Flower Types, Pollen Morphology, and In Vitro Pollen Germination of Longan (Dimocarpus longan Lour.)
Abstract
Longan (Dimocarpus longan Lour.) is an important tropical and subtropical fruit tree in Southeast Asia and China. We investigated flower type, morphology, and development, pollen morphology, and pollen vigor of a popular Chinese cultivar 'Honghezi'. Results showed that the cultivar developed three types of flower, i.e. two types of male flowers (M1 and M2) and female flower (F). The stamens of M2 were longer than that of M1 and F. The rudimentary pistil of M2 was longer than that of M1. Flowers in the terminal inflorescences opened in three waves in time, and the M1 and M2 flowers did not let to seed-setting. The pollen grains of the three types of longan flowers were trizonocolporate, and the size of a viable hydrated pollen grain ranged from 20.18 (± 1.94) to 27.40 (± 1.44) µm. An in vitro evaluation of pollen vigor was used for pollen grains of M1 flower. Medium sucrose concentrations and temperatures significantly affected pollen germination and pollen tube growth in vitro, and the optimized conditions were the medium containing 15% sucrose and incubated at 25 ℃ for 12 hrs. The flower developmental timing and the underdeveloped and rudimentary structures in relation to pollen vigor and seed-setting, point to an evolutionary significance, which together with the pollen vigor techniques are useful for molecular analysis and breeding of this specialty crop.
Keywords
Chinese longan, Pollen viability and vigor, Sapindaceae, Sucrose, Temperature
Introduction
Dimocarpus longan Lour., commonly known as longan, belongs to the family Sapindaceae and is one of the most important tropical and subtropical fruit trees indigenous to the mountains of Myanmar (Burma) and southern China [1]. The primary center of longan origin in China was Yunnan, and the secondary centers were Guangdong, Guangxi, and Hainan provinces [2]. Longan bears delicious and aromatic fruits that can be eaten fresh, frozen, canned or dried, and the fruit is also valuable in traditional Chinese medicine [3,4]. The world longan production in 2010 was more than 2500 million tons, of which China produced about 1300 million tons and Vietnam and Thailand produced more than 600 and 500 million tons, respectively [5]. Because of its nutritional and pharmaceutical values, longan production is expected to significantly increase in the future [6].
Longan is an underutilized specialty crop, and little effort has been made to improve this species worldwide [7]. It is a cross-pollinated species; its chromosome number is 2n = 2x = 30 [1]. Longan is reported to have three types of flower (staminate, female flower with underdeveloped stamens, and male flower with rudimentary pistil) borne on terminal inflorescences or panicles [6]. Staminate flowers open first, followed by female flower, and finally male with rudimentary pistil flowers [6,8]. There is an overlapping between male and female flower opening time depending on cultivars and environmental conditions [6,8]. Pollination is mainly carried out by insects, particularly stingless bees and honeybees and also by wind, and fruit set is heavily dependent on the viability of pollen grains and weather conditions [9]. Thus far, information regarding pollen morphology, viability, germination, and tube growth in this species is limited [10].
The differential development of structures such as stamens and pistil could be used as a model for molecular analysis and breeding. Moreover, the cascade of gene expression will be detected since the organs cease their development in different stages.
In vitro methods have been widely used for studying pollen vigor [11]. Pollen grains were cultured on a selected medium and tested for viability based on the germination percentage and tube growth [12]. The medium compositions include sucrose as carbon source, boron and calcium, and Polyethylene Glycol (PEG) [13]. Pollen from different species often requires different medium components and different concentrations of the components [11,14]. Among them, sucrose is one of the essential components, serving to control the osmotic potential of the germination medium and also providing a base for polysaccharide synthesis and metabolic energy [15]. Different species may need different sucrose concentration for example 5% is optimal for pecan [16], 10% for Salix species [17], 15% for Prunus laurocerasus [18], 20% for okra [19], 30% for Pithecellobium dulce [20], and 40% for Areca catechu [21]. Therefore, in addition to selecting an appropriate culture medium, sucrose concentrations in the medium should be optimized for evaluating pollen vigor of a particular species.
Temperature is an important environmental factor influencing pollen germination and pollen tube growth during the programic phase [22,23]. Different plant species need different optimal temperatures for pollen germination and tube growth. Temperature ranging from 18 to 20 ℃ is appropriate for wheat pollen germination [24], 22 ℃ for Arabidopsis [25], 25 to 30 ℃ for cocoa [26], 25 to 29 ℃ for avocado [27], and 30 ℃ for pistachio [28]. In longan, Pham, et al. [10] reported that 30 ℃ was the appropriate temperature for in vitro germination of Thai cultivars 'Choompoo', 'Fuk How', 'Biew Kiew', and 'Duan Yu'. In Chinese cultivar 'Shixia', the optimal temperature for in vitro germination is between 23 to 37 ℃ [29]. For the Chinese longan, however, information regarding sucrose concentration and culture temperature in vitro evaluation of pollen viability is limited.
The objectives of this study were to document flower types and developmental structure, and pollen morphology of a representative Chinese cultivar 'Honghezi', and determine the effects of sucrose concentration and culture temperature on in vitro culture pollen germination and pollen tube growth. Information gained from this study could help us improve longan breeding programs in China.
Materials and Methods
Plant material
Flowers of longan cultivar 'Honghezi' grown in the longan collection garden at Fujian Agriculture and Forestry University, China were closely examined during April and May 2015. The newly opened flowers i.e. male 1 (M1), male 2 (M2), female (F) were collected between 08:00 and 10:00 hrs and brought to the laboratory for recording, photographing and other studies. After recording floral types, structure and development, flowers were left in the laboratory at room temperature and relative humidity of 70% for 30 to 60 minutes for anther dehiscence.
Morphology and development of flowers and pollen
The flowers (M1, M2 and F) were collected, observed under stereo-microscope and took photographs. To observe pollen grains under a microscope, they were collected from dehiscent anthers of newly opened M1 and M2 flowers. Indehiscent anthers of F flowers were squashed on the microscope slide and observed under a microscope. Micrographs were taken using a digital camera attached Leica DMIL inverted microscope (Germany). To document the size of the pollen, longest and shortest diameters were measured using Image J software IJ1.48r (NIH, Maryland, USA).
Sucrose concentrations, pollen germination and pollen tube growth
Pollen grains, from M1 anthers which were carefully chosen to ensure healthy and dehisced, were being used. A basal medium, which was used by Vivian-Smith, et al. [30] for in vitro germination of litchi (Litchi chinensis Sonn.) pollen, was modified in this study. This basal liquid pollen germination medium originally contained 150 g•L-1 PEG 4000, 4.88 g•L-1 MES-KOH (pH 6.4), 409.52 mg•L-1 MgSO4.7H2O, 100 mg•L-1 KNO3, 100 mg•L-1 H3BO3, 1.007 g•L-1 Ca(NO3)2.4H2O, and 200 g•L-1 sucrose with a final pH of 5.5. We modified the medium sucrose concentrations to 5%, 10%, 15%, 20%, 25%, or 30% (w/v), thus media with six sucrose concentrations were prepared. The sitting drop culture technique described by Shivanna and Rangaswamy [31] was used for pollen culture. Twenty dehisced anthers were dipped and slightly shaken one after another in 100 µL of each sucrose concentration. A 30 µL aliquot of each pollen mixture was placed on a clean dry glass slide. There were three replicates per sucrose concentration. Slides for each treatment were kept across the two supporting glass rods in large Petri plates (ca. 15 cm diameter) with a moist filter paper per plate. The slides were incubated at 25 ℃ in darkness for 12 hrs in an incubator (Bluepard, Shanghai, China). A minimum of 100 pollen grains per slide was randomly counted under the microscope at X200 magnification. According to Stanley and Linskens [15], pollen grains were considered germinated when the length of the pollen tube was equal to or exceeded its diameter. Subsequently, the length of the pollen tubes was measured using Image J software IJ1.48r (NIH, Maryland, USA).
Temperature, pollen germination and tube growth
After optimizing sucrose concentrations in the basic germination medium, pollen grains of 'Honghezi' were mixed with the medium containing 15% sucrose and cultured at 20, 25, 30, or 35 ℃, respectively for 12 hrs in the darkness using the aforementioned method. There were three replicates for each temperature regime. Pollen germination percentage and pollen tube length were determined.
Data analysis
Pollen germination percentage data were arcsine-square root transformed and pollen tube lengths were analyzed using SPSS 19.00 statistical software (SPSS Inc., Chicago, USA). Means were separated by Tukey HSD test at P ≤ 0.05.
Results and Discussion
Floral types, morphology and development
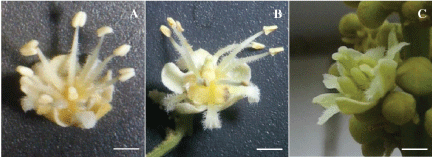
The flowering season of 'Honghezi' went from late April to late May. The stamens of M2 were longer than that of M1 and F. The rudimentary pistil of M2 was longer than that of M1. Viable pollen grains were developed from M1 and M2 but not from F which produced immature ones. The dehiscence of anthers started before noon but it largely depends on the temperature, and then the pollen grains were lost quickly since pollinators taken them away. The flower types and flowering open sequence in 'Honghezi' resemble those reported in other longan cultivars [6,8,32]. The development of the pistil in each kind of flowers might be useful in the work of molecular breeding. In order to study the genes and proteins involved in the early stage of the pistil, the rudimentary pistil of M1 should be used since it ceased the development in very early stage (Figure 1A). And for the middle stage, the undeveloped pistil of M2 might be used (Figure 1B).
Pollen morphology and development
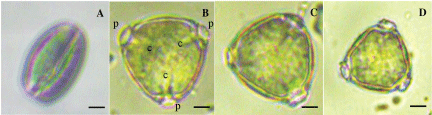
Pollen grains collected from all types of flowers (M1, M2 and F) were tricolporate. In M1 and M2, the pollen grains were elongate at dehiscence (Figure 2A) but become rounded triangular or nearly circular after being hydrated in water or in the liquid medium (Figure 2B, Figure 2C and Figure 2D). However, the anthers of F never dehisce and never release pollen since they were underdeveloped. The average size of M1 pollen at dehiscence was 15.66 ± 1.36 µm × 25.66 ± 1.32 µm (n = 120), while hydrated pollen grains were 24.21 ± 1.46 µm × 27.40 ± 1.44 µm (n = 106). The size of hydrated pollen grains of M2 and F were 20.18 ± 1.94 µm × 21.46 ± 1.43 µm (n = 136) and 17.89 ± 2.34 × 20.03 ± 1.48 (n = 153), respectively. Among pollen grains of three kinds of flower, the size of M1 pollen was the largest while F pollen was the smallest. Mature anthers of M1 and M2 produced viable and mature pollen grains. We found that, however, in addition to the non-dehiscence of F anther, most of the F pollen grains were still together with generative tissues, clumped together and had thick pollen wall suggesting that F pollen grains were still at the young stage. So we can conclude that F anther produced immature pollen grains since anther was not mature enough while the pistil was ready to function. These immature pollen grains from F might be used in vitro pollen maturation. The size and morphology of longan pollen grains measured in the present study were similar to those of the litchi which also belongs to the family Sapindaceae [33].
Sucrose concentration effects on pollen vigor
Sucrose concentrations significantly affected both pollen germination and pollen tube growth. At 5% sucrose concentration, the average germination percentage was only 4.83%, and the average pollen tube length was 98.68 µm (Figure 3A and Figure 3B). At 15% of sucrose concentration, however, pollen germination increased to 95.13% and pollen tube length was 273.63 µm (Figure 3A and Figure 3B). Sucrose concentrations ranging from 10% to 20% did not result in significant difference in pollen tube growth (Figure 3B). Thus, 15% of sucrose concentration was considered to be optimal for testing 'Honghezi' longan pollen vigor. This sucrose concentration, however, differed from the report of Pham, et al. [10] for Thai longan cultivars where 10% of sucrose was optimal. The difference may be attributed to cultivar differences and may also be due to the difference in PEG concentrations. The present study had 15% PEG 4000, while the medium in the report of Pham, et al. [10] had 23% PEG 8000. High concentrations of PEG reportedly require relatively low concentrations of sucrose [16]. Pollen vigor refers to the speed of pollen grain germination and the rate of pollen tube growth [34]. In vitro pollen germination tests have been widely used for evaluating pollen vigor [11,12]. In the present study, we used a basal medium that was initially developed for testing the pollen vigor of litchi [30]. Since longan is a close relative of litchi, and the present study found that this basal medium that was also appropriate for evaluating longan pollen vigor. Sucrose plays a vital role as osmoregulator and nutritive compound [11]. Our result showed that sucrose is important for pollen germination and pollen growth of 'Honghezi', and the optimal sucrose concentrations for evaluating pollen vigor of this cultivar range from 10 to 20%.
Effects of temperature on pollen germination and pollen tube growth
Longan pollen vigor was evaluated at 20, 25, 30, and 35 ℃, respectively using the basal medium containing 15% sucrose. Pollen germination occurred after incubation in the darkness for 12 hrs (Figure 4). The average percentages of the pollen germination and average length of pollen tubes at 25 ℃ scored highest while those are less at lower and higher temperature (Figure 5A and Figure 5B). Results from the present study showed that the optimal temperature for both pollen germination and tube growth of longan 'Honghezi' is 25 ℃. Our results concur with those of Zhao, et al. [29] in which optimal temperatures for in vitro pollen germination of a Chinese longan cultivar 'Shixia' were between 23 and 27 ℃ but differ from the report of Pham, et al. [10] where 30 ℃ was considered to be optimal for longan cultivars 'Choompoo', 'Fuk How', 'Biew Kiew', and 'Duan Yu'. The temperatures used by Pham, et al. [10] were 10, 20, 30, 35, and 40 ℃; it is unknown if 25 ℃ is also appropriate for the four cultivars. On the other hand, the optimal temperatures for in vitro pollen germination and tube growth of litchi were around 30 ℃ [35] and between 25 and 30 ℃ [33]. The differences in temperature requirement may be attributed the cultivar adaptation to different climatic conditions. 'Honghezi' and 'Shixia' were produced in Fujian Province, China where it is subtropical climate. However, 'Choompoo', 'Fuk How', 'Biew Kiew', and 'Duan Yu' were originated in Thailand where it is tropical climate. The adaptation to the subtropical climate may result in these cultivars requiring a relatively mild temperature for pollen germination.
With the awareness of its fruit nutritional and pharmaceutical values, there is an increased interest in longan research [7,10]. To genetically improve longan, however, pollen morphology and pollen vigor should be investigated. Using a basal medium, the present study established a protocol for in vitro evaluation of pollen vigor of 'Honghezi'. This protocol consists of 150 g•L-1 PEG 4000, 4.88 g•L-1 MES-KOH (pH 6.4), 409.52 mg•L-1 MgSO4.7H2O, 100 mg•L-1 KNO3, 100 mg•L-1 H3BO3, 1.007 g•L-1Ca(NO3)2.4H2O, and 15% of sucrose (150 g•L-1) with a pH of 5.5. Pollen should be cultured on the medium and incubated at 25 ℃ for 12 hrs to evaluate pollen germination rates and pollen tube growth. This method is simple and could be used for pollen selection [36] such as heat tolerance and for improving breeding efficiency of this underutilized specialty crop.
Only Sucrose concentration and temperature were optimized in this study. Better germination rate and longer pollen tube may be achieved by using an all-round optimized medium.
Implications in longan evolution and breeding
The present observations reveal that longan M1, M2 and F flowers ceased the development in different developmental stages: The M1 pistils at early stage (Figure 1A), the M2 pistils at middle stage (Figure 1B), and the F pistils at mature stage (Figure 1C), whereas the F stamens at early stage and the M1 and M2 stamens at mature stage. Besides the differences of the three types of flower structure and morphology, it is observed that the longan flowering showed three waves, M1 flower at the beginning and the M2 flower the last, F flowers occur in between. Hither to it is unknown why longan has three types of flowers with unique developmental process and structure, and three flowering waves. A possible explanation of having three kinds of flowers and three flowering waves is to promote cross pollination and increase seed setting leading to higher fruit production. And the timing of blooming was to save the energy while making successful seed-setting with least energy utilization. There was further evidence to explain that the energy was not equally distributed, for example, out of two carpels of ovary in F flowers (Figure 1C), only one carpel developed into fruit. In addition to the one out of two carpels developed into mature fruit, F flowers also produced immature pollen grains to save developmental energy while enlarging genetic basis by accepting pollen from other flowers. Based on these characteristics, the longan flowers might be served as a model for molecular analysis and breeding. Genes involved in different stages of the pistil and stamen can be cascaded by comparison of normal and the rudimentary structures, thus, more evolution information and breeding clues can be obtained.
Acknowledgment
Special thanks go to the Research Funds for the National Natural Science Foundation of China (31572088 and 31672127), Science and Technology Plan Major Projects of Fujian Province (2015NZ0002-1), and Horticultural Postdoctoral Research Funding of Fujian Agriculture and Forestry University (150797).
References
- Choo WK, Ketsa S (1992) Edible fruits and nuts. In: Verheij EWM, RE Coronel, Plant resources of South-east Asia No. 2. Prosea Foundation, Bogor, Indonesia, 146-151.
- Ke GW, Wang CC, Tang ZF (1994) Palynological studies on the origin of longan cultivation. Acta Hort Sinica 21: 323-328.
- Huang G, Wang B, Lin W, et al. (2012) Antioxidant and anti-inflammatory properties of longan (Dimocarpus longan Lour.) pericarp. Evidence-Based Complementary and Alternative Medicine 2012: 709483.
- Lin C, Chung Y, Hsu C (2012) Potential roles of longan flower and seed extracts for anti-cancer. World J Exp Med 2: 78-85.
- FAO (2011) Tropical fruits compendium. Food and Agricultural Organization of the United Nations.
- Wong KC (2000) Longan production in Asia. Food and Agricultural Organization of the United Nations, Bangkok, Thailand.
- Litz RE, Raharjo S (2005) Dimocarpus longan and Litchi chinensis litchi. In: Litz RE, Biotechnology of fruit and nut crops. CABI Publishing, Wallingford, UK, 628-636.
- Pham HD (2012) Pollination biology of jujubes and longans and the importance of insects in the pollination of crops in Vietnam. The University of Guelph, Canada.
- Blanche KR, Ludwig JA, Cunningham SA (2006) Proximity to rainforest enhances pollination and fruit set in orchards. Journal of Applied Ecology 43: 1182-1187.
- Pham VT, Herrero M, Homaza JI (2015) Effect of temperature on pollen germination and pollen tube growth in longan (Dimocarpus longan Lour.). Scientia Horticulturae 197: 470-475.
- Taylor LP, Hepler PK (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48: 461-491.
- Rodriguez Enriquez MJ, Mehdi S, Dickinson HG, et al. (2013) A novel method for efficient in vitro germination and tube growth of Arabidopsis thaliana pollen. New Phytol 197: 668-679.
- Stone LM, Seaton KA, Kuo J, et al. (2004) Fast pollen tube growth in Conospermum species. Ann Bot 93: 369-378.
- Acar I, Kakani VG (2010) The effects of temperature on in vitro pollen germination and pollen tube growth of Pistacia spp. Scientia Horticulturae 125: 569-572.
- Stanley RG, Linskens KF (1974) Pollen: Biology, biochemistry and management. Springer-Verlag, New York.
- Conner PJ (2011) Optimization of in vitro pecan pollen germination. HortScience 46: 571-576.
- Kopp RF, Maynard CA, de Niella PR, et al. (2002) Collection and storage of pollen from salix (Salicaceae). Am J Bot 89: 248-252.
- Mondal S, Ghanta R (2012) Effect of sucrose and boric acid on in vitro pollen germination of Solanum Macranthum Dunal. Indian Journal of Fundamental and Applied Life Sciences 2: 202-206.
- Baloch MJ, Lakoh AR, Hidavatullah B, et al. (2001) Impact of sucrose concentration on in vitro pollen germination of okra, Hibiscus esculentus. Pakistan Journal of Biological Sciences 4: 402-403.
- Rao AN, Ong ET (1972) Germination of compound pollen grains. Grana 12: 113-120.
- Liu L, Huang L, Li Y (2013) Influence of boric acid and sucrose on the germination and growth of areca pollen. American Journal of Plant Sciences 4: 1669-1674.
- Hedhly A, Hormaza JI, Herrero M (2005) Influence of genotype-temperature interaction on pollen performance. Journal of Evolutionary Biology 18: 1494-1502.
- Kakani VG, Reddy KR, Koti S, et al. (2005) Differences in in-vitro pollen germination and pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann Bot 96: 59-67.
- Chakrabarti B, Singh SD, Nagarajan S, et al. (2011) Impact of temperature on phenology and pollen sterility of wheat varieties. AJCS 5: 1039-1043.
- Boavida LC, Mc Cormick S (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570-582.
- Aneja M, Gianfagna T (1992) Carbon dioxide and temperature influence pollen germination and fruit set in cocoa. HortScience 27: 1038-1040.
- Sahar N, Spiegel Roy P (1984) In vitro germination of avocado pollen. HortScience 19: 886-888.
- Kardoush M, Dairy MA, Shdeifat S, et al. (2009) Effect of some local pollinators on nuts characteristics of three pistachio cultivars in Aleppo area. Res J Agr Biol Sci 5: 255-260.
- Zhao C, Peng N, Li X, et al. (2002) Studies on the pollen viability of Dimocarpus longan. Fujian Fruits 4: 8-10.
- Vivian Smith A, Mc Conchie CA, Batten DJ (1992) In vitro germination and growth of lychee (Litchi chinensis Sonn.). In: Control of fruit set and fruit retention in lychee. Rural Industries Research and Development Corporation Report CSIRO, Brisbane, 9-16.
- Shivanna KR, Rangaswamy NS (1992) Pollen biology: A laboratory manual. Springer-Verlag, New York.
- Pham VT, Tran MH, Herrero M, et al. (2013) The reproductive biology of the longan. In: Proceedings of the 5th National Scientific Conference on Ecology and Biological Resources, Hanoi, 1242-1246.
- Sukhvibul N, Dasananda M (2014) Morphology and factors affecting germination of litchi pollen. Acta Hort 1024: 171-176.
- Ottaviano E, Mulcahy DL (1989) Genetics of angiosperm pollen. Advances in Genetics 26: 1-64.
- Stern RA, Gazit S (1998) Pollen viability in lychee. JASHS 123: 41-46.
- Hormaza JI, Herrero M (1992) Pollen selection. Theoretical and Applied Genetics 83: 663-672.
Corresponding Author
Zhongxiong Lai, Institute of Horticultural Biotechnology, Fujian Agriculture and Forestry University, Fuzhou, Fujian 350002, China, Tel: +86-591-8378-9484.
Copyright
© 2017 Thu MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.