography, equilibrium relative humidity eRH, moisture content and morphology were conducted.
Seed x-ray radiography was performed on three replicates ofeach seed lot by placing random samples of seeds on a 240 x 180 x 2 mm transparent acrylic plate at a distance of 28.6 cm from radiation source (Faxitron MX-20DC12 cabinet X-ray system, FaxitronBioptics, Tucson, AZ, USA). The seed radiographs were used to assess seed quality by examining the embryonic and endospermic tissues for: emptiness, mouldiness, insect infestation, damaged embryo or seed coat and diseased tissues [38].
The seed eRH of T. Fassoglense was measured on random samples of three replicates from the three seed lots in the drying room operating at ca. 15% Relative Humidity (RH) and 15℃ [39]. The sample vial was three quarter filled and then inserted into the rotronic chamber (Rotronic AWVC-DIO Sensor Manufactured by Rotronic Limited, UK), with an accuracy of ± 2% eRH for 30 minutes to allow the seeds to equilibrate before recording the eRH% [40].
Moisture content, MC, was determined on ten seeds weighed singly using a microbalance (0.0001 g; UMT2 Mettler, Toledo). The seeds were put in metal cups and then dried in hot air oven operating at 103℃ for 17 hours [38]. The oven dried seeds were allowed to cool inside a closed drum containing silica gel ca. 0% RH for 30 minutes before reweighing. Seed moisture content (MC) was calculated on fresh weight basis (fwb) according to ISTA, (2017) (Eq.1).
where, M1=wet seed weight and M2=dry seed weight
Seed morphological measurement was performed by using a Carl Zeiss camera to take images from different angles of 10 seeds randomly sampled from each seed lot and mounted on a cardboard. From the images, seed dimensions (i.e. length, width and thickness) were estimated using Axio vision computer software (Axio Vs40; Carl Zeiss Micro imaging 2010, Germany). Seed shape was expressed as the variance of length L, width W and thickness T according to Thompson and Hodgson, et al. (1993) [32] whereby a value of 0 = dimensionless or perfectly spherical and ≥ 0.3 = needle or disc-shaped. Length, width and thickness were first transformed by dividing each by length after which the variance was obtained as the average of the squared differences from the mean (Eq.2).
Where S2 = sample variance, Xi = the value of the one observation, = the mean value of all observations and n = The number of observations.
Imbibition of water
To determine seed coat permeability to water, imbibition rates of water were measured for scarified and non-scarified seeds. For scarification treatment, small part of the seed coat opposite to the micropyle was chipped off using a nail clipper. Ten individual seeds for each treatment (scarified and non-scarified) from the three seed lots were used in the experiment. Initial weight of an individual seed was measured using the microbalance (0.0001 g; UMT2 Mettler, Toledo). The seeds were thereafter sown onto moistened anchor steel blue seed germination blotter (Anchor Paper Company, Seed Solutions; Saint Paul, MN, USA) in sandwich boxes (17 x 11 x 5 cm) placed at 25℃. Seeds were removed, blotted dry and weighed after 1, 3, 8, 23, 28, 32, 47 and 52 hours of keeping on moist media. Fifty two hours corresponded to the time when signs of radicle protrusion were observed. Seeds were watered when needed by adding 20 ml and 10 ml of deionized distilled water on scarified and non-scarified seeds respectively. The amount of water uptake (g) was calculated following modified formula by Baskin and Baskin, et al. (2004) [41] (Eq. 2):
where ΔMt is the mass increment at a given time "t", Mt represents the mass at a time "t" and Mi is the initial mass at time "t" = 0 (dry at 15%RH).
Effect of manual scarification on germination
The experiment of dormancy-breaking comprised four replicates of 10 seeds each for scarified and non-scarified (one replicate was the same used during the imbibition test). The seeds were sown onto moist germination paper in sandwich boxes (as described in imbibition tests), wrapped in self-sealing polythene and placed randomly in germination cabinet. The boxes were incubated at 25℃ plus 12/12 hour light/dark photoperiod (radiometric flux density 50-100 W.m-2). Seed germination was monitored daily for a period of 14 days. The final germination percentage was calculated as follows:
Seed germination under light and darkness
The role of light on germination was determined on scarified seeds of each seed lot sown onto moist germination paper in sandwich boxes in four replicates (as described in imbibition tests). For each seed lot, one set of four sandwich boxes was kept under 12/12 hour (light/dark) (radiometric flux density 50-100 W.m-2) and a second set under 0/24 hour (total darkness). For the dark treatment/photoperiod, seeds were wrapped in aluminium foil while the other set were kept in light penetrating sandwich boxes and incubated at 25℃. Germination for seed samples under dark photoperiod was scored in an isolated dark room under "safe" green light. The sandwich boxes were wrapped in self-sealing polythene bags to prevent moisture loss while the media was kept moist by adding 20 ml of deionised distilled water when needed. Germination percentages were calculated using (Eq. 3) and germination under light was compared against its absence using the relative light germination index (RLG) [31] (Eq. 5):
where Gl = proportion of germinated seeds in light conditions and Gd is its equivalent in dark conditions. The average seed germination percentage in presence of light and dark conditions was used for each seed lot. The RLG can range from zero (negative photoblastism) to one (positive photoblastism)
Germination evaluation
Germination was recorded daily for the three seed lots until no more seeds germinated for two weeks. Seed germination was defined as, radicle protrusion (>2 mm) and the germinated seeds were left in the sandwich boxes to allow them to develop into normal seedling. According to ISTA (2017) [38], a normal seedling is that which has grown to show essential structures (i.e. cotyledons, hypocotyls and roots) necessary for further development into a satisfactory plant and lacks visible abnormalities. Viability of non-germinated seeds were evaluated by cut test and seeds that were firm and full were reported as viable while rotten seeds were reported as dead.
Data analyses
All percentage data (seed eRH, MC, and germination) were arcsine transformed before analysis. Analysis of Variance (ANOVA) was conducted and the means separated with Tukey's HSD test where significance difference were observed (p < 0.05). Data was analysed using SAS Version 2002-2003 (SAS Institute Inc. Cary, NC, USA)
Results
Seed lot characteristics
The examined x-ray radiographs indicated high seed quality in the three seed lots, they had no signs of mouldiness or physical damage. There was no observable insect infestation and embryo damage across the three seed lots. The equilibrium relative humidity (eRH) of seeds sampled from Siaya seed lot was significantly higher (p < 0.05) than Migori seed lots. The mean eRH of T. fassoglense seed lots ranged from 56.03% (Migori seed lot) to 59.0% (Siaya seed lot) (Table 2). Seed moisture content of T. fassoglense did not differ (p>0.05) significantly among the three seed lots. The mean moisture content of seeds sampled from the three seed lots ranged from 5.9 % (Migori seed lot) to 6.3% (Siaya seed lot). The seed shape ranged from 0.32 (Busia seed lot) to 0.35 (Migori seed lot).
Imbibition of water
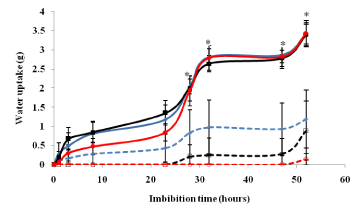
The average water uptake differed significantly (p < 0.05) between scarified and non-scarified seeds after 1, 3, 8, 28, 32, 47 and 52 hours of keeping on moist media (Figure 1). Scarified seeds from Busia and Siaya seed lots imbibed three to four times more water compared to the non-scarified seeds from the same lot after 52 hours of incubation. Scarified seeds from Migori seed lot imbibed 22 times more compared to the non-scarified seeds from the same seed lot after the same time. After 52 hours, the average amount of water imbibed by scarified seeds was between 3.41 g (Busia) and 3.44 g (Migori) while non-scarified seeds ranged from 0.15 g (Migori) to 1.08 g (Busia). Non-scarified seeds from Migori seed lot imbibed the least amount of water.
Effect of manual scarification on germination
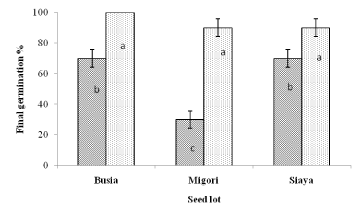
Seed scarification significantly (p < 0.05) improved the final germination percentages in the three seed lots (Figure 2). The mean final germination percentage of scarified seeds ranged from 90% (both Migori and Siaya seed lots) to100% (Busia seed lot) and non-scarified seeds ranged from 30% (Migori seed lot) to 70% (both Busia and Siaya seed lots). A cut test on non-germinated seeds at the end of the experiment showed high viability of 90% in all the seed lots tested.
Light sensitivity on germination
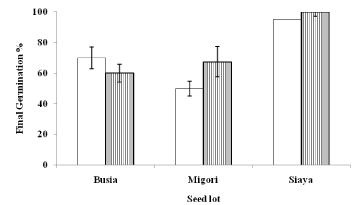
Seed germination under the two photoperiods of light/dark and total darkness was not significantly (p>0.05) different among the seed lots. The final germination percentage of T. fassoglense seeds under both light/dark and darkness was over 50% (Figure 3). Similarly, seed germination sensitivity to light determined by calculating relative light germination index, RLG ranged from 0.46 (Busia seed lot) to 0.57 (Migori seed lot).
Discussion
Seed dormancy and dormancy-breaking
This study has demonstrated that seed scarification is effective in breaking dormancy in T. fassoglense. It confirms that T. fassoglense seeds have moderate physical dormancy [9]. Physical dormancy increases chances of seedling survival by promoting temporal distribution of seed germination due to variation in degree of coat permeability and different germination timing for each seed population [9]. Previous studies have been conducted to determine seed dormancy and effectiveness of dormancy breaking pre-treatment in legume species [9,14,42,43]. For example, Abubakar and Muhammad, et al. (2013) [42] reported that scarification of Tamarindusindica (Fabaceae- Caesalpinoideae) using 50% sulphuric acid for 30 minutes resulted in higher germination percentage compared to hot water (100℃) treatment for 30 minutes and the control. Travlos, et al. (2007) [14] reported that mechanical scarification using sandpaper, soaking seeds in cold water for 20 hours or in concentrated sulphuric acid for 20 minutes greatly increased speed and percentage germination of T. esculentum (Fabaceae- Caesalpinoideae). Similarly, Long, et al. (2012) [43] reported that scarification improved germination of Astralagus arpilobus (Fabaceae- Papilionoideae) from 4% to nearly 100%. The positive response to manual scarification by the three seed lots confirms the presence of some level of physical dormancy in T. fassoglense.
The level of physical dormancy has been reported to vary among seed lots of a few legumespecies [17,26,29]. Richard, et al. (2018) [29] reported that variation in "hard seededness" and seed coat thickness in three seed lots of Desmanthusvirgatus (Fabaceae- Mimosoideae) was linked to the environmental aridity of the seed source. Hradilová, et al. (2019) [26] reported variation in germination (ranging between 0-100%) and seed coat thickness (80-140 mm) across the different environment envelopes in 96 accessions of wild pea (Pisumsativumsbsp. elatius). Similarly, Lacerda, et al. (2004) [17] reported dormancy variation in Sennamultijuga (Fabaceae- Caesalpinioideae) and Plathymeniareticulata (Fabaceae- Mimosoideae) at population level with seed germination of 9-35% and 40-62% respectively for non-scarified seeds and germination improved > 84% for scarified seeds. Additionally, the air temperatures during seed filling phase strongly affects seed coat pigmentation and dormancy [18,20,21]. For example, Toorop, et al. (2012) [21] reported a transition in seed coat colour during seed set that alters dormancy and correlates with flowering in Carpsella bursa-pastoris. Similarly, MacGregor, et al. (2015) [18] reported seed coat pigmentation is affected by temperature during seed development in Arabidopsis sp. which greatly impacted on dormancy level. Springthorpe and Penfield, et al. (2015) [20] reported that lower temperatures during seed filling almost always result in lower germination in Arabidopsis thaliana. Besides temperature, seed collection year and storage duration are known to influence seed dormancy even when working with same population [17,44]. Among the seed lots of T. fassoglense, non-scarified seeds from Migori imbibed the least and had the lowest germination percentage than both Busia and Siaya seed lots. The observed difference in dormancy level between Migori seed lot and Busia as well as Siaya seed lots may be attributed to the prevailing temperatures during seed filling phase since seeds from Busia and Migori were collected and stored for similar duration. The mean monthly maximum temperatures preceding seed maturity in Migori were 1℃ lower than both Busia and Siaya. However, altitude, salinity, nutrient levels and genotype can also affect dormancy level but were not considered in this experiment [16,22]. Although further research needs to be done, the air temperature during seed filling phase seems to strongly influence seed dormancy in T. fassoglense. Hard seed coat is an undesirable trait in agricultural production and in food processing, therefore this information will be important in selecting seed production sites for domestication programs.
Sensitivity to light presence
Regarding light, this study has demonstrated that T. Fassoglense seeds are probably neutral photoblastic [9]. The results of this experiment showed that seeds of T. fassoglense incubated under both light/dark and dark conditions had high germination percentages. Several species have been shown to be indifferent to light, however a small proportion exist in a seed population of neutral photoblastic that require light for germination [23]. For example, Bhatt, et al. (2016) [45] and Sánchez-Bayo and King, et al. (1994) reported that light or light/dark condition did not affect germination of Lotus glinoides (Fabaceae-Papilionoideae) and Acacia horrid (Fabaceae- Mimosoideae) respectively. Motsa, et al. (2015) [36] reported light positively affected germination of Brassica rapaL. subsp. Chinensis, Citrulluslanatus Thunb. And SolanumretroflexumDun. seed and optimal germination would occur if seeds were sown at or near soil surface. Gairola, et al. (2017) [35] reported seeds of Lotononisplatycarpa (Fabaceae) germinated to higher percentage under light condition than in total darkness.
Additionally, seed mass and shape have also been shown to be correlated to germination light requirement and soil seed bank formation for other taxa [31,32]. The results of T. Fassoglense seed weight, relative light germination (RLG) index range and seed shape were characteristic of neutral photoblastic species. For example, Milberg, et al. (2000) [31] reported that germination became less dependent on light with increasing seed mass in 54 species. Gómez-Barreiro, et al. (2019) [46] reported light had an impact at constant temperature on germination of Flacourtiaindica (Salicaceae) based on relative light germination index. Thompson and Hodgson, et al. (1993) [32] reported that seed weighing less than 15 mg and had a variance of less than 0.18 were likely to form soil seed bank in 97 species. Similarly, Flores, et al. (2016) [34] reported that seed with higher seed mass were less dependent on light for germination in 13 species (10-Asparagaceae sp; 3-Cactaceae sp.).
From the results of this study, regeneration of T. Fassoglense under natural habitat is not affected by seed positioning on the soil and germination can probably occur under or at the soil surface, under leaf litter or in vegetation gaps [23]. However, further studies considering habitat, seed coat colour, varied lighting regimes and seed germination in relation to sowing depth may provide additional information. This information will be useful in propagation and domestication of T. fassoglense.
Conclusion
The results suggest that T. Fassoglense possess moderate physical dormancy that can potentially affect germination and stand establishment under crop production. Seed dormancy variation was also observed among the three seed lots, a trait that can be exploited in selecting seed production sites for domestication. Scarification positively increased water imbibition and the final germination of T. Fassoglense seeds which will help improve its propagation. The study indicates that seed positioning of T. Fassoglense at the soil layer does not affect its regeneration in the wild and germination can occur when buried or at the soil surface, under leaf litter or in vegetation gaps. This trait is common in cultivated crops and may positively facilitate its domestication and propagation under cropping ecosystem. The information generated in this study can help the propagation and domestication of T. Fassoglense to benefit small-holder farmers of Kenya through food security and sustainable agriculture.
Funding
This study has been funded by MGU, a philanthropist based in Spain, as part of Project MGU - the Useful Plants Project, managed by the Royal Botanic Gardens, Kew. The authors have no conflict of interests to declare.
Acknowledgments
The authors sincerely acknowledge Pablo Gómez-Barreiro for his support during the experimentation.
References
- Castro S, Silveira P, Coutinho AP, et al. (2005) Systematic studies in tylosema (Leguminosae). Botanical Journal of the Linnean Society 147: 99-115.
- Maundu M, Ngugi W, Kabuye H (1999) Traditional food plants of Kenya: National Museums of Kenya.
- Hartley ML, Tshamekeng E, Thomas SM (2002) Functional heterostyly in Tylosema esculentum (Caesalpinioideae). Ann Bot 89: 67-76.
- Brink M (2006) Cereals and Pulses. PROTA.
- Dubois M, Lognay G, Baudart E, et al. (1995) Chemical characterisation of Tylosema fassoglensis (Kotschy) Torre & Hillc oilseed. Journal of the Science of Food and Agriculture 67: 163-167.
- Okumu TO (2011) Investigation of tylosema fassoglensis seed as an alternative source of protein in animal feed. MSc. Thesis. University of Nairobi, Kenya.
- Otieno OD, Awuor OB, Wafula WG (2015) Quality evaluation of oil from seeds of wild plant tylosema fassoglensis in Kenya. Journal of Food Processing 2015.
- Adongo JO, Omolo JO, Njue AW, et al. (2012) Antimicrobial activity of the root extracts of Tylosema fassoglensis Schweinf. Torre &Hillc (Caesalpiniaceae).
- Baskin CC, Baskin JM (2014) Seeds: Ecology, biogeography and evolution of dormancy and germination. (2nd edn) Academic Press: San Diego, CA, USA.
- Bewley JD, Bradford KJ, Hilhorst HW, et al. (2013) Germination. In Seeds. Springer, New York, NY 133-181.
- Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytologist 171: 501-523.
- Bewley JD, Black M (1994) Dormancy and the control of germination. In Seeds. Springer, Boston, MA 199-271.
- Rodríguez-Arévalo I, Mattana E, García L, et al. (2017) Conserving seeds of useful wild plants in Mexico: main issues and recommendations. Genetic Resources and Crop Evolution 64: 1141-1190.
- Travlos IS, Economou G, Karamanos AI (2007) Germination and emergence of the hard seed coated Tylosema esculentum (Burch) A. Schreib in response to different pre-sowing seed treatments. Journal of Arid Environments, 68: 501-507.
- Willis CG, Baskin CC, Baskin JM, et al. (2014) The evolution of seed dormancy: environmental cues, evolutionary hubs, and diversification of the seed plants. New Phytol, 203: 300-309.
- Fenner M (1991) The effects of the parent environment on seed germinability. Seed Science Research 1: 75-84.
- Lacerda DR, Lemos Filho JP, Goulart MF, et al. (2004) Seed-dormancy variation in natural populations of two tropical leguminous tree species: Senna multijuga (Caesalpinoideae) and Plathymenia reticulata (Mimosoideae). Seed Science Research 14: 127-135.
- MacGregor DR, Kendall SL, Florance H, et al. (2015) Seed production temperature regulation of primary dormancy occurs through control of seed coat phenylpropanoid metabolism. New Phytol 205: 642-652.
- Smýkal P, Vernoud V, Blair MW, et al. (2014) The role of the testa during development and in establishment of dormancy of the legume seed. Front Plant Sci 5: 351.
- Springthorpe V, Penfield S (2015) Flowering time and seed dormancy control use external coincidence to generate life history strategy. Elife 4: e05557.
- Toorop PE, Campos Cuerva R, Begg GS, et al. (2012) Co-adaptation of seed dormancy and flowering time in the arable weed Capsella bursa-pastoris (shepherd's purse). Ann Bot 109: 481-489.
- Wang L, Baskin JM, Baskin CC, et al. (2012) Seed dimorphism, nutrients and salinity differentially affect seed traits of the desert halophyte Suaeda aralocaspica via multiple maternal effects. BMC Plant Biology 12: 1-11.
- Fenner M, Thompson K (2005) The ecology of seeds: Cambridge University Press.
- de Morais LF, Almeida JC, Deminicis BB, et al. (2014) Methods for breaking dormancy of seeds of tropical forage legumes. American Journal of Plant Sciences 5: 1831-1835.
- Gama-Arachchige N, Baskin J, Geneve R, et al. (2013) Identification and characterization of ten new water gaps in seeds and fruits with physical dormancy and classification of water-gap complexes. Ann Bot 112: 69-84.
- Hradilová I, Duchoslav M, Brus J, et al. (2019) Variation in wild pea (Pisum sativum subsp. elatius) seed dormancy and its relationship to the environment and seed coat traits. Peer J 7: e6263.
- Jayasuriya KG, Wijetunga AS, Baskin JM, et al. (2013) Seed dormancy and storage behaviour in tropical Fabaceae: a study of 100 species from Sri Lanka. Seed Science Research 23: 257-269.
- Kimura E, Islam M (2012) Seed scarification methods and their use in forage legumes. Research Journal of Seed Science 5: 38-50.
- Richard GA, Zabala JM, Cerino MC, et al. (2018) Variability in hardseededness and seed coat thickness of three populations of Desmanthus virgatus (Fabaceae, Mimosoideae). Grass and Forage Science 73: 938-946.
- Otieno V, Ulian T, Nzuve F, et al. (2020) Germination response to temperature and water potential for Sprawling bauhinia (Tylosema fassoglense), a potential crop for Kenya. South African Journal of Botany 132: 463-470.
- Milberg P, Andersson L, Thompson K (2000) Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Science Research 10: 99-104.
- Thompson K, Band S, Hodgson J (1993) Seed size and shape predict persistence in soil. Functional Ecology 236-241.
- Costa CRX, Pivetta KFL, de Souza GRB, et al. (2018) Effects of temperature, light and seed moisture content on germination of euterpe precatoria palm. American Journal of Plant Sciences 9: 98-106.
- Flores J, González-Salvatierra C, Jurado E et al. (2016) Effect of light on germination and seedling shape of succulent species from Mexico. Journal of Plant Ecology 9: 174-179.
- Gairola S, Shabana HA, Mahmoud T, et al. (2018) Effects of seed colour heterogeneity on germination behaviour of the desert plant Lotononisplatycarpa (Fabaceae). Nordic Journal of Botany 36: njb-01617.
- Motsa MM, Slabbert MM, Van Averbeke W, et al. (2015) Effect of light and temperature on seed germination of selected African leafy vegetables. South African Journal of Botany 99: 29-35.
- Sánchez-Bayo F, King GW (1994) Imbibition and germination of seeds of three Acacia species from Ethiopia. South African Journal of Plant and Soil 11: 20-25.
- International Rules for Seed Testing (ISTA) (2017) The International Seed Testing Association, (ISTA), Bassersdorf, CH-Switzerland.
- FAO IPGRI (1994) Genebank standards. FAO/IPGRI, Rome.
- Karrfalt RP (2010) Equilibrium relative humidity as a tool to monitor seed moisture. In: Riley LE, Pinto JR, Dumroese RK, tech. cords. National Proceedings: Forest and Conservation Nursery Associations-2009. Proc. RMRS-P-62. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station. 45-47.
- Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Science Research 14: 1-16.
- Abubakar ZA, Muhammad A (2013) Breaking seed dormancy in Tamarind (Tamarindus indica) a case study of gombe local government area. Journal of Applied Sciences and Environmental Management 17: 83-87.
- Long Y, Tan DY, Baskin CC, et al. (2012) Seed dormancy and germination characteristics of Astragalus arpilobus (Fabaceae, subfamily Papilionoideae), a central Asian desert annual ephemeral. South African Journal of Botany 83: 68-77.
- Penfield S, MacGregor DR (2017) Effects of environmental variation during seed production on seed dormancy and germination. Journal of Experimental Botany 68: 819-825.
- Bhatt A, Gairola S, El-Keblawy AA (2016) Seed colour affects light and temperature requirements during germination in two Lotus species (Fabaceae) of the Arabian subtropical deserts. Rev biol trop 64: 483-492.
- Gómez-Barreiro P, Otieno V, Mattana E, et al. (2019) Interaction of functional and environmental traits on seed germination of the multipurpose tree Flacourtia indica. South Africa Journal of Botany 125: 427-433.
Corresponding Author
Victor Otieno, Department of Plant Science and Crop Protection, College of Agriculture and Veterinary Sciences, University of Nairobi, P. O. Box 30197-00100, Nairobi, Kenya.
Copyright
© 2022 Otieno V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.